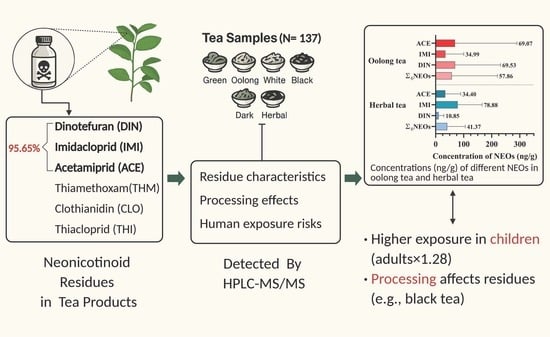
Neonicotinoid Residues in Tea Products from China: Contamination Patterns and Implications for Human Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling and Sample Preparation
2.3. Instrumental Analysis
2.4. Quality Assurance and Quality Control (QA/QC)
2.5. Health Risk Assessments
2.6. Data Analysis
3. Results and Discussion
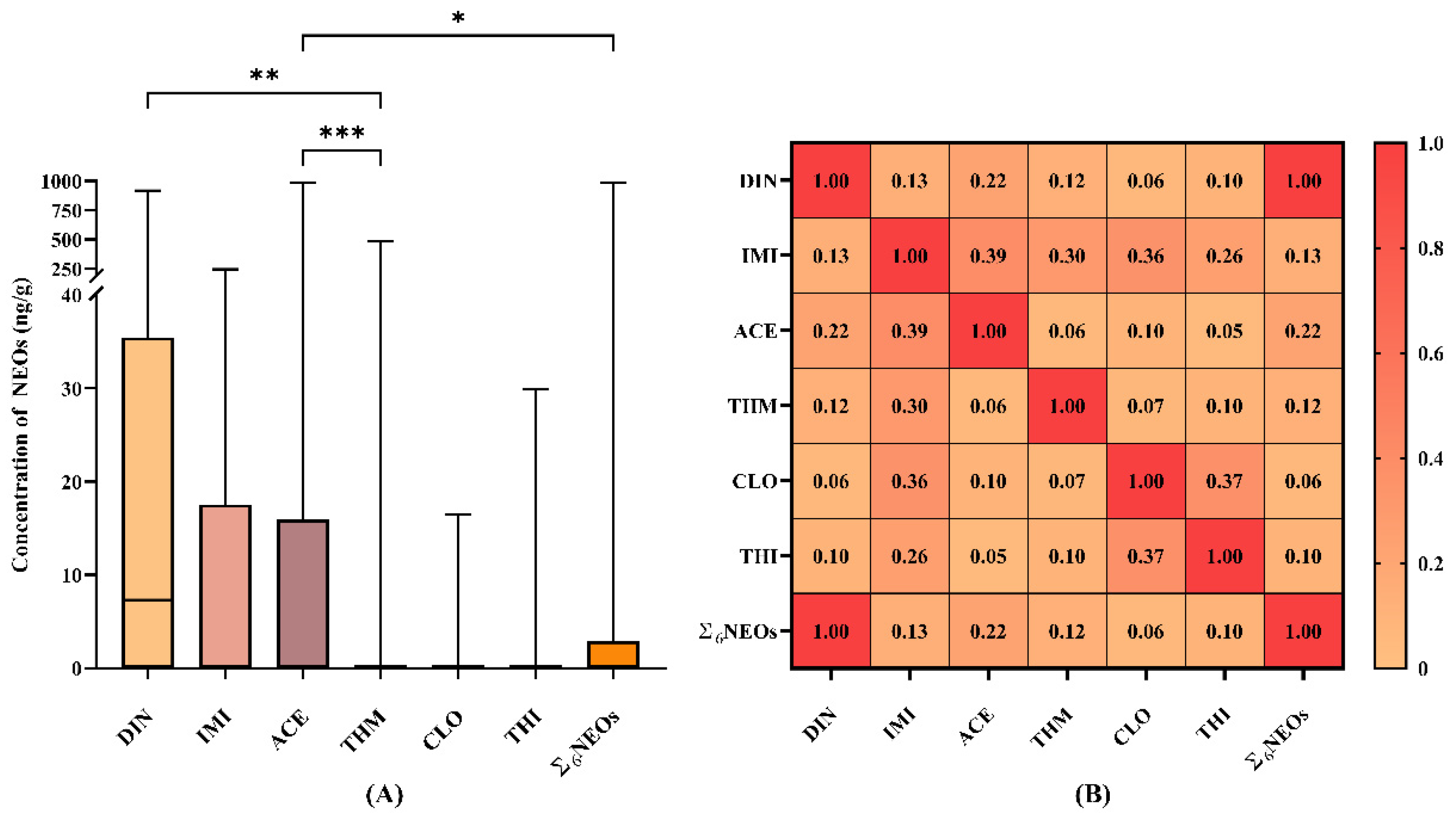
3.1. Distribution of Neonicotinoid Residue Concentrations in Tea Samples
3.2. Contamination Profiles of Six Neonicotinoids
3.3. Variation in Neonicotinoid Residues Among Different Tea Types
3.4. Exposure Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, M.; Wang, Y.; Yang, Z.; Wang, Y.; Huang, M.; Luo, B.; Wang, H.; Chen, Y.; Jiang, Q. Neonicotinoids residues in the honey circulating in Chinese market and health risk on honey bees and human. Environ. Pollut. 2022, 313, 120146. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, X.; Tu, C.; Long, T.; Bu, Y.; Wang, H.; Jeyakumar, P.; Jiang, J.; Deng, S. Remediation technologies for neonicotinoids in contaminated environments: Current state and future prospects. Environ. Int. 2023, 178, 108044. [Google Scholar] [CrossRef] [PubMed]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef]
- Wang, X.; Goulson, D.; Chen, L.; Zhang, J.; Zhao, W.; Jin, Y.; Yang, S.; Li, Y.; Zhou, J. Occurrence of Neonicotinoids in Chinese Apiculture and a Corresponding Risk Exposure Assessment. Environ. Sci. Technol. 2020, 54, 5021–5030. [Google Scholar] [CrossRef]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Ihara, M.; Matsuda, K. Neonicotinoids: Molecular mechanisms of action, insights into resistance and impact on pollinators. Curr. Opin. Insect Sci. 2018, 30, 86–92. [Google Scholar] [CrossRef]
- Bandeira, F.O.; Alves, P.R.L.; Hennig, T.B.; Brancalione, J.; Nogueira, D.J.; Matias, W.G. Chronic effects of clothianidin to non-target soil invertebrates: Ecological risk assessment using the species sensitivity distribution (SSD) approach. J. Hazard. Mater. 2021, 419, 126491. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.-F.; Wang, S.; Liu, L.-Y.; Zeng, E.Y. The human and ecological risks of neonicotinoid insecticides in soils of an agricultural zone within the Pearl River Delta, South China. Environ. Pollut. 2021, 284, 117358. [Google Scholar] [CrossRef]
- Cook, S.C. Compound and Dose-Dependent Effects of Two Neonicotinoid Pesticides on Honey Bee (Apis mellifera) Metabolic Physiology. Insects 2019, 10, 18. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Huang, K.; Zhu, W.; Ye, G.; Qi, J.; Shu, Y.; Chen, X.; Wang, Z.; Maimaiti, S.; et al. Neonicotinoid residues in fruits and vegetables in Shenzhen: Assessing human exposure and health risks. Chemosphere 2024, 364, 143267. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.; Fan, X.; Fantke, P.; Zhao, S.; Hu, J. Dynamics and dietary risk assessment of thiamethoxam in wheat, lettuce and tomato using field experiments and computational simulation. Environ. Pollut. 2020, 256, 113285. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, X.; Lou, J.; Ying, Z.; Wang, Y.; Dai, W. Occurrence, distribution and potential risk to infants of neonicotinoids in breast milk: A case study in Hangzhou, China. Sci. Total Environ. 2023, 878, 163044. [Google Scholar] [CrossRef] [PubMed]
- Loser, D.; Hinojosa, M.G.; Blum, J.; Schaefer, J.; Brüll, M.; Johansson, Y.; Suciu, I.; Grillberger, K.; Danker, T.; Möller, C.; et al. Functional alterations by a subgroup of neonicotinoid pesticides in human dopaminergic neurons. Arch. Toxicol. 2021, 95, 2081–2107. [Google Scholar] [CrossRef]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-H.; Li, Y.; Huang, X.-F.; Zheng, J.-F.; Yang, J.; Diao, H.; Yuan, Y.; Xu, Y.; Liu, M.; Shi, H.-J.; et al. Reproductive effects of two neonicotinoid insecticides on mouse sperm function and early embryonic development in vitro. PLoS ONE 2013, 8, e70112. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S. Human exposure to neonicotinoids and the associated health risks: A review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef]
- Butler, D. EU expected to vote on pesticide ban after major scientific review. Nature 2018, 555, 150–151. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Llorent-Martínez, E.J.; Martínez-Soliño, S.; Ruiz-Medina, A. Automated Photochemically Induced Method for the Quantitation of the Neonicotinoid Thiacloprid in Lettuce. Molecules 2019, 24, 4089. [Google Scholar] [CrossRef]
- Zheng, K.; Lin, R.; Liu, X.; Wu, X.; Chen, R.; Yang, M. Multiresidue Pesticide Analysis in Tea Using GC-MS/MS to Determine 12 Pesticide Residues (GB 2763-2021). Molecules 2022, 27, 8419. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Wang, Y.; Lin, G.; Wen, X.; Xu, X.; Hong, S.; Chen, Y.; Lin, H.; Yang, Z.; et al. Exposure of pregnant women to neonicotinoids in Wenzhou City, East China: A biomonitoring study. Environ. Int. 2024, 189, 108811. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Meng, Q.; Shi, J.; Zhou, M.; Zhu, Y.; You, Q.; Xu, P.; Wu, W.; Lin, Z.; Lv, H. Special tea products featuring functional components: Health benefits and processing strategies. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1686–1721. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, D.; Wang, F.; Luo, L.; Chen, Y.; Lu, S. Risk assessment of metal(loid)s in tea from seven producing provinces in China. Sci. Total Environ. 2023, 856, 159140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Zhang, B.; Liu, K.; Sun, J.; Li, Q.; Zhao, L. Herbal tea, a novel adjuvant therapy for treating type 2 diabetes mellitus: A review. Front. Pharmacol. 2022, 13, 982387. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.; Zhang, T.; Liang, G.; Hu, B.; Han, P.; Gong, W. Assessing transfer of pesticide residues from chrysanthemum flowers into tea solution and associated health risks. Ecotoxicol. Environ. Saf. 2020, 187, 109859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, Y.; An, H.; Li, J.; Li, Q.; Huang, J.; Liu, Z. Analysis of Volatile Components of Jasmine and Jasmine Tea during Scenting Process. Molecules 2022, 27, 479. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Guo, A.; Wu, G. Screening and confirmation of pesticide residues in substitutional tea such as orange peel, lotus leaf, pueraria lobata and Pangdahai. Wei Sheng Yan Jiu = J. Hyg. Res. 2020, 49, 815–822. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, X.; Xu, S.; Chen, X.; Xu, Y.; Lu, Y.; Liu, L.; Lin, L.; Ma, H.; Lu, S. Neonicotinoids in tea leaves and infusions from China: Implications for human exposure. Sci. Total Environ. 2023, 905, 166114. [Google Scholar] [CrossRef]
- Li, S.; Ren, J.; Li, L.; Chen, R.; Li, J.; Zhao, Y.; Chen, D.; Wu, Y. Temporal variation analysis and risk assessment of neonicotinoid residues from tea in China. Environ. Pollut. 2020, 266, 115119. [Google Scholar] [CrossRef]
- Hou, R.-Y.; Jiao, W.-T.; Qian, X.-S.; Wang, X.-H.; Xiao, Y.; Wan, X.-C. Effective extraction method for determination of neonicotinoid residues in tea. J. Agric. Food Chem. 2013, 61, 12565–12571. [Google Scholar] [CrossRef]
- Anjos, C.S.; Lima, R.N.; Porto, A.L.M. An overview of neonicotinoids: Biotransformation and biodegradation by microbiological processes. Environ. Sci. Pollut. Res. Int. 2021, 28, 37082–37109. [Google Scholar] [CrossRef]
- Gupta, M.; Shanker, A. Fate of imidacloprid and acetamiprid residues during black tea manufacture and transfer into tea infusion. Food Addit. Contam. 2009, 26, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Lin, T.; Fan, C.; Li, Y.; Zhang, Z.; Li, J. Migration behavior and dietary exposure risk assessment of pesticides residues in honeysuckle (Lonicera japonica Thunb.) based on modified QuEChERS method coupled with tandem mass spectrometry. Food Res. Int. 2023, 166, 112572. [Google Scholar] [CrossRef]
- Xiao, J.; Xu, X.; Wang, F.; Ma, J.; Liao, M.; Shi, Y.; Fang, Q.; Cao, H. Analysis of exposure to pesticide residues from Traditional Chinese Medicine. J. Hazard. Mater. 2019, 365, 857–867. [Google Scholar] [CrossRef]
- Cui, K.; Wu, X.; Wei, D.; Zhang, Y.; Cao, J.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Health risks to dietary neonicotinoids are low for Chinese residents based on an analysis of 13 daily-consumed foods. Environ. Int. 2021, 149, 106385. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.-K.; Behra, P.; Nhu-Trang, T.-T. Quantification of 397 pesticide residues in different types of commercial teas: Validation of high accuracy methods and quality assessment. Food Chem. 2022, 370, 130986. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T.; Gupta, R.K. Effect of light on the degradation of two neonicotinoids viz acetamiprid and thiacloprid in soil. Bull. Environ. Contam. Toxicol. 2008, 81, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-H.; Yu, M.-T.; Ikenaka, Y.; Chen, Y.-H.; Nakayama, S.F.; Chan, C.-C. Characteristics of neonicotinoid and metabolite residues in Taiwanese tea leaves. J. Sci. Food Agric. 2022, 102, 341–349. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q.; Jiang, Y.; Wang, C.; Yin, P.; Liu, X.; Lu, C. Monitoring and risk assessment of 74 pesticide residues in Pu-erh tea produced in Yunnan, China. Food Addit. Contam. 2015, 8, 56–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Li, S.; Zhao, Y.; Chen, D.; Wu, Y. Simultaneous determination of neonicotinoids and fipronils in tea using a modified QuEChERS method and liquid chromatography-high resolution mass spectrometry. Food Chem. 2020, 329, 127159. [Google Scholar] [CrossRef]
- Li, N.; Zhou, X.; Wang, J.; Chen, J.; Lu, Y.; Sun, Y.; Song, Y.; Tan, X.; Xie, G.; Chen, Y.; et al. White tea alleviates non-alcoholic fatty liver disease by regulating energy expenditure and lipid metabolism. Gene 2022, 833, 146553. [Google Scholar] [CrossRef]
- Wei, Y.; Yin, X.; Wu, H.; Zhao, M.; Huang, J.; Zhang, J.; Li, T.; Ning, J. Improving the flavor of summer green tea (Camellia sinensis L.) using the yellowing process. Food Chem. 2022, 388, 132982. [Google Scholar] [CrossRef]
- Ng, K.-W.; Cao, Z.-J.; Chen, H.-B.; Zhao, Z.-Z.; Zhu, L.; Yi, T. Oolong tea: A critical review of processing methods, chemical composition, health effects, and risk. Crit. Rev. Food Sci. Nutr. 2018, 58, 2957–2980. [Google Scholar] [CrossRef]
- Li, B.; Lin, J.; Zheng, Z.; Duan, H.; Li, D.; Wu, M. Effects of different drying methods on drying kinetics and physicochemical properties of Chrysanthemum morifolium Ramat. Int. J. Agric. Biol. Eng. 2019, 12, 187–193. [Google Scholar] [CrossRef]
- Okeke, E.S.; Olisah, C.; Malloum, A.; Adegoke, K.A.; Ighalo, J.O.; Conradie, J.; Ohoro, C.R.; Amaku, J.F.; Oyedotun, K.O.; Maxakato, N.W.; et al. Ecotoxicological impact of dinotefuran insecticide and its metabolites on non-targets in agroecosystem: Harnessing nanotechnology- and bio-based management strategies to reduce its impact on non-target ecosystems. Environ. Res. 2024, 243, 117870. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhou, J.; Li, Y.; Zhang, D.; Ntezimana, B.; Zhu, J.; Wang, X.; Xu, W.; Wen, X.; Chen, Y.; et al. The aroma characteristics of oolong tea are jointly determined by processing mode and tea cultivars. Food Chem. X 2023, 18, 100730. [Google Scholar] [CrossRef] [PubMed]
- Physiology for children. JAMA 2014, 311, 1811. [CrossRef]
- Gao, W.; Yan, M.; Xiao, Y.; Lv, Y.; Peng, C.; Wan, X.; Hou, R. Rinsing Tea before Brewing Decreases Pesticide Residues in Tea Infusion. J. Agric. Food Chem. 2019, 67, 5384–5393. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Chang, C.-H.; Yu, C.; Wang, X.; Lu, C. Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children. Environ. Int. 2019, 126, 672–681. [Google Scholar] [CrossRef]
- Li, L.; Fu, Q.-L.; Achal, V.; Liu, Y. A comparison of the potential health risk of aluminum and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi, China. Environ. Monit. Assess. 2015, 187, 228. [Google Scholar] [CrossRef]
- Miri, M.; Bhatnagar, A.; Mahdavi, Y.; Basiri, L.; Nakhaei, A.; Khosravi, R.; Eslami, H.; Ghasemi, S.M.; Balarak, D.; Alizadeh, A.; et al. Probabilistic risk assessment of exposure to fluoride in most consumed brands of tea in the Middle East. Food Chem. Toxicol. 2018, 115, 267–272. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, Y. Pesticide residues in honey and their potential reproductive toxicity. Sci. Total Environ. 2020, 741, 139953. [Google Scholar] [CrossRef] [PubMed]

| DIN | IMI | ACE | THM | CLO | THI | ∑6NEOs | |
|---|---|---|---|---|---|---|---|
| Total samples (n = 137) | |||||||
| DF(%) | 57.7 | 36.5 | 49.6 | 12.4 | 6.57 | 8.76 | - |
| Mean (SD) | 43.9 (0.02) | 30.8 (0.01) | 47.4 (0.03) | 4.83 (0.01) | 0.30 (0.01) | 0.43 (0.01) | 21.3(0.01) |
| Min | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| Median | 7.31 | N.d. | N.d. | N.d. | 0.02 | 0.01 | N.d. |
| Max | 918 | 244 | 988 | 482 | 16.5 | 29.9 | 988 |
| Chronic Exposure Risk | Acute Exposure Risk | Cumulative Exposure Risk | ||||
|---|---|---|---|---|---|---|
| EDI a (µg/kg BW/day) | cHQ a | ESTI (mg/kg BW/day) | aHQ | cHI a | aHI | |
| DIN | 9.75 × 10−4 (0.00, 1.22 × 10−1) | 3.90 × 10−5 (0.00, 4.88 × 10−3) | 1.47 × 10−4 | 1.47 × 10−4 | 3.99 × 10−5 (0.00, 1.18 × 10−2) | 1.97 × 10−3 |
| IMI | 2.67 × 10−6 (0.00, 3.25 × 10−2) | 4.68 × 10−8 (0.00, 5.70 × 10−4) | 2.35 × 10−11 | 5.88 × 10−11 | ||
| ACE | 2.67 × 10−6 (0.00, 1.32 × 10−1) | 3.81 × 10−8 (0.00, 1.89 × 10−3) | 1.58 × 10−4 | 1.58 × 10−3 | ||
| THM | 1.33 × 10−6 (0.00, 1.92 × 10−2) | 2.22 × 10−7 (0.00, 3.20 × 10−3) | 7.71 × 10−5 | 7.71 × 10−5 | ||
| CLO | 2.67 × 10−6 (0.00, 2.20 × 10−3) | 2.72 × 10−7 (0.00, 2.24 × 10−4) | 2.64 × 10−6 | 4.39 × 10−6 | ||
| THI | 1.33 × 10−6 (0.00, 3.99 × 10−3) | 3.33 × 10−7 (0.00, 9.97 × 10−4) | 4.78 × 10−6 | 1.59 × 10−4 | ||
| Chronic Exposure Risk | Acute Exposure Risk | Cumulative Exposure Risk | ||||
|---|---|---|---|---|---|---|
| EDI a (µg/kg BW/day) | cHQ a | ESTI (mg/kg BW/day) | aHQ | cHI a | aHI | |
| DIN | 1.25 × 10−3 (0.00, 1.57 × 10−1) | 4.99 × 10−5 (0.00, 6.27 × 10−3) | 1.96 × 10−4 | 1.96 × 10−4 | 5.11 × 10-5 (0.00, 1.51 × 10−2) | 2.76 × 10−3 |
| IMI | 3.41 × 10−6 (0.00, 4.16 × 10−2) | 5.99 × 10−8 (0.00, 7.31 × 10−4) | 5.21 × 10−5 | 1.30 × 10−4 | ||
| ACE | 3.41 × 10−6 (0.00, 1.69 × 10−1) | 4.88 × 10−8 (0.00, 2.41 × 10−3) | 2.11 × 10−4 | 2.11 × 10−3 | ||
| THM | 1.71 × 10−6 (0.00, 2.46 × 10−2) | 2.85 × 10−7 (0.00, 4.10 × 10−3) | 1.03 × 10−4 | 1.03 × 10−4 | ||
| CLO | 3.41 × 10−6 (0.00, 2.81 × 10−3) | 3.48 × 10−7 (0.00, 2.87 × 10−4) | 3.51 × 10−6 | 5.86 × 10−6 | ||
| THI | 1.71 × 10−6 (0.00,5.10 × 10−3) | 4.27 × 10−7 (0.00, 1.28 × 10−3) | 6.38 × 10−6 | 2.13 × 10−4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Jin, H.; Chen, J.; Lin, K.; Zhu, L.; Guo, Y.; Ji, J.; Chen, X. Neonicotinoid Residues in Tea Products from China: Contamination Patterns and Implications for Human Exposure. Toxics 2025, 13, 550. https://doi.org/10.3390/toxics13070550
Fan Y, Jin H, Chen J, Lin K, Zhu L, Guo Y, Ji J, Chen X. Neonicotinoid Residues in Tea Products from China: Contamination Patterns and Implications for Human Exposure. Toxics. 2025; 13(7):550. https://doi.org/10.3390/toxics13070550
Chicago/Turabian StyleFan, Yulong, Hongwei Jin, Jinru Chen, Kai Lin, Lihua Zhu, Yijia Guo, Jiajia Ji, and Xiaming Chen. 2025. "Neonicotinoid Residues in Tea Products from China: Contamination Patterns and Implications for Human Exposure" Toxics 13, no. 7: 550. https://doi.org/10.3390/toxics13070550
APA StyleFan, Y., Jin, H., Chen, J., Lin, K., Zhu, L., Guo, Y., Ji, J., & Chen, X. (2025). Neonicotinoid Residues in Tea Products from China: Contamination Patterns and Implications for Human Exposure. Toxics, 13(7), 550. https://doi.org/10.3390/toxics13070550