Assessment of Antibiotic and Pesticides Residues in Breast Milk of Syrian Refugee Lactating Mothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Area
2.2. Ethical Approval
2.3. Questionnaire
2.4. Sample Collection
2.5. Chemicals and Reagents
2.6. Sample Extraction for Antibiotics Residues Analysis
2.7. Sample Extraction for Pesticide Residues Analysis: QuEChERS Extraction
2.8. LC-MS/MS Equipment
2.9. LC/MS/MS Parameters
2.10. GC/MSMS Parameters
2.11. Recovery Test
3. Results and Discussion
3.1. Validation of the Arabic Version of the Questionnaire
3.2. Survey Results
3.3. Assessment of Antibiotic Residues in Breast Milk
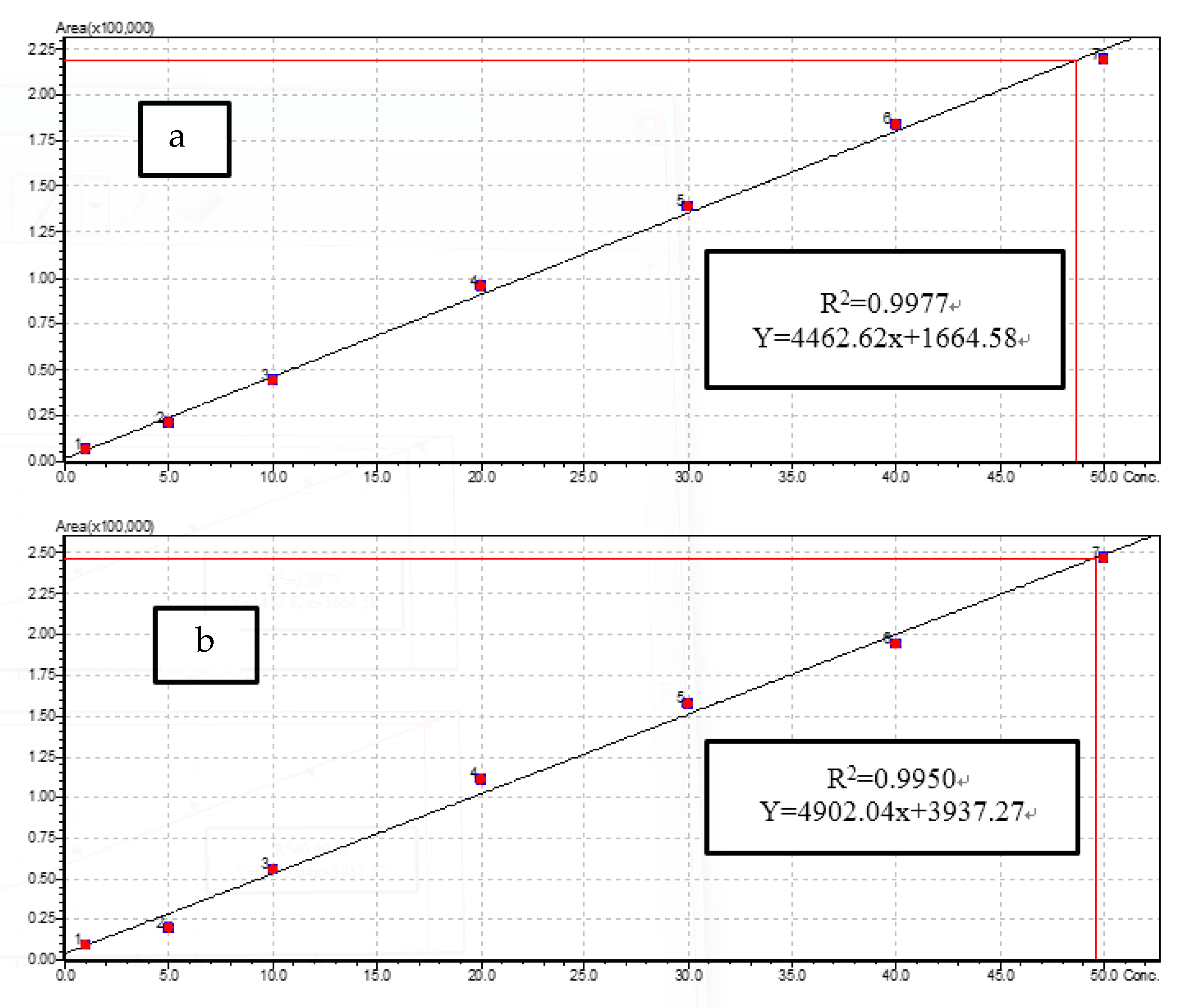
3.3.1. LC-MS/MS Method Performance for Antibiotic Residues
3.3.2. Occurrence of Antibiotic Residues in Breast Milk Samples
3.3.3. Mean Concentrations of Antibiotic Residues for the Different Families in Breast Milk Samples
3.4. Assessment of Pesticide Residues in Breast Milk
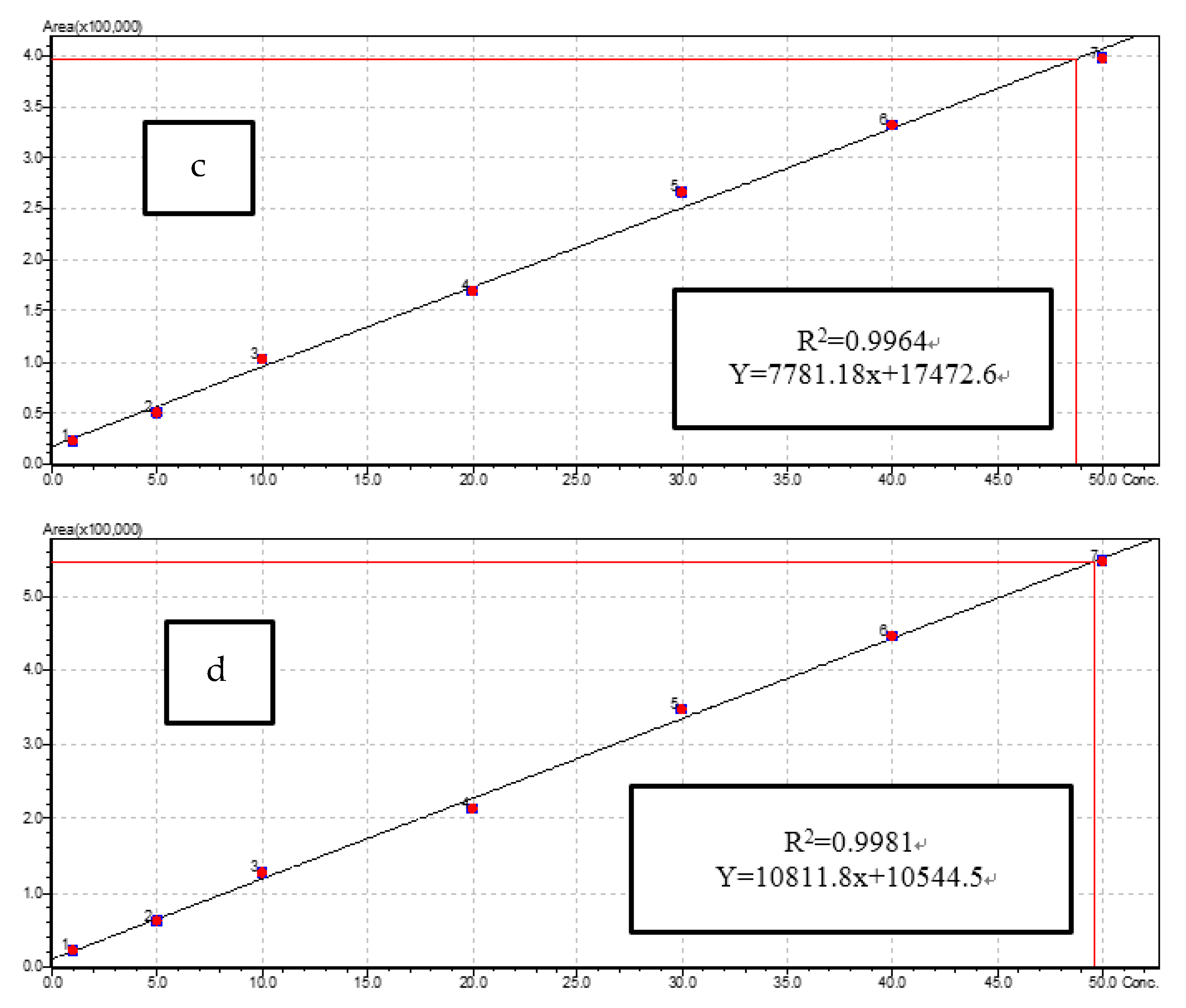
3.4.1. LC-MS/MS Method Performance for Pesticide Residuess
3.4.2. Occurrence of Pesticide Residues in Breast Milk Samples
3.4.3. Mean Concentrations of Pesticide Residues in Breast Milk Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hörnell, A.; Lagström, H.; Lande, B.; Thorsdottir, I. Breastfeeding, introduction of other foods and effects on health: A systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr. Res. 2013, 57, 20823. [Google Scholar] [CrossRef] [PubMed]
- WHO. 2008. Available online: https://www.who.int/maternal_child_adolescent/documents/9789241596664/en/ (accessed on 26 April 2019).
- Agostoni, C.; Bergman, R.; Bresson, J.-L.; Michaelsen, K.F.; Przyrembel, H.; Sanz, Y.; Tomé, D. Scientific opinion on the appropriate age for introduction of complementary feeding of infants: EFSA panel on dietetic products, nutrition and allergies (NDA). EFSA J. 2009, 7, 1423. [Google Scholar]
- Hamade, H.; Naja, F.; Keyrouz, S.; Hwalla, N.; Karam, J.; Al-Rustom, L.; Nasreddine, L. Breastfeeding knowledge, attitude, perceived behavior, and intention among female undergraduate university students in the Middle East: The case of Lebanon and Syria. Food Nutr. Bull. 2014, 35, 179–190. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. UNICEF. At a Glance: Syrian Arab Republic. 2013. Available online: http://www.unicef.org/infobycountry/syria_statistics. htmL (accessed on 26 April 2019).
- Forste, R.; Weiss, J.; Lippincott, E. The decision to breastfeed in the United States: Does race matter? Pediatrics 2001, 108, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250. [Google Scholar] [CrossRef]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Dinleyici, M.; Yildirim, G.K.; Aydemir, O.; Kaya, T.B.; Bildirici, Y.; Carman, K.B. Human milk antibiotic residue levels and their relationship with delivery mode, maternal antibiotic use and maternal dietary habits. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6560–6566. [Google Scholar]
- Jammoul, A.; El Darra, N. Evaluation of Antibiotics Residues in Chicken Meat Samples in Lebanon. Antibiotics 2019, 8, 69. [Google Scholar] [CrossRef]
- Anderson, H.A.; Wolff, M.S. Environmental contaminants in human milk. J. Expo. Sci. Environ. Epidemiol. 2000, 10, 755. [Google Scholar] [CrossRef]
- Szyrwińska, K.; Lulek, J. Exposure to specific polychlorinated biphenyls and some chlorinated pesticides via breast milk in Poland. Chemosphere 2007, 66, 1895–1903. [Google Scholar] [CrossRef]
- Zhou, J.; Zeng, X.; Zheng, K.; Zhu, X.; Ma, L.; Xu, Q.; Zhang, X.; Yu, Y.; Sheng, G.; Fu, J. Musks and organochlorine pesticides in breast milk from Shanghai, China: Levels, temporal trends and exposure assessment. Ecotoxicol. Environ. Saf. 2012, 84, 325–333. [Google Scholar] [CrossRef]
- Rojas-Squella, X.; Santos, L.; Baumann, W.; Landaeta, D.; Jaimes, A.; Correa, J.C.; Sarmiento, O.L.; Ramos-Bonilla, J.P. Presence of organochlorine pesticides in breast milk samples from Colombian women. Chemosphere 2013, 91, 733–739. [Google Scholar] [CrossRef]
- Müller, M.H.B.; Polder, A.; Brynildsrud, O.B.; Karimi, M.; Lie, E.; Manyilizu, W.B. Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human breast milk and associated health risks to nursing infants in Northern Tanzania. Environ. Res. 2017, 154, 425–434. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, Y.; Yin, S.; Li, J.; Zhao, Y.; Zhang, L.; Chen, H.; Liu, Y.; Yang, X.; Li, X. National survey of the levels of persistent organochlorine pesticides in the breast milk of mothers in China. Environ. Pollut. 2011, 159, 524–531. [Google Scholar] [CrossRef]
- Örün, E.; Yalçın, S.S.; Aykut, O.; Orhan, G.; Morgil, G.K.; Yurdakök, K.; Uzun, R. Breast milk lead and cadmium levels from suburban areas of Ankara. Sci. Total Environ. 2011, 409, 2467–2472. [Google Scholar] [CrossRef]
- Cantú-Cornelio, F.; Aguilar-Toalá, J.E.; de León-Rodríguez, C.I.; Esparza-Romero, J.; Vallejo-Cordoba, B.; González-Córdova, A.F.; García, H.S.; Hernández-Mendoza, A. Occurrence and factors associated with the presence of aflatoxin M1 in breast milk samples of nursing mothers in central Mexico. Food Control 2016, 62, 16–22. [Google Scholar] [CrossRef]
- Radonić, J.R.; Kocić Tanackov, S.D.; Mihajlović, I.J.; Grujić, Z.S.; Vojinović Miloradov, M.B.; Škrinjar, M.M.; Turk Sekulić, M.M. Occurrence of aflatoxin M1 in human milk samples in Vojvodina, Serbia: Estimation of average daily intake by babies. J. Environ. Sci. Health Part B 2017, 52, 59–63. [Google Scholar] [CrossRef]
- Bassil, M.; Daou, F.; Hassan, H.; Yamani, O.; Abi, J.; Attieh, Z.; Elaridi, J. Chemosphere Lead, cadmium and arsenic in human milk and their socio-demographic and lifestyle determinants in Lebanon. Chemosphere 2018, 191, 911–921. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar]
- Fleiss, J.L.; Wallenstein, S.; Chillton, N.W.; Goodson, J.M. A re-examination of within-mouth correlations of attachment level and of change in attachment level. J. Clin. Periodontol. 1988, 15, 411–414. [Google Scholar] [CrossRef]
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002; pp. 12–19. [Google Scholar]
- Ramatla, T.; Ngoma, L.; Adetunji, M.; Mwanza, M. Evaluation of Antibiotic Residues in Raw Meat Using Different Analytical Methods. Antibiotics 2017, 6, 34. [Google Scholar] [CrossRef]
- Olatoye, I.O.; Daniel, O.F.; Ishola, S.A. Screening of antibiotics and chemical analysis of penicillin residue in fresh milk and traditional dairy products in Oyo state, Nigeria. Vet. World 2016, 9, 948–954. [Google Scholar] [CrossRef]
- Obaidat, M.M.; Salman, A.E.B.; Davis, M.A.; Roess, A.A. Major diseases, extensive misuse, and high antimicrobial resistance of Escherichia coli in large-and small-scale dairy cattle farms in Jordan. J. Dairy Sci. 2018, 101, 2324–2334. [Google Scholar] [CrossRef]
- Elgueta, S.; Moyano, S.; Sepúlveda, P.; Quiroz, C.; Correa, A. Pesticide residues in leafy vegetables and human health risk assessment in North Central agricultural areas of Chile. Food Addit. Contam. Part B 2017, 10, 105–112. [Google Scholar] [CrossRef]

| Antibiotic Family | Antibiotic | Precursor Ion (m/z) | Collision Energy for Precursor Ion (eV) | Product Ion (m/z) | Collision Energy for Production Ion (eV) | Cone Voltage (V) | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| Sulphonamides | Sulfamethazine | 279.0 > 186.1 | 17 | 279.0 > 184.0 | 23 | Default | 5.012 |
| Tetracyclines | Tetracycline | 445.1 > 410.25 | 20 | 445.1 > 427.15 | 12 | 4.886 | |
| Oxytetracycline | 461.1 > 443.3 | 14 | 461.1 > 426.15 | 18 | 4.934 | ||
| Beta-Lactam | Ampicillin | 350.3 > 106.05 | 20 | 350.3 > 159.9 | 13 | 4.944 |
| Scale | Mean Scores | Correlation between Scores at T1 and T2 | Intraclass Correlation (ICC) | |||
|---|---|---|---|---|---|---|
| Arabic T1 | Arabic T2 | Paired t-Test | ||||
| Mean ± SD | p-Value | Correlation Coefficient | ICC | 95% CI | ||
| Dietary Habits of Lactating Mothers | 71.2 ± 10.21 | 71.43 ± 10.23 | 0.032 | 0.998 | 0.998 | 0.996–0.999 |
| Smoking Status | 5.16 ± 0.94 | 5.14 ± 0.92 | 0.000 | 0.999 | 0.999 | 0.998–1.000 |
| Demographic Characteristics | 3.33 ± 0.711 | 3.32 ± 0.743 | 0.001 | 1.000 | 1.000 | 1.000 |
| Pesticides | 2.866 ± 0.860 | 2.867 ± 0.862 | 0.000 | 1.000 | 1.000 | 1.000 |
| Medical History and Supplements Intake | 6.566 ± 1.381 | 6.565 ± 1.380 | 0.000 | 0.999 | 0.999 | 0.998–1.000 |
| Characteristics | Frequency | Percent |
|---|---|---|
| Residence years in Tripoli Lebanon camps (1–5 years) | 40 | 100 |
| Age (<30 years) | 40 | 100 |
| Gestational Age (= 9 months) | 40 | 100 |
| Lactation time (>= 120 days) | 40 | 100 |
| Level of education (Below secondary) | 40 | 100 |
| Occupation Not employed (Housekeeper) | 40 | 100 |
| Number of newborn (=1 child) | 40 | 100 |
| Infant Gender | ||
| Male | 16 | 40 |
| Female | 24 | 60 |
| Age of newborn | ||
| =< 5months | 11 | 27.5 |
| >5 months | 29 | 72.5 |
| Birth weight (g) | ||
| =< 3.5 kg | 39 | 97.5 |
| > 3.5kg | 1 | 2.5 |
| Irregular newborn Sleep pattern | 40 | 100 |
| Colic Crying of newborn | 40 | 100 |
| Fish intake | ||
| Never | 40 | 100 |
| Sea food intake | ||
| Never | 40 | 100 |
| Cereals intake | ||
| Twice a week | 39 | 97.5 |
| Daily | 1 | 2.5 |
| Potatoes intake | ||
| Once a week | 7 | 17.5 |
| Twice a week | 2 | 5.0 |
| More than twice a week | 18 | 45 |
| Daily | 13 | 32.5 |
| Fresh Vegetables intake | ||
| Once a week | 26 | 65 |
| Twice a week | 14 | 35 |
| Milk intake | ||
| Never | 39 | 97.5 |
| Daily | 1 | 2.5 |
| Dairy Product intake | ||
| Never | 11 | 27.5 |
| Once a week | 19 | 47.5 |
| > once a week | 4 | 10 |
| Twice a week | 6 | 15 |
| Pasta intake | ||
| Once a week | 15 | 37.5 |
| More than Once a week | 5.0 | 5.0 |
| Twice a week | 37.5 | 37.5 |
| More than Twice a week | 20.0 | 20.0 |
| Rice intake | ||
| More than Twice a week | 10 | 25.0 |
| Daily | 30 | 75.0 |
| Grains | ||
| Once a week | 4 | 10.0 |
| Twice a week | 13 | 32.5 |
| More than Twice a week | 23 | 57.5 |
| Soft Drink intake | ||
| Never | 12 | 30.0 |
| Once a week | 20 | 50.0 |
| More than Once a week | 2 | 5.0 |
| Twice a week | 6 | 15.0 |
| Jam intake | ||
| Never | 27 | 67.5 |
| Once a week | 13 | 32.5 |
| Coffee intake | ||
| Never | 23 | 57.5 |
| Once a week | 9 | 22.5 |
| Twice a week | 8 | 20.0 |
| Tea intake | ||
| Once a week | 1 | 2.5 |
| More than Once a week | 1 | 2.5 |
| Twice a week | 11 | 27.5 |
| More than Twice a week | 12 | 30.0 |
| Daily | 15 | 37.5 |
| Fruits intake | ||
| Once a week | 22 | 55.0 |
| Twice a week | 18 | 45.0 |
| Salty Snack intake | ||
| Never | 19 | 47.5 |
| Once a week | 21 | 52.5 |
| Chocolate intake | ||
| Never | 21 | 52.5 |
| Once a week | 17 | 42.5 |
| Twice a week | 2 | 5.0 |
| Meat and Poultry intake | ||
| Never | 40 | 100 |
| Eggs intake | ||
| Never | 16 | 40.0 |
| Once a week | 13 | 32.5 |
| More than Once a week | 6 | 15.0 |
| Twice a week | 5 | 12.5 |
| Beverages | ||
| Never | 11 | 27.5 |
| Once a week | 17 | 42.5 |
| More than Once a week | 3 | 7.5 |
| Twice a week | 9 | 22.5 |
| Smoking status | ||
| Before pregnancy | 40 | 100 |
| No | 40 | 100 |
| During pregnancy | 40 | 100 |
| No | ||
| Random smoke exposure | ||
| Yes | ||
| Nearby Waste Disposal | ||
| Yes | 40 | 100 |
| Nearby Cultivation Activity | ||
| Yes | 40 | 100 |
| Spray indoor to prevent mosquitoes | ||
| Yes | 40 | 100 |
| Spray outdoor with pesticides | ||
| Yes | 40 | 100 |
| Vitamin supplement in pregnancy | ||
| Yes | 40 | 100 |
| Iron supplement in pregnancy | ||
| Yes | 40 | 100 |
| Vitamin_supplement_in_postpartum_2_months | ||
| No | 40 | 100 |
| Iron_supplement_in_postpartum_2_months | ||
| No | 40 | 100 |
| Antibiotic intake in pregnancy | ||
| No | 40 | 100 |
| Antibiotic intake after pregnancy | ||
| No | 40 | 100 |
| Anemia at any time | ||
| Yes | 40 | 100 |
| Drinking water | ||
| Well artesian water | 40 | 100 |
| Water bottles intake | ||
| 1–2 bottle | 40 | 100 |
| Antibiotic Family | Antibiotic | Spiking Level (50 µg/L) | Mean Recoveries (%) | STDEV | RSD (%) | LOQ (µg/L) |
|---|---|---|---|---|---|---|
| Sulphonamides | Sulfamethazine | 49.075 | 98.15 | 5.72 | 5.82 | 2 µg/L |
| Tetracyclines | Tetracycline | 53.85 | 107.71 | 6.96 | 6.46 | |
| Oxytetracycline | 48.664 | 97.328 | 18.22 | 18.72 | ||
| Beta-lactam | Ampicillin | 26.475 | 52.95 | 0.627 | 1.18 |
| Breast Milk Samples (n = 120) | Sulphonamides | Tetracyclines | β-Lactams | |
|---|---|---|---|---|
| Sulfamethazine | Tetracycline | Oxytetracycline | Ampicillin | |
| Mean * (µg/L) | 0 | 0 | 5.04 | 0 |
| min (µg/L) | 0 | 0 | 0 | 0 |
| max (µg/L) | 0 | 0 | 6 | 0 |
| n positive | 0 | 0 | 3 | 0 |
| % positive | 0 | 0 | 2.5 | 0 |
| Breast Milk Samples (n = 120) | Lufeneron | Methamidophos | Chlorpyriphos |
|---|---|---|---|
| Mean * (µg/L) | 5.8754 | 2.198 | 2.05 |
| min (µg/L) | 5.1208 | 0 | 0 |
| max (µg/L) | 12.0447 | 13.1927 | 12.32 |
| n positive | 4 | 1 | 1 |
| % positive | 3.33 | 0.83 | 0.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smadi, N.; Jammoul, A.; El Darra, N. Assessment of Antibiotic and Pesticides Residues in Breast Milk of Syrian Refugee Lactating Mothers. Toxics 2019, 7, 39. https://doi.org/10.3390/toxics7030039
Smadi N, Jammoul A, El Darra N. Assessment of Antibiotic and Pesticides Residues in Breast Milk of Syrian Refugee Lactating Mothers. Toxics. 2019; 7(3):39. https://doi.org/10.3390/toxics7030039
Chicago/Turabian StyleSmadi, Nadia, Adla Jammoul, and Nada El Darra. 2019. "Assessment of Antibiotic and Pesticides Residues in Breast Milk of Syrian Refugee Lactating Mothers" Toxics 7, no. 3: 39. https://doi.org/10.3390/toxics7030039
APA StyleSmadi, N., Jammoul, A., & El Darra, N. (2019). Assessment of Antibiotic and Pesticides Residues in Breast Milk of Syrian Refugee Lactating Mothers. Toxics, 7(3), 39. https://doi.org/10.3390/toxics7030039





