Emerging Pollutants in Chinstrap Penguins and Krill from Deception Island (South Shetland Islands, Antarctica)
Abstract
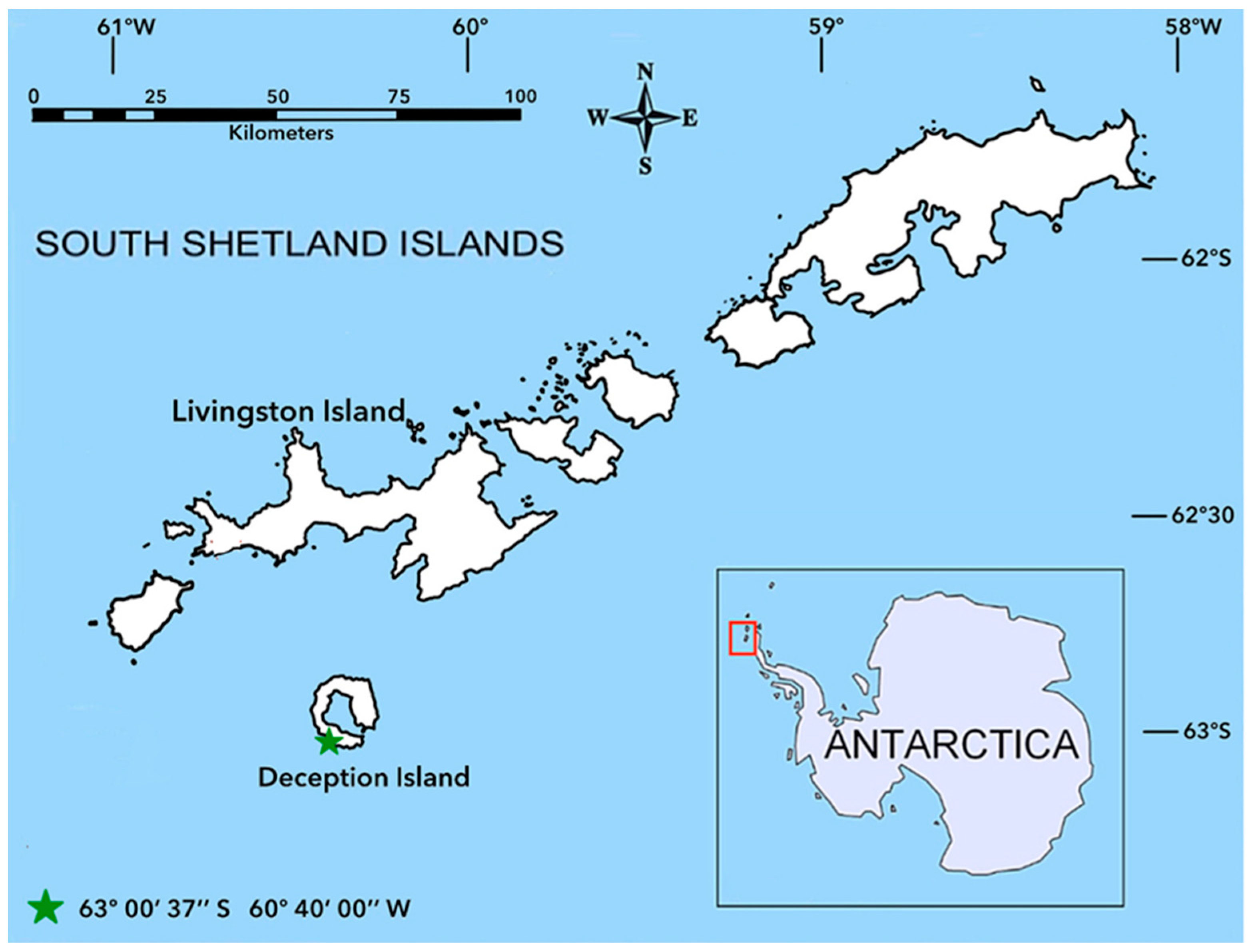
1. Introduction
2. Materials and Methods
- -
- Introduction of samples in duplicate at random;
- -
- Introduction of reagent blanks at the beginning and every 5 samples;
- -
- Initial and periodic analysis of calibration standards. Standard solutions of the compounds were prepared at 4 different concentrations. When the upper limit of a calibration line was insufficient because levels higher than this were detected in the problem samples, higher concentration standards were prepared or the sample in question was diluted;
- -
- Addition of internal standards for the analysis of BPA;
- -
- Analysis of matrices spiked with known quantities of the compounds studied. Spiked matrices were used for each type of sample analyzed. Recovery percentages were 87–92% for PFOS, 85–91% for PFOA and 85–90% for phthalates and BPA.
3. Results and Discussion
3.1. Relative Contributions of Different Contaminant Families in Pygoscelis Antarctica and Krill
3.2. Perfluorinated Compounds, Phthalates and Bisphenol a Levels in Pygoscelis Antarctica and Krill
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sladen, W.J.L.; Menzie, C.M.; Reichel, W.L. DDT Residues in Adelie Penguins and a Crabeater Seal from Antarctica. Nature 1966, 210, 670–673. [Google Scholar] [CrossRef]
- Bargagli, R. Environmental contamination in Antarctic ecosystems. Sci. Total Environ. 2008, 400, 212–226. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, A.L.; Kamstra, J.H.; Fluitsma, D.M.; Legler, J. Dithiocarbamates are teratogenic to developing zebrafish through inhibition of lysyl oxidase activity. Toxicol. Appl. Pharmacol. 2010, 244, 156–161. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, H.; Kim, K.; Kim, S.; Kim, J.H.; Ko, Y.W.; Hawes, I.; Oh, J.-E.; Kim, J.-T. Persistent organic pollutants in the Antarctic marine environment: The influence impacts of human activity, regulations, and climate change. Environ. Pollut. 2024, 363, 125100. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Eulaers, I.; Covaci, A.; Bossi, R.; Hawker, D.; Cropp, R.; Southwell, C.; Emmerson, L.; Lepoint, G.; Eisenmann, P.; et al. South polar skua (Catharacta maccormicki) as biovectors for long-range transport of persistent organic pollutants to Antarctica. Environ. Pollut. 2022, 292, 118358. [Google Scholar] [CrossRef] [PubMed]
- Wania, F.; Mackay, D. The global fractionation of persistent organic pollutants. NILU TR 1996, 1–25. Available online: https://nilu.brage.unit.no/nilu-xmlui/handle/11250/2762224?locale-attribute=en (accessed on 1 June 2025).
- Luarte, T.; Gómez-Aburto, V.A.; Poblete-Castro, I.; Castro-Nallar, E.; Huneeus, N.; Molina-Montenegro, M.; Egas, C.; Azcune, G.; Pérez-Parada, A.; Lohmann, R.; et al. Levels of persistent organic pollutants (POPs) in the Antarctic atmosphere over time (1980 to 2021) and estimation of their atmospheric half-lives. Atmos. Chem. Phys. 2023, 23, 8103–8118. [Google Scholar] [CrossRef]
- Corsolini, S.; Covaci, A.; Ademollo, N.; Focardi, S.; Schepens, P. Occurrence of organochlorine pesticides (OCPs) and their enantiomeric signatures, and concentrations of polybrominated diphenyl ethers (PBDEs) in the Adélie penguin food web, Antarctica. Environ. Pollut. 2006, 140, 371–382. [Google Scholar] [CrossRef]
- Corsolini, S.; Focardi, S. Bioconcentration of Polychlorinated Biphenyls in the Pelagic Food Chain of the Ross Sea. In Ross Sea Ecology: Italiantartide Expeditions (1987–1995); Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 575–584. [Google Scholar]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Moody, C.A.; Martin, J.W.; Kwan, W.C.; Muir, D.C.G.; Mabury, S.A. Monitoring Perfluorinated Surfactants in Biota and Surface Water Samples Following an Accidental Release of Fire-Fighting Foam into Etobicoke Creek. Environ. Sci. Technol. 2002, 36, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, P.B.; Jensen, A.A.; Wallström, E.; Aps, E. More environmentally friendly alternatives to PFOS-compounds and PFOA. Environ. Proj. 2005, 1013, 2005. [Google Scholar]
- Casal, P.; Casas, G.; Vila-Costa, M.; Cabrerizo, A.; Pizarro, M.; Jiménez, B.; Dachs, J. Snow Amplification of Persistent Organic Pollutants at Coastal Antarctica. Environ. Sci. Technol. 2019, 53, 8872–8882. [Google Scholar] [CrossRef]
- Casas, G.; Martinez-Varela, A.; Vila-Costa, M.; Jiménez, B.; Dachs, J. Rain Amplification of Persistent Organic Pollutants. Environ. Sci. Technol. 2021, 55, 12961–12972. [Google Scholar] [CrossRef] [PubMed]
- Tickner, J.A.; Schettler, T.; Guidotti, T.; McCally, M.; Rossi, M. Health risks posed by use of Di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: A critical review. Am. J. Ind. Med. 2001, 39, 100–111. [Google Scholar] [CrossRef]
- Cini, R.; Innocenti, N.D.; Loglio, G.; Stortini, A.M.; Tesei, U. Spectrofluorimetric Evidence of the Transport of Marine Organic Matter in Antarctic Snow Via Air-Sea Interaction. Int. J. Environ. Anal. Chem. 1994, 55, 285–295. [Google Scholar] [CrossRef]
- Desideri, P.; Lepri, L.; Cecchini, L. Identification and determination of organic compounds in sea water in Terra Nova Bay (Antartica). Ann. Chim. 1989, 79, 589–605. [Google Scholar]
- Bubba, M.D.; Alessandra, C.; Leonardo, C.; Luciano, L.; Desideri, P. Horizontal and vertical distributions of Biogenic and Anthropogenic Organic compounds in the Ross Sea (Antarctica). Int. J. Environ. Anal. Chem. 2004, 84, 441–456. [Google Scholar] [CrossRef]
- Kang, J.-H.; Daisuke, A.; Katayama, Y. Bisphenol A in the Aquatic Environment and Its Endocrine-Disruptive Effects on Aquatic Organisms. Crit. Rev. Toxicol. 2007, 37, 607–625. [Google Scholar] [CrossRef]
- Emnet, P.; Gaw, S.; Northcott, G.; Storey, B.; Graham, L. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base. Environ. Res. 2015, 136, 331–342. [Google Scholar] [CrossRef]
- Balakrishna, K.; Praveenkumarreddy, Y.; Nishitha, D.S.; Gopal, C.M.; Shenoy, J.K.; Bhat, K.; Khare, N.; Dhangar, K.; Kumar, M. Occurrences of UV filters, endocrine disruptive chemicals, alkyl phenolic compounds, fragrances, and hormones in the wastewater and coastal waters of the Antarctica. Environ. Res. 2023, 222, 115327. [Google Scholar] [CrossRef]
- Burger, J. Metals in avian feathers: Bioindicators of environmental pollution. Rev. Environ. Toxicol. 1993, 5, 203–311. [Google Scholar]
- Carlini, A.R.; Coria, N.R.; Santos, M.M.; Negrete, J.; Juares, M.A.; Daneri, G.A. Responses of Pygoscelis adeliae and P. papua populations to environmental changes at Isla 25 de Mayo (King George Island). Polar Biol. 2009, 32, 1427–1433. [Google Scholar] [CrossRef]
- Corsolini, S. Industrial contaminants in Antarctic biota. J. Chromatogr. A 2009, 1216, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Corsolini, S.; Borghesi, N.; Ademollo, N.; Focardi, S. Chlorinated biphenyls and pesticides in migrating and resident seabirds from East and West Antarctica. Environ. Int. 2011, 37, 1329–1335. [Google Scholar] [CrossRef]
- Morales, P.; Roscales, J.L.; Muñoz-Arnanz, J.; Barbosa, A.; Jiménez, B. Evaluation of PCDD/Fs, PCBs and PBDEs in two penguin species from Antarctica. Chemosphere 2022, 286, 131871. [Google Scholar] [CrossRef]
- Herman, R.W.; Valls, F.C.L.; Hart, T.; Petry, M.V.; Trivelpiece, W.Z.; Polito, M.J. Seasonal consistency and individual variation in foraging strategies differ among and within Pygoscelis penguin species in the Antarctic Peninsula region. Mar. Biol. 2017, 164, 115. [Google Scholar] [CrossRef]
- Deheyn, D.D.; Gendreau, P.; Baldwin, R.J.; Latz, M.I. Evidence for enhanced bioavailability of trace elements in the marine ecosystem of Deception Island, a volcano in Antarctica. Mar. Environ. Res. 2005, 60, 1–33. [Google Scholar] [CrossRef]
- Baker, P.E.; Davies, T.G.; Roobol, M.J. Volcanic Activity at Deception Island in 1967 and 1969. Nature 1969, 224, 553–560. [Google Scholar] [CrossRef]
- Barbosa, A.; Benzal, J.; De León, A.; Moreno, J. Population decline of chinstrap penguins (Pygoscelis antarctica) on Deception Island, South Shetlands, Antarctica. Polar Biol. 2012, 35, 1453–1457. [Google Scholar] [CrossRef]
- Cipro, C.V.Z.; Taniguchi, S.; Montone, R.C. Occurrence of organochlorine compounds in Euphausia superba and unhatched eggs of Pygoscelis genus penguins from Admiralty Bay (King George Island, Antarctica) and estimation of biomagnification factors. Chemosphere 2010, 78, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Corsolini, S.; Borghesi, N.; Schiamone, A.; Focardi, S. Polybrominated diphenyl ethers, polychlorinated dibenzo-dioxins, -furans, and -biphenyls in three species of antarctic penguins. Environ. Sci. Pollut. Res. 2007, 14, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Corsolini, S.; Borghesi, N.; Focardi, S. Contamination profiles of selected PCB congeners, chlorinated pesticides, PCDD/Fs in Antarctic fur seal pups and penguin eggs. Chemosphere 2009, 76, 264–269. [Google Scholar] [CrossRef]
- Weichbrodt, M.; Vetter, W.; Scholza, E.; Luckas, B.; Reinhardt, K. Determination of Organochlorine Levels in Antarctic Skua and Penguin Eggs by Application of Combined Focused Open-Vessel Microwave-Assisted Extraction, Gel-Permeation Chromatography, Adsorption Chromatography, and GC/ECD. Int. J. Environ. Anal. Chem. 1999, 73, 309–328. [Google Scholar] [CrossRef]
- Gesi, M. Distribuzione di PCB e Pesticidi Clorurati in Tessuti del Pinguino di Adèlia (Pygoscelis adeliae), del Pinguino Antartico (Pygoscelis antarctica) e del Pinguino Papua (Pygoscelis papua). Bachelor’s Thesis, University of Siena, Siena, Italy, 2009. [Google Scholar]
- Maisano, F. PCB e Pesticidi Clorurati in Uova e Tessuti di Pinguino di Adelia (Pygoscelis adéliae), di Pinguino Papua (Pygoscelis papua) e Krill (Euphasia superba) Provenienti Dallo Stretto di Bransfield. Bachelor’s Thesis, University of Siena, Siena, Italy, 2009. [Google Scholar]
- Gales, R.P. Validation of the stomach-flushing technique for obtaining stomach contents of Penguins. IBIS 1987, 129, 335–343. [Google Scholar] [CrossRef]
- Wilson, R. An improved stomach pump for penguins and other seabirds. J. Field Ornithol. 1984, 55, 109–112. [Google Scholar]
- Corsolini, S.; Guerranti, C.; Perra, G.; Focardi, S. Polybrominated Diphenyl Ethers, Perfluorinated Compounds and Chlorinated Pesticides in Swordfish (Xiphias gladius) from the Mediterranean Sea. Environ. Sci. Technol. 2008, 42, 4344–4349. [Google Scholar] [CrossRef]
- Takatori, S.; Kitagawa, Y.; Kitagawa, M.; Nakazawa, H.; Hori, S. Determination of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate in human serum using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2004, 804, 397–401. [Google Scholar] [CrossRef]
- Prins, G.S.; Ye, S.-H.; Birch, L.; Ho, S.-M.; Kannan, K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague–Dawley rats. Reprod. Toxicol. 2011, 31, 1–9. [Google Scholar] [CrossRef]
- Coughlin, J.L.; Winnik, B.; Buckley, B. Measurement of bisphenol A, bisphenol A ß-d-glucuronide, genistein, and genistein 4′-ß-d-glucuronide via SPE and HPLC-MS/MS. Anal. Bioanal. Chem. 2011, 401, 995–1002. [Google Scholar] [CrossRef]
- Jerez Rodríguez, S. Los Pingüinos: Bioindicadores de la Contaminación Ambiental en la Península Antártica e Islas Asociadas. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain. Available online: https://www.tdx.cat/bitstream/handle/10803/116920/TSJR.pdf;jsessionid=ECB68E04704B253C003952C5E3FA9CA4?sequence=1 (accessed on 1 June 2012).
- Tao, L.; Kannan, K.; Kajiwara, N.; Costa, M.M.; Fillmann, G.; Takahashi, S.; Tanabe, S. Perfluorooctanesulfonate and Related Fluorochemicals in Albatrosses, Elephant Seals, Penguins, and Polar Skuas from the Southern Ocean. Environ. Sci. Technol. 2006, 40, 7642–7648. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Corsolini, S.; Kannan, K.; Tao, L.; Trivelpiece, W.; Torres, D.; Focardi, S. Perfluorinated contaminants in fur seal pups and penguin eggs from South Shetland, Antarctica. Sci. Total Environ. 2009, 407, 3899–3904. [Google Scholar] [CrossRef]
- Muir, D.C.G.; de Wit, C.A. Trends of legacy and new persistent organic pollutants in the circumpolar arctic: Overview, conclusions, and recommendations. Sci. Total Environ. 2010, 408, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Bossi, R.; Riget, F.F.; Dietz, R.; Sonne, C.; Fauser, P.; Dam, M.; Vorkamp, K. Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands. Environ. Pollut. 2005, 136, 323–329. [Google Scholar] [CrossRef]
- Butt, C.M.; Mabury, S.A.; Muir, D.C.; Braune, B.M. M. Prevalence of Long-Chained Perfluorinated Carboxylates in Seabirds from the Canadian Arctic between 1975 and 2004. Environ. Sci. Technol. 2007, 41, 3521–3528. [Google Scholar] [CrossRef]
- Löfstrand, K.; Jörundsdóttir, H.; Tomy, G.; Svavarsson, J.; Weihe, P.; Nygård, T.; Bergman, Å. Spatial trends of polyfluorinated compounds in guillemot (Uria aalge) eggs from North-Western Europe. Chemosphere 2008, 72, 1475–1480. [Google Scholar] [CrossRef]
- Tomy, G.T.; Budakowski, W.; Halldorson, T.; Helm, P.A.; Stern, G.A.; Friesen, K.; Pepper, K.; Tittlemier, S.A.; Fisk, A.T. Fluorinated Organic Compounds in an Eastern Arctic Marine Food Web. Environ. Sci. Technol. 2004, 38, 6475–6481. [Google Scholar] [CrossRef]
- Verreault, J.; Berger, U.; Gabrielsen, G.W. Trends of Perfluorinated Alkyl Substances in Herring Gull Eggs from Two Coastal Colonies in Northern Norway: 1983–2003. Environ. Sci. Technol. 2007, 41, 6671–6677. [Google Scholar] [CrossRef]
- Verreault, J.; Houde, M.; Gabrielsen, G.W.; Berger, U.; Haukås, M.; Letcher, R.J.; Muir, D.C.G. Perfluorinated Alkyl Substances in Plasma, Liver, Brain, and Eggs of Glaucous Gulls (Larus hyperboreus) from the Norwegian Arctic. Environ. Sci. Technol. 2005, 39, 7439–7445. [Google Scholar] [CrossRef]
- Haukås, M.; Berger, U.; Hop, H.; Gulliksen, B.; Gabrielsen, G.W. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ. Pollut. 2007, 148, 360–371. [Google Scholar] [CrossRef]
- Martin, J.W.; Smithwick, M.M.; Braune, B.M.; Hoekstra, P.F.; Muir, D.C.G.; Mabury, S.A. Identification of Long-Chain Perfluorinated Acids in Biota from the Canadian Arctic. Environ. Sci. Technol. 2004, 38, 373–380. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Q.; Chen, X.; Fu, X.; Ji, L.; Wang, J.; Li, T.; Zhang, Q. Different transport behaviors and mechanisms of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in saturated porous media. J. Hazard. Mater. 2021, 402, 123435. [Google Scholar] [CrossRef]
- Kennedy, G.L.; Butenhoff, J.L.; Olsen, G.W.; O’Connor, J.C.; Seacat, A.M.; Perkins, R.G.; Biegel, L.B.; Murphy, S.R.; Farrar, D.G. The Toxicology of Perfluorooctanoate. Crit. Rev. Toxicol. 2004, 34, 351–384. [Google Scholar] [CrossRef]
- Newsted, J.L.; Beach, S.A.; Gallagher, S.P.; Giesy, J.P. Pharmacokinetics and Acute Lethality of Perfluorooctanesulfonate (PFOS) to Juvenile Mallard and Northern Bobwhite. Arch. Environ. Contam. Toxicol. 2006, 50, 411–420. [Google Scholar] [CrossRef]
- Ciccioli, P.; Cecinato, A.; Brancaleoni, E.; Brachetti, A.; Frattoni, M. Polar volatile organic compounds (VOC) of natural origin as precursors of ozone. Environ. Monit. Assess. 1994, 31, 211–217. [Google Scholar] [CrossRef]
- Cincinelli, A.; Stortini, A.M.; Checchini, L.; Martellini, T.; Bubba, M.D.; Lepri, L. Enrichment of organic pollutants in the sea surface microlayer (SML) at Terra Nova Bay, Antarctica: Influence of SML on superficial snow composition. J. Environ. Monit. 2005, 7, 1305–1312. [Google Scholar] [CrossRef]
- Mackintosh, C.E.; Maldonado, J.; Hongwu, J.; Hoover, N.; Chong, A.; Ikonomou, M.G.; Gobas, F.A.P.C. Distribution of Phthalate Esters in a Marine Aquatic Food Web: Comparison to Polychlorinated Biphenyls. Environ. Sci. Technol. 2004, 38, 2011–2020. [Google Scholar] [CrossRef]
- Han, X.; Liu, D. Detection of the toxic substance dibutyl phthalate in Antarctic krill. Antarct. Sci. 2017, 29, 511–516. [Google Scholar] [CrossRef]
- Jörg, O.; Ulrike, S.-O.; Werner, K.; Oana, J.; Ilka, L.; Kresten, O.K.; Leah, W.; Eduarda, M.S.; Gregory, C.P.; Katrien, J.W.V.L.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Barron, M.G.; Schultz, I.R.; Hayton, W.L. Presystemic branchial metabolism limits di-2-ethylhexyl phthalate accumulation in fish. Toxicol. Appl. Pharmacol. 1989, 98, 49–57. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.D.; Ikonomou, M.G.; Kelly, B.C.; Surridge, B.; Gobas, F.A.P.C. Ultra-Trace Determination of Phthalate Ester Metabolites in Seawater, Sediments, and Biota from an Urbanized Marine Inlet by LC/ESI-MS/MS. Environ. Sci. Technol. 2009, 43, 6262–6268. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Kawamura, K. Ubiquity of bisphenol A in the atmosphere. Environ. Pollut. 2010, 158, 3138–3143. [Google Scholar] [CrossRef]
| # Sample | Life Stage | Weight (kg) | Tissue |
|---|---|---|---|
| 1A | adult | 2.5 | B |
| 2A | adult | A | |
| 3A | adult | 3.5 | A |
| 4A | adult | A | |
| 1C | chick | 2.6 | A |
| 2C | chick | 2.1 | A |
| 3C | chick | A | |
| 4C | chick | 1.8 | A |
| 5C | chick | 2.0 | A |
| 6C | chick | A | |
| Chicks | |||
| tissue | no. of pool | no. of specimen | |
| liver | 3 | 1C + 6C, 3C + 4C, 2C + 5C | |
| kidney | 3 | 1C + 2C, 3C + 4C, 5C + 6C | |
| muscle | 5 single samples | (sample no. 4C: n.a.) | |
| heart | 2 | 1C + 2C + 3C, 4C + 5C + 6C | |
| brain | 2 | 1C + 2C + 3C, 4C + 5C + 6C | |
| Krill | 5.1 g | ||
| Samples | PFOA | PFOA * | (%) | PFOS | PFOS * | (%) | MEHP | MEHP * | (%) |
|---|---|---|---|---|---|---|---|---|---|
| Liver (A) | 2.5 ± 1.3 1.1–4.3 | 111.6 ± 60.9 49.8–195.3 | (100) | <0.5 | <0.5 | (0) | 11.0 ± 20.0 <2.0–40.9 | 497.5 ± 904.4 <2.0–1854.2 | (25) |
| Liver (C) | 2.2 ± 1.6 <0.5–3.6 | 69.7 ± 49.3 <0.5–112.0 | (66) | <0.5 | <0.5 | (0) | <2.0 | <2.0 | (0) |
| Kidney (A) | 3.3 ± 1.4 1.8–5.2 | 102.8 ± 45.1 57.0–165.0 | (100) | 09 ± 0.7 <0.5–1.8 | 26.5 ± 21.0 <0.5–58.0 | (25) | 4.8 ± 7.7 <2.0–16.4 | 155.2 ± 246.6 <2.0–525.1 | (25) |
| Kidney (C) | 3.4 ± 1.0 2.5–4.5 | 81.4 ± 24.5 60.3–108.3 | (100) | <0.5 | <0.5 | (0) | 50.6 ± 29.1 18.8–75.8 | 1217.5 ± 699.6 451.2–1822.1 | (100) |
| Muscle (A) | 3.4 ± 1.7 2.1–6.0 | 249.3 ± 125.8 150.3–432.7 | (100) | <0.5 | <0.5 | (0) | <2.0 | <2.0 | (0) |
| Muscle (C) | 2.3 ± 1.4 <0.5–4.3 | 126.6 ± 78.4 <0.5–234.4 | (80) | 1.7 ± 2.7 <0.5–6.5 | 97.3 ± 145.5 <0.5–352.5 | (20) | <2.0 | <2.0 | (0) |
| Heart (A) | 2.2 ± 1.5 <0.5–4.1 | 144.5 ± 101.6 <0.5–280.0 | (75) | <0.5 | <0.5 | (0) | <2.0 | <2.0 | (0) |
| Heart (C) | 2.8 ± 0.4 2.5–3.1 | 113.1 ± 16.7 101.3–124.9 | (100) | <0.5 | <0.5 | (0) | 45.3 ± 24.2 28.3–62.4 | 1809.3 ± 962.3 1128.8–2489.8 | (100) |
| Brain (A) | 1.8 ± 0.5 1.2–2.3 | 42.8 ± 12.5 29.2–53.7 | (100) | <0.5 | <0.5 | (0) | 25.9 ± 23.4 <2.0–47.5 | 609.4 ± 550.9 <2.0–1116.8 | (66) |
| Brain (C) | 1.4 ± 0.9 0.8–2.0 | 67.4 ± 43.2 36.9–98.0 | (100) | <0.5 | <0.5 | (0) | <2.0 | <2.0 | (0) |
| Krill | 1.3 | 32.8 | (100) | <0.5 | <0.5 | (0) | <2.0 | <2.0 | (0) |
| Samples | DEHP | DEHP * | (%) | BPA | BPA * | (%) | |||
| Liver (A) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Liver (C) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Kidney (A) | <10.0 | <10.0 | (0) | <0.500 | <0.5 | (0) | |||
| Kidney (C) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Muscle (A) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Muscle (C) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Heart (A) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Heart (C) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Brain (A) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Brain (C) | <10.0 | <10.0 | (0) | <0.5 | <0.5 | (0) | |||
| Krill | 28.8 | 700.6 | (100) | <0.5 | <0.5 | (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motas, M.; Jerez-Rodríguez, S.; Veiga-del-Baño, J.M.; Ramos, J.J.; Oliva, J.; Cámara, M.Á.; Andreo-Martínez, P.; Corsolini, S. Emerging Pollutants in Chinstrap Penguins and Krill from Deception Island (South Shetland Islands, Antarctica). Toxics 2025, 13, 549. https://doi.org/10.3390/toxics13070549
Motas M, Jerez-Rodríguez S, Veiga-del-Baño JM, Ramos JJ, Oliva J, Cámara MÁ, Andreo-Martínez P, Corsolini S. Emerging Pollutants in Chinstrap Penguins and Krill from Deception Island (South Shetland Islands, Antarctica). Toxics. 2025; 13(7):549. https://doi.org/10.3390/toxics13070549
Chicago/Turabian StyleMotas, Miguel, Silvia Jerez-Rodríguez, José Manuel Veiga-del-Baño, Juan José Ramos, José Oliva, Miguel Ángel Cámara, Pedro Andreo-Martínez, and Simonetta Corsolini. 2025. "Emerging Pollutants in Chinstrap Penguins and Krill from Deception Island (South Shetland Islands, Antarctica)" Toxics 13, no. 7: 549. https://doi.org/10.3390/toxics13070549
APA StyleMotas, M., Jerez-Rodríguez, S., Veiga-del-Baño, J. M., Ramos, J. J., Oliva, J., Cámara, M. Á., Andreo-Martínez, P., & Corsolini, S. (2025). Emerging Pollutants in Chinstrap Penguins and Krill from Deception Island (South Shetland Islands, Antarctica). Toxics, 13(7), 549. https://doi.org/10.3390/toxics13070549








