Real-Time Measurement of Herbicides in the Atmosphere: A Case Study of MCPA and 2,4-D during Field Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Measurements
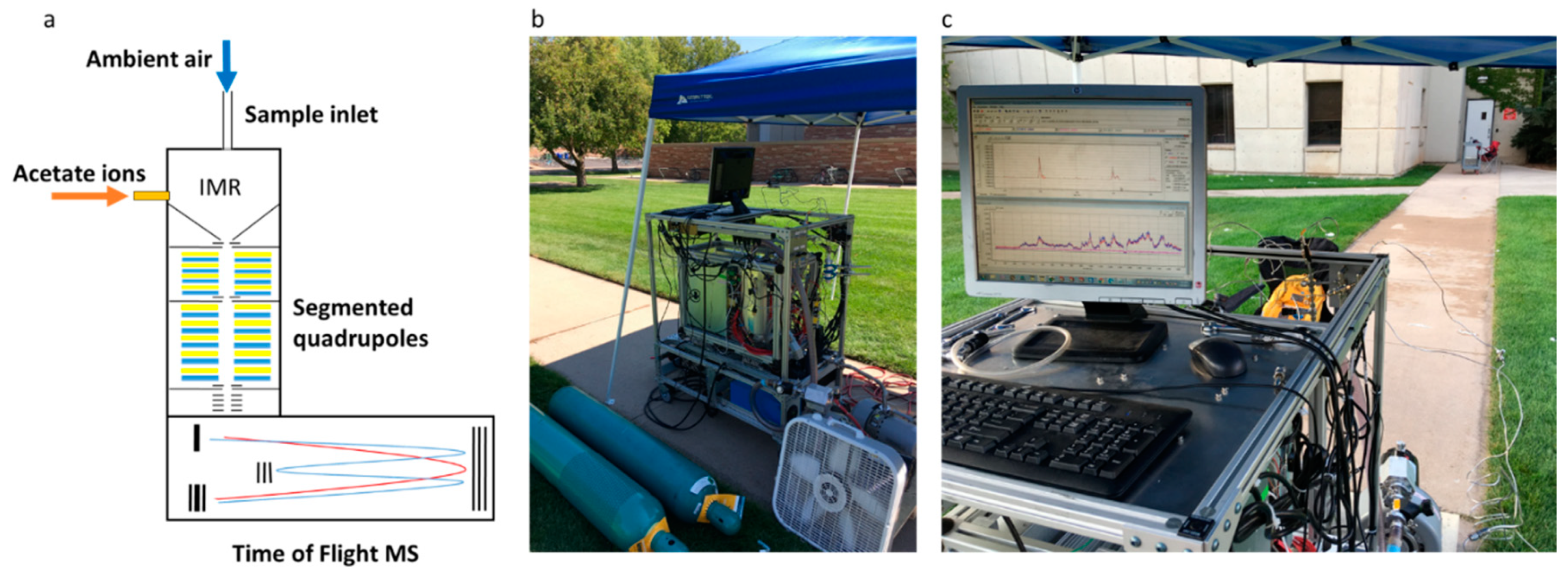
2.2. Acetate-CIMS
2.3. Data Analysis
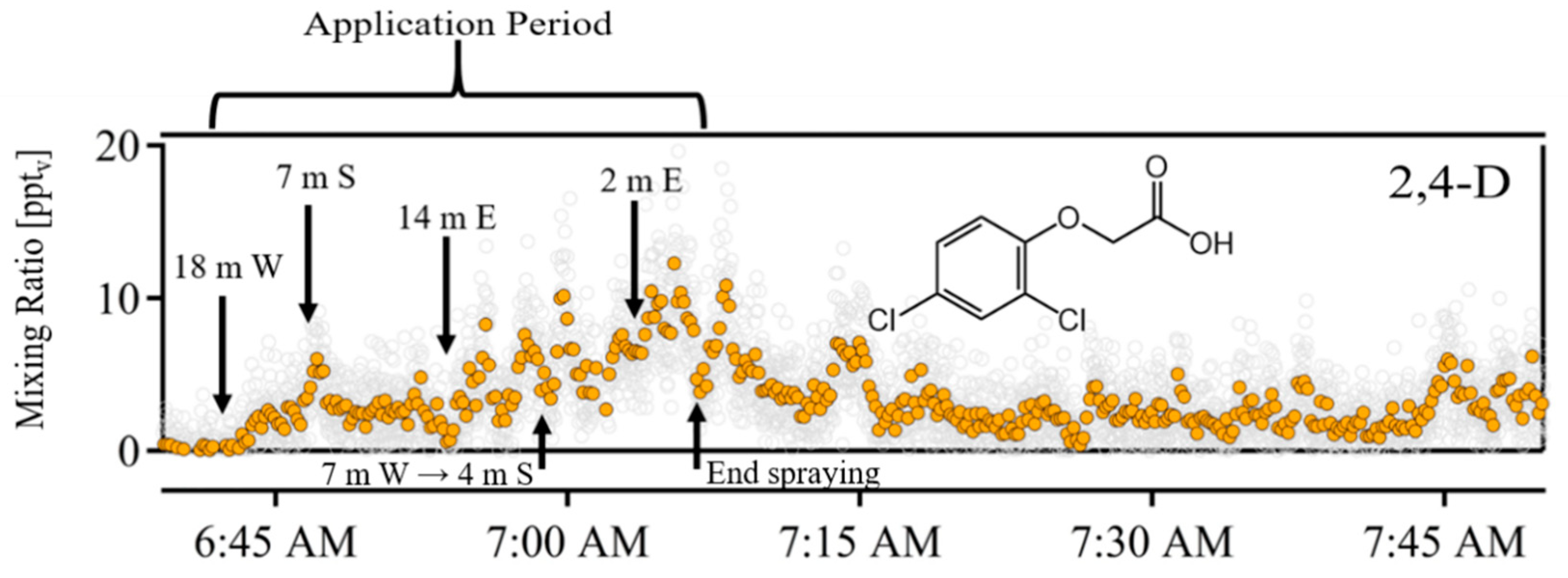
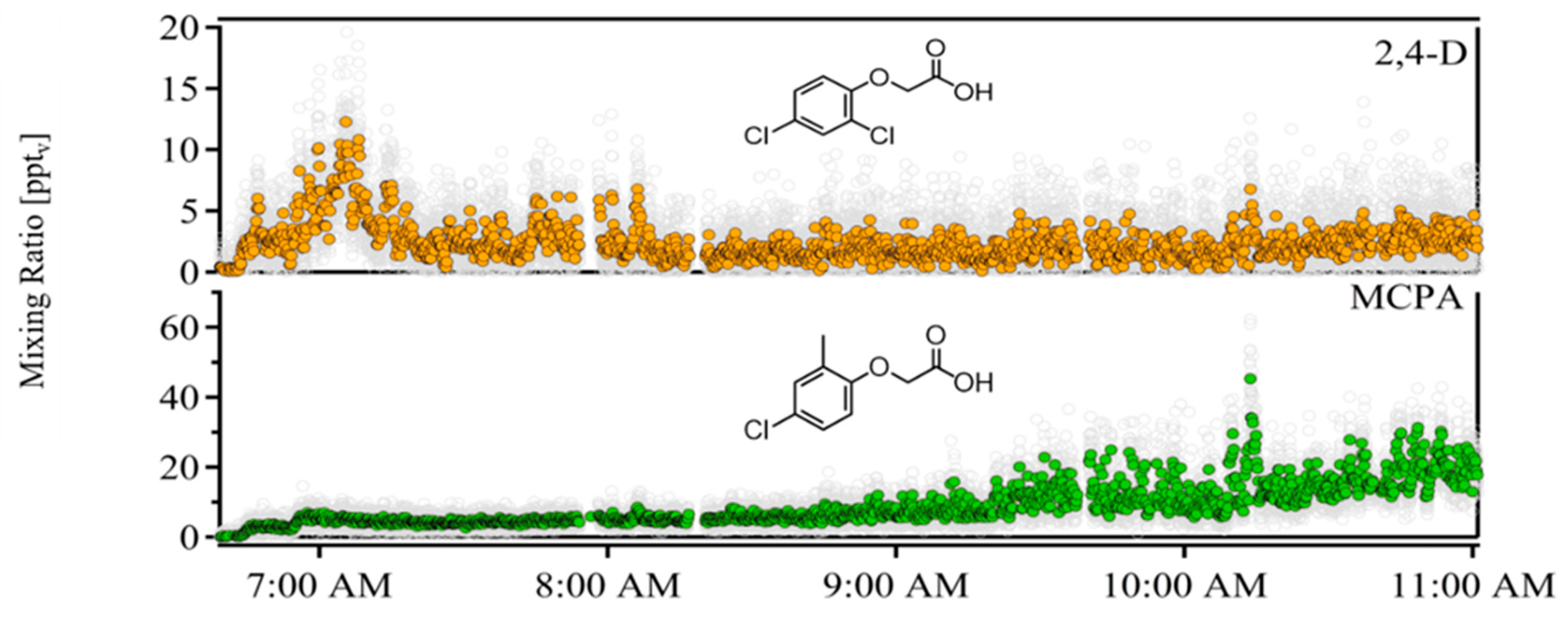
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atwood, D.P.J.; Paisley-Jones, C. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates; U.S. Environmental Protection Agency: Washington, DC, USA, 2017; pp. 4–5.
- Moon, J.K.; Choi, H.; Kim, J.H. Assessment of the Exposure of Workers to the Insecticide Imidacloprid during Application on Various Field Crops by a Hand-Held Power Sprayer. J. Agric. Food Chem. 2013, 61, 10642–10648. [Google Scholar]
- Harris, S.A.; Villeneuve, P.J.; Crawley, C.D.; Mays, J.E.; Yeary, R.A.; Hurto, K.A.; Meeker, J.D. National Study of Exposure to Pesticides among Professional Applicators: An Investigation Based on Urinary Biomarkers. J. Agric. Food Chem. 2010, 58, 10253–10261. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, J.; Wauchope, R.; Klein, A.; Dorn, E.; Zeeh, B.; Yeh, S.; Akerblom, M.; Racke, K.; Rubin, B. Significance of the long range transport of pesticides in the atmosphere. Pest Manag. Sci. 2002, 58, 314. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Vanengelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef] [PubMed]
- Coscollà, C.; Colin, P.; Yahyaoui, A.; Petrique, O.; Yusà, V.; Mellouki, A.; Pastor, A. Occurrence of currently used pesticides in ambient air of Centre Region (France). Atmos. Environ. 2010, 44, 3915–3925. [Google Scholar] [CrossRef]
- Foreman, W.; Majewski, M.; Goolsby, D.; Wiebe, F.; Coupe, R. Pesticides in the atmosphere of the Mississippi River Valley, part II—Air. Sci. Total. Environ. 2000, 248, 213–226. [Google Scholar] [CrossRef]
- Majewski, M.S.; Coupe, R.H.; Foreman, W.T.; Capel, P.D. Pesticides in Mississippi air and rain: A comparison between 1995 and 2007. Environ. Toxicol. Chem. 2014, 33, 1283–1293. [Google Scholar] [CrossRef]
- Ottar, B.; Oehme, M. The long range transport of polyclorinated hydrocarbons to the Arctic. Geophys. Res. Lett. 1984, 11, 1133–1136. [Google Scholar]
- Scheyer, A.; Morville, S.; Mirabel, P.; Millet, M. Variability of atmospheric pesticide concentrations between urban and rural areas during intensive pesticide application. Atmos. Environ. 2007, 41, 3604–3618. [Google Scholar] [CrossRef]
- Tabor, E.C. Pesticides in Urban Atmospheres. J. Air Pollut. Control. Assoc. 1965, 15, 415–418. [Google Scholar] [CrossRef] [PubMed]
- White, L.M.; Ernst, W.R.; Julien, G.; Garron, C.; Leger, M. Ambient air concentrations of pesticides used in potato cultivation in Prince Edward Island, Canada. Pest Manag. Sci. 2006, 62, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Harner, T.; Blanchard, P.; Tuduri, L.; Waite, D.; Poissant, L.; Murphy, C.; Belzer, W.; Aulagnier, F.; Sverko, E. Pesticides in the atmosphere across Canadian agricultural regions. Environ. Sci. Technol. 2008, 42, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Hageman, K.J.; Hafner, W.D.; Campbell, D.H.; Jaffe, D.A.; Landers, D.H.; Simonic, S.L.M. Variability in Pesticide Deposition and Source Contributions to Snowpack in Western US National Parks. Environ. Sci. Technol. 2010, 44, 4452–4458. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Da Rocha, G.O.; De Andrade, J.B.; Rocha, G. Pesticides in the atmospheric environment: An overview on their determination methodologies. Anal. Methods 2018, 10, 4484–4504. [Google Scholar] [CrossRef]
- Spencer, W.F.; Farmer, W.J.; Cliath, M.M. Pesticide Volatilization. In Residue Reviews: Residues of Pesticides and Other Contaminants in the Total Environment; Gunther, F.A., Ed.; Springer: New York, NY, USA, 1973; pp. 1–47. [Google Scholar]
- Peck, A.M.; Hornbuckle, K.C. Gas-phase Concentrations of Current-use Pesticides. Environ. Sci. Technol. 2005, 39, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.T.; Cessna, A.J.; Grover, R.; Kerr, L.A.; Snihura, A.D. Environmental Concentrations of Agricultural Herbicides. J. Environ. Qual. 2002, 31, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Harner, T.; Farrar, N.J.; Shoeib, M.; Jones, K.C.; Gobas, F.A.P.C. Characterization of Polymer-Coated Glass as a Passive Air Sampler for Persistent Organic Pollutants. Environ. Sci. Technol. 2003, 37, 2486–2493. [Google Scholar] [CrossRef]
- Murschell, T.; Fulgham, S.R.; Farmer, D.K. Gas-phase pesticide measurement using iodide ionization time-of-flight mass spectrometry. Atmos. Meas. Tech. 2017, 10, 2117–2127. [Google Scholar] [CrossRef]
- Vesin, A.; Bouchoux, G.; Quivet, E.; Temime-Roussel, B.; Wortham, H. Use of the HS-PTR-MS for online measurements of pyrethroids during indoor insecticide treatments. Anal. Bioanal. Chem. 2012, 403, 1907–1921. [Google Scholar] [CrossRef]
- Cypel, Y.; Kang, H. Mortality Patterns of Army Chemical Corps Veterans Who were Occupationally Exposed to Herbicides in Vietnam. Ann. Epidemiol. 2010, 20, 339–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cypel, Y.S.; Kress, A.M.; Eber, S.M.; Schneiderman, A.I.; Davey, V.J. Herbicide Exposure, Vietnam Service, and Hypertension Risk in Army Chemical Corps Veterans. J. Occup. Environ. Med. 2016, 58, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Dalager, N.A.; Needham, L.L.; Patterson, D.G.; Lees, P.S.; Yates, K.; Matanoski, G.M. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am. J. Ind. Med. 2006, 49, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Rawn, D.F.K.; Halldorson, T.H.J.; Lawson, B.D.; Muir, D.C.G. A Multi-Year Study of Four Herbicides in Air and Precipitation from a Small Prairie Watershed. J. Environ. Qual. 1999, 28, 898–906. [Google Scholar] [CrossRef]
- Waite, D.; Bailey, P.; Sproull, J.; Quiring, D.; Chau, D.; Bailey, J.; Cessna, A. Atmospheric concentrations and dry and wet deposits of some herbicides currently used on the Canadian Prairies. Chemosphere 2005, 58, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Brophy, P.; Farmer, D.K. A switchable reagent ion high resolution time-of-flight chemical ionization mass spectrometer for real-time measurement of gas phase oxidized species: Characterization from the 2013 southern oxidant and aerosol study. Atmos. Meas. Tech. 2015, 8, 2945–2959. [Google Scholar] [CrossRef]
- Veres, P.; Roberts, J.M.; Warneke, C.; Welsh-Bon, D.; Zahniser, M.; Herndon, S.; Fall, R.; De Gouw, J. Development of negative-ion proton-transfer chemical-ionization mass spectrometry (NI-PT-CIMS) for the measurement of gas-phase organic acids in the atmosphere. Int. J. Mass Spectrom. 2008, 274, 48–55. [Google Scholar] [CrossRef]
- Bertram, T.H.; Kimmel, J.R.; Crisp, T.A.; Ryder, O.S.; Yatavelli, R.L.N.; Thornton, J.A.; Cubison, M.J.; Gonin, M.; Worsnop, D.R. A field-deployable, chemical ionization time-of-flight mass spectrometer: Application to the measurement of gas-phase organic and inorganic acids. Atmos. Meas. Tech. Discuss. 2011, 4, 1963–1987. [Google Scholar] [CrossRef]
- Lee, B.H.; Lopez-Hilfiker, F.D.; Mohr, C.; Kurtén, T.; Worsnop, D.R.; Thornton, J.A. An Iodide-Adduct High-Resolution Time-of-Flight Chemical-Ionization Mass Spectrometer: Application to Atmospheric Inorganic and Organic Compounds. Environ. Sci. Technol. 2014, 48, 6309–6317. [Google Scholar] [CrossRef]
- Yatavelli, R.L.N.; Lopez-Hilfiker, F.; Wargo, J.D.; Kimmel, J.R.; Cubison, M.J.; Bertram, T.H.; Jiménez, J.L.; Gonin, M.; Worsnop, D.R.; Thornton, J.A. A Chemical Ionization High-Resolution Time-of-Flight Mass Spectrometer Coupled to a Micro Orifice Volatilization Impactor (MOVI-HRToF-CIMS) for Analysis of Gas and Particle-Phase Organic Species. Aerosol Sci. Technol. 2012, 46, 1313–1327. [Google Scholar] [CrossRef]
- Mohr, C.; Lopez-Hilfiker, F.D.; Zotter, P.; Prévôt, A.S.H.; Xu, L.; Ng, N.L.; Herndon, S.C.; Williams, L.R.; Franklin, J.P.; Zahniser, M.S.; et al. Contribution of nitrated phenols to wood burning brown carbon light absorption in Detling, United Kingdom during winter time. Environ. Sci. Technol. 2013, 47, 6316–6324. [Google Scholar] [CrossRef] [PubMed]
- Link, M.F.; Friedman, B.; Fulgham, R.; Brophy, P.; Galang, A.; Jathar, S.H.; Veres, P.; Roberts, J.M.; Farmer, D.K. Photochemical processing of diesel fuel emissions as a large secondary source of isocyanic acid (HNCO). Geophys. Res. Lett. 2016, 43, 4033–4041. [Google Scholar] [CrossRef]
- Yao, Y.; Tuduri, L.; Harner, T.; Blanchard, P.; Waite, D.; Poissant, L.; Murphy, C.; Belzer, W.; Aulagnier, F.; Li, Y.F.; et al. Spatial and temporal distribution of pesticide air concentrations in Canadian agricultural regions. Atmos. Environ. 2006, 40, 4339–4351. [Google Scholar] [CrossRef]
- Murschell, T.; Farmer, D.K. Atmospheric OH Oxidation of Three Chlorinated Aromatic Herbicides. Environ. Sci. Technol. 2018, 52, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Murschell, T.; Farmer, D.K. Atmospheric OH oxidation chemistry of trifluralin and acetochlor. Environ. Sci. Process. Impacts 2019, 21, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Bedos, C.; Cellier, P.; Calvet, R.; Barriuso, E. Mass transfer of pesticides into the atmosphere by volatilization from soils and plants: Overview. Agronomie 2002, 22, 21–33. [Google Scholar] [CrossRef]
- Spencer, W.F.; Cliath, M.M.; Jury, W.A.; Zhang, L.Z. Volatilization of Organic Chemicals from Soil as Related to Their Henry’s Law Constants. J. Environ. Qual. 1988, 17, 504–509. [Google Scholar] [CrossRef]
- Mayer, R.; Letey, J.; Farmer, W.J. Models for Predicting Volatilization of Soil-Incorporated Pesticides1. Soil Sci. Soc. Am. J. 1974, 38, 563–568. [Google Scholar] [CrossRef]
- Jury, W.A.; Spencer, W.F.; Grover, R.; Farmer, W.J. Modeling Vapor Losses of Soil-Incorporated Triallate1. Soil Sci. Soc. Am. J. 1980, 44, 445–450. [Google Scholar] [CrossRef]
- Woodrow, J.E.; Seiber, J.N.; Baker, L.W. Correlation Techniques for Estimating Pesticide Volatilization Flux and Downwind Concentrations. Environ. Sci. Technol. 1997, 31, 523–529. [Google Scholar] [CrossRef]
- Watanabe, T.J. Relationship between Volatilization Rates and Physicochemical Properties of Some Pesticides. J. Pestic. Sci. 1993, 18, 201–209. [Google Scholar] [CrossRef][Green Version]
- Kurtz, D.A. Long Range Transport of Pesticides; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Mackay, D.; Shiu, W.Y.; Lee, S.C.; Ma, K.C. Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Yalkowsky, S.H.; Dannenfelser, R.M. Aquasol Database of Aqueous Solubility; College of Pharmacy, University of Arizona: Tucson, AZ, USA, 1992. [Google Scholar]
- Helweg, A. Degradation and adsorption of 14C-MCPA in soil—Influence of concentration, temperature and moisture content on degradation. Weed Res. 1987, 27, 287–296. [Google Scholar] [CrossRef]
- Macbean, C. e-Pesticide Manual, 15th ed.; British Crop Protection Council: Alton, UK, 2010. [Google Scholar]
- Davidson, J.M.; Rao, P.S.; Ou, L.T.; Wheeler, W.B.; Rothwell, D.F. Adsorption, Movement, and Biological Degradation of Large Concentrations of Selected Pesticides in Soils; EPA-600/2-80-124; Municipal Environmental Research Laboratory, US Environmental Protection Agency: Cincinatti, OH, USA, 1980.
- Amer, S.M.; Aly, F.A. Genotoxic effect of 2,4-dichlorophenoxy acetic acid and its metabolite 2,4-dichlorophenol in mouse. Mutat. Res. Toxicol. Environ. Mutagen. 2001, 494, 1–12. [Google Scholar] [CrossRef]
- Faustini, A.; Settimi, L.; Pacifici, R.; Fano, V.; Zuccaro, P.; Forastiere, F. Immunological changes among farmers exposed to phenoxy herbicides: Preliminary observations. Occup. Environ. Med. 1996, 53, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Weick, J.; Thorn, R.S. Effects of Acute Sublethal Exposure to Coumaphos or Diazinon on Acquisition and Discrimination of Odor Stimuli in the Honey Bee (Hymenoptera: Apidae). J. Econ. Èntomol. 2002, 95, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.E.; Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and sublethal effects of seven insecticides on three beneficial insects in laboratory assays and field trials. Chemosphere 2016, 156, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.H. Agrochemicals Desk Reference: Environmental Data; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Abeleira, A.; Pollack, I.B.; Sive, B.; Zhou, Y.; Fischer, E.V.; Farmer, D.K. Source characterization of volatile organic compounds in the Colorado Northern Front Range Metropolitan Area during spring and summer 2015. J. Geophys. Res. Atmos. 2017, 122, 3595–3613. [Google Scholar] [CrossRef]
- Davie-Martin, C.L.; Hageman, K.J.; Chin, Y.P.; Rougé, V.; Fujita, Y. Influence of Temperature, Relative Humidity, and Soil Properties on the Soil–Air Partitioning of Semivolatile Pesticides: Laboratory Measurements and Predictive Models. Environ. Sci. Technol. 2015, 49, 10431–10439. [Google Scholar] [CrossRef]
- Socorro, J.; Durand, A.; Temime-Roussel, B.; Gligorovski, S.; Wortham, H.; Quivet, E. The persistence of pesticides in atmospheric particulate phase: An emerging air quality issue. Sci. Rep. 2016, 6, 33456. [Google Scholar] [CrossRef]
| Compound | Chemical Formula | Chemical Form | Vapor Pressure (mmHg, 25 °C) | Henry’s Law Constant (atm m3/mole) | Water Solubility (mg/L) | KOC (ml/g) |
|---|---|---|---|---|---|---|
| MCPA | C9H9ClO3 | Dimethyl amine salt of 2-methyl-4-chlorophenoxyacetic acid | 5.9 × 10−6 [44] | 4.8 × 10−10 [45] | 630 (25 °C) [46] | 52–60 [47] |
| 2,4-D | C8H6Cl2O3 | 2,4-dichlorophenoxyacetic acid | 1.4 × 10−7 [48] | 9.8 ×10−8 [48] | 851 (25 °C) [45] | 72–135 [49] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murschell, T.; Farmer, D.K. Real-Time Measurement of Herbicides in the Atmosphere: A Case Study of MCPA and 2,4-D during Field Application. Toxics 2019, 7, 40. https://doi.org/10.3390/toxics7030040
Murschell T, Farmer DK. Real-Time Measurement of Herbicides in the Atmosphere: A Case Study of MCPA and 2,4-D during Field Application. Toxics. 2019; 7(3):40. https://doi.org/10.3390/toxics7030040
Chicago/Turabian StyleMurschell, Trey, and Delphine K. Farmer. 2019. "Real-Time Measurement of Herbicides in the Atmosphere: A Case Study of MCPA and 2,4-D during Field Application" Toxics 7, no. 3: 40. https://doi.org/10.3390/toxics7030040
APA StyleMurschell, T., & Farmer, D. K. (2019). Real-Time Measurement of Herbicides in the Atmosphere: A Case Study of MCPA and 2,4-D during Field Application. Toxics, 7(3), 40. https://doi.org/10.3390/toxics7030040





