Exposure to Environmental Chemicals from Environmental Tobacco Smoking in Korean Adolescents
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Environmental Tobacco Smoking
2.3. Environmental Chemical Analysis
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
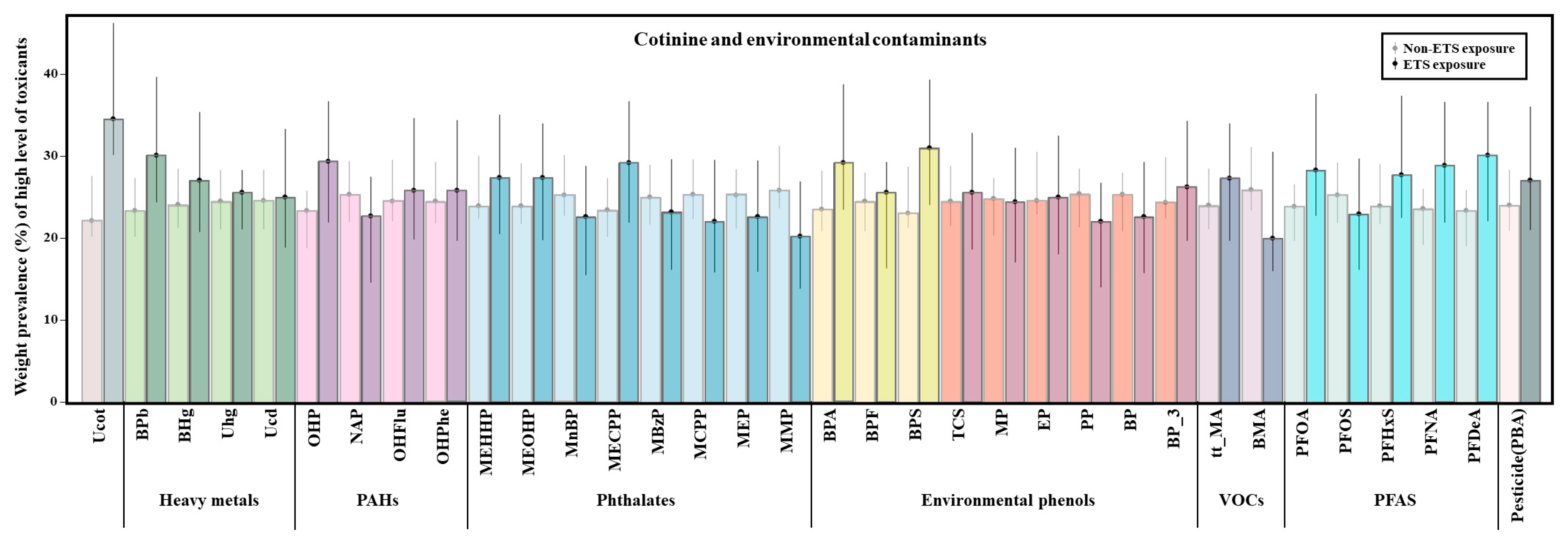
3.2. Environmental Chemicals Associated with ETS
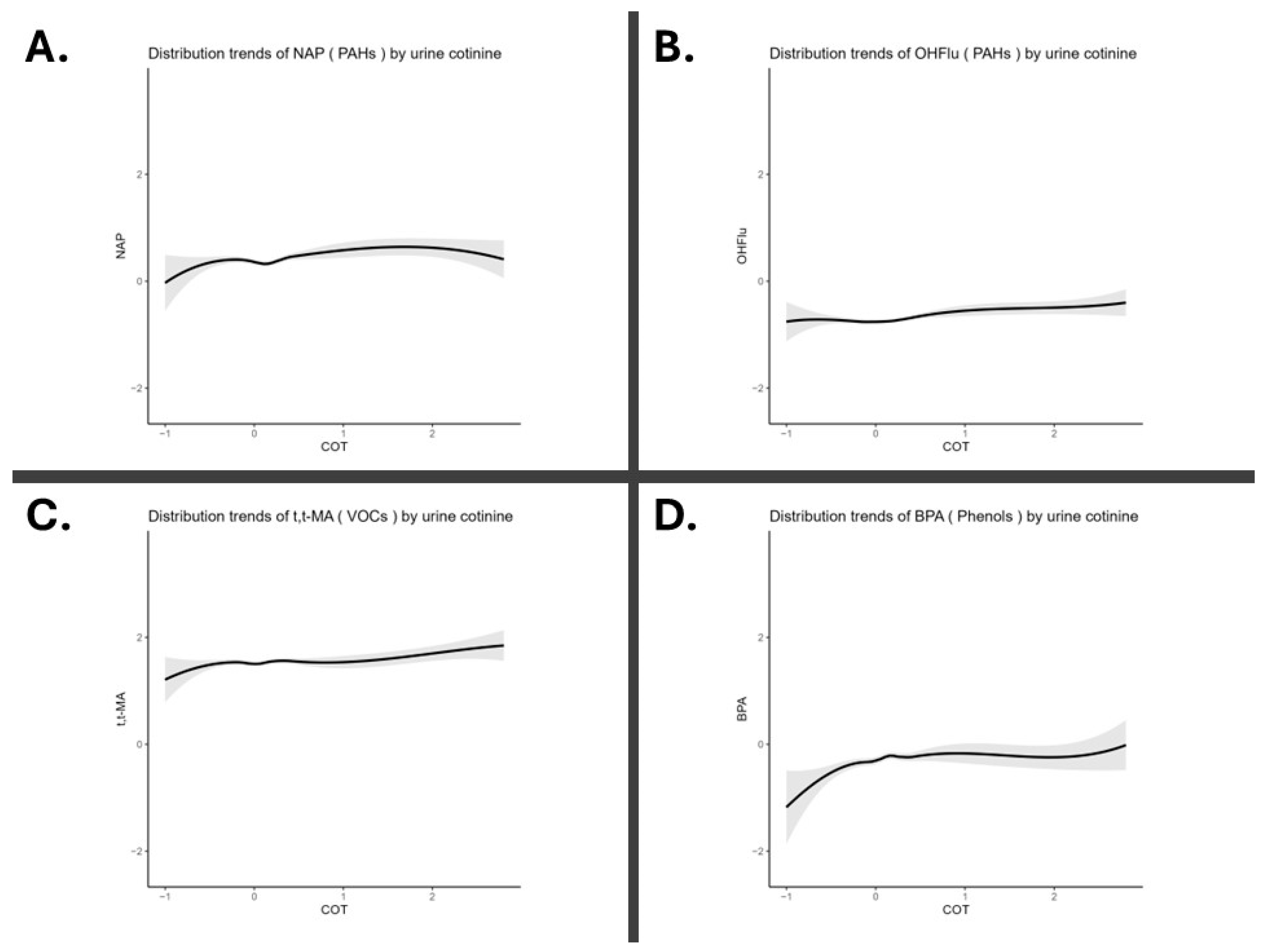
3.3. Environmental Chemicals Correlated with Urinary Cotinine Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Al-Delaimy, W. Hair as a biomarker for exposure to tobacco smoke. Tob. Control 2002, 11, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.T.; Kodet, J. A systematic review of secondhand tobacco smoke exposure and smoking behaviors: Smoking status, susceptibility, initiation, dependence, and cessation. Addict. Behav. 2015, 47, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, E.M.; Kim, J.; Min, J.; Kim, I. Lung Cancer Risk in Female School Cooks: A Nationwide Retrospective Cohort Study in the Republic of Korea. Saf. Health Work 2025, 16, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Torre, A.; Foti Randazzese, S.; Mollica, S.A.; Motta, F.; Busceti, D.; Ferrante, F.; Caminiti, L.; Crisafulli, G.; Manti, S. Association between Second-Hand Exposure to E-Cigarettes at Home and Exacerbations in Children with Asthma. Children 2024, 11, 356. [Google Scholar] [CrossRef]
- Twum, F.; Tome, J.; Ledel, E.; Roy, V.; Mallhi, A.K.; Aguirre, D.; Wei, Y.; Zhang, J. The diverging trend in exposure to environmental tobacco smoke among US children. J. Racial Ethn. Health Disparities 2024, 11, 1718–1729. [Google Scholar] [CrossRef]
- Rubinstein, M.L.; Delucchi, K.; Benowitz, N.L.; Ramo, D.E. Adolescent exposure to toxic volatile organic chemicals from e-cigarettes. Pediatrics 2018, 141, e20173557. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Bashardoust, P.; Nayeri, D.; Ghalhari, M.R.; Yazdi, N.B.; Jajarmi, F.; Karri, R.R.; Mubarak, N.M. A comprehensive review of the potential outcomes of exposure to tobacco smoke or secondhand smoke. Health Eff. Indoor Air Pollut. 2024, 167–189. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef]
- Öksüz, A.; Kutlu, R.; Reisli, İ.; Kılınc, İ. Use of urinary cotinine and cotinine/creatinine ratio as a biomarker of environmental tobacco exposure. Cukurova Med. J. 2022, 47, 961–971. [Google Scholar] [CrossRef]
- Hong, S.; Kim, O.-J.; Jung, S.K.; Jeon, H.L.; Kim, S.; Kil, J. The Exposure Status of Environmental Chemicals in South Korea: The Korean National Environmental Health Survey 2018–2020. Toxics 2024, 12, 829. [Google Scholar] [CrossRef]
- Jaakkola, M.; Jaakkola, J. Assessment of exposure to environmental tobacco smoke. Eur. Respir. J. 1997, 10, 2384–2397. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment. 4th ('18-'20) Korean National Health and Nutrition Examination Survey Manual for Analysis of Environmental Pollutants in Biological Samples (Heavy Metals); National Institute of Environmental Research: Incheon, Republic of Korea, 2022. [Google Scholar]
- Ministry of Environment. 4th ('18-'20) Korean National Health and Nutrition Examination Survey Manual for Analysis of Environmental Pollutants in Biological Samples (Organic Chemicals); National Institute of Environmental Research: Incheon, Republic of Korea, 2022. [Google Scholar]
- Golub, M.S. Adolescent health and the environment. Environ. Health Perspect. 2000, 108, 355–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheraghi, M.; Salvi, S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur. J. Pediatr. 2009, 168, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Deng, X.; Li, W.; Liu, S.; Chen, Y.; Yang, B.; Liu, Q. Internal exposure levels of polycyclic aromatic hydrocarbons in children and adolescents: A systematic review and meta-analysis. Environ. Health Prev. Med. 2019, 24, 1–15. [Google Scholar] [CrossRef]
- Jacob, P., 3rd; Wilson, M.; Benowitz, N.L. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal. Chem. 2007, 79, 587–598. [Google Scholar] [CrossRef]
- Wilhelm, M.; Hardt, J.; Schulz, C.; Angerer, J. New reference value and the background exposure for the PAH metabolites 1-hydroxypyrene and 1- and 2-naphthol in urine of the general population in Germany: Basis for validation of human biomonitoring data in environmental medicine. Int. J. Hyg. Environ. Health 2008, 211, 447–453. [Google Scholar] [CrossRef]
- Descatha, A.; Dousseau, H.; Pitet, S.; Magnolini, F.; McMillan, N.; Mangelsdorf, N.; Swan, R.; Steve, J.-M.; Pourret, D.; Fadel, M. Work Exposome and Related Disorders of Firefighters: An Overview of Systematized Reviews. Saf. Health Work 2025, 16, 145–155. [Google Scholar] [CrossRef]
- Lin, T.J.; Guo, Y.L.; Hsu, J.C.; Wang, I.J. 2-Naphthol Levels and Allergic Disorders in Children. Int. J. Environ. Res. Public Health 2018, 15, 1449. [Google Scholar] [CrossRef]
- Melikian, A.A.; Prahalad, A.K.; Hoffmann, D. Urinary trans, trans-muconic acid as an indicator of exposure to benzene in cigarette smokers. Cancer Epidemiol. Biomark. Prev. 1993, 2, 47–51. [Google Scholar] [PubMed]
- Kicinski, M.; Saenen, N.D.; Viaene, M.K.; Den Hond, E.; Schoeters, G.; Plusquin, M.; Nelen, V.; Bruckers, L.; Sioen, I.; Loots, I.; et al. Urinary t,t-muconic acid as a proxy-biomarker of car exhaust and neurobehavioral performance in 15-year olds. Environ. Res. 2016, 151, 521–527. [Google Scholar] [CrossRef]
- Amin, M.M.; Rafiei, N.; Poursafa, P.; Ebrahimpour, K.; Mozafarian, N.; Shoshtari-Yeganeh, B.; Hashemi, M.; Kelishadi, R. Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ. Sci. Pollut. Res. Int. 2018, 25, 34046–34052. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010; ISBN 978 92 890 0213 4. [Google Scholar]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Bellisario, V.; Tassinari, R.; Squillacioti, G.; Manetta, T.; Bugiani, M.; Migliore, E.; Piccioni, P. Bisphenol A, Tobacco Smoke, and Age as Predictors of Oxidative Stress in Children and Adolescents. Int. J. Environ. Res. Public Health 2019, 16, 2025. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Bruckers, L.; Covaci, A.; Schoeters, G.; Fierens, T.; Sioen, I.; Vanermen, G.; Baeyens, W.; Morrens, B.; Loots, I.; et al. Determinants of bisphenol A and phthalate metabolites in urine of Flemish adolescents. Environ. Res. 2014, 134, 110–117. [Google Scholar] [CrossRef]
- Wang, Y.; Aimuzi, R.; Nian, M.; Zhang, Y.; Luo, K.; Zhang, J. Bisphenol A substitutes and sex hormones in children and adolescents. Chemosphere 2021, 278, 130396. [Google Scholar] [CrossRef]
- Duty, S.M.; Ackerman, R.M.; Calafat, A.M.; Hauser, R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect. 2005, 113, 1530–1535. [Google Scholar] [CrossRef]
- Ha, M.; Kwon, H.-J.; Leem, J.-H.; Kim, H.-C.; Lee, K.J.; Park, I.; Lim, Y.-W.; Lee, J.-H.; Kim, Y.; Seo, J.-H.; et al. Korean Environmental Health Survey in Children and Adolescents (KorEHS-C): Survey design and pilot study results on selected exposure biomarkers. Int. J. Hyg. Environ. Health 2014, 217, 260–270. [Google Scholar] [CrossRef]
- Lee, B.-c.; Yoon, H.; Lee, B.; Kim, P.; Moon, H.-B.; Kim, Y. Occurrence of bisphenols and phthalates in indoor dust collected from Korean homes. J. Ind. Eng. Chem. 2021, 99, 68–73. [Google Scholar] [CrossRef]
- Chang, W.-H.; Herianto, S.; Lee, C.-C.; Hung, H.; Chen, H.-L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef]
- Runkel, A.A.; Stajnko, A.; Tratnik, J.S.; Mazej, D.; Horvat, M.; Přibylová, P.; Kosjek, T. Exposure of children and adolescents from Northeastern Slovenia to per-and polyfluoroalkyl substances. Chemosphere 2023, 321, 138096. [Google Scholar] [CrossRef]
- Rosato, I.; Bonato, T.; Fletcher, T.; Batzella, E.; Canova, C. Estimation of per-and polyfluoroalkyl substances (PFAS) half-lives in human studies: A systematic review and meta-analysis. Environ. Res. 2024, 242, 117743. [Google Scholar] [CrossRef] [PubMed]
- Dereumeaux, C.; Saoudi, A.; Goria, S.; Wagner, V.; De Crouy-Chanel, P.; Pecheux, M.; Berat, B.; Zaros, C.; Guldner, L. Urinary levels of pyrethroid pesticides and determinants in pregnant French women from the Elfe cohort. Environ. Int. 2018, 119, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Fábelová, L.; Beneito, A.; Casas, M.; Colles, A.; Dalsager, L.; Den Hond, E.; Dereumeaux, C.; Ferguson, K.; Gilles, L.; Govarts, E.; et al. PFAS levels and exposure determinants in sensitive population groups. Chemosphere 2023, 313, 137530. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, B.; Zhu, X.; Su, Q. Determination of pyrethroid residues in tobacco and cigarette smoke by capillary gas chromatography. J. Chromatogr. A 2002, 964, 205–211. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, S.W.; Lee, J.-G.; Joo, J.H.; Lee, J.-H.; Lee, K.-J. The exposure level of environmental harmful substances related to the secondhand smoke in Korean non-smoker adults: Data from the second Korean National Environmental Health Survey (KoNEHS 2012–2014): A cross-sectional study. Ann. Occup. Environ. Med. 2019, 31, 41–50. [Google Scholar] [CrossRef]
- Min, G.; Shin, J.; Kim, D.; Woo, J.; Sung, K.; Cho, M.; Yang, W. Assessment of Heavy Metal Exposure Levels (Pb, Hg, Cd) among South Koreans and Contribution Rates by Exposure Route—Korean National Environmental Health Survey (KoNEHS) Cycle 4 (2018~2020). J. Environ. Health Sci. 2023, 49, 262–274. [Google Scholar] [CrossRef]
- Richterová, D.; Govarts, E.; Fábelová, L.; Rausová, K.; Martin, L.R.; Gilles, L.; Remy, S.; Colles, A.; Rambaud, L.; Riou, M.; et al. PFAS levels and determinants of variability in exposure in European teenagers–Results from the HBM4EU aligned studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 247, 114057. [Google Scholar] [CrossRef]
- Cox, B.; Wauters, N.; Rodríguez-Carrillo, A.; Portengen, L.; Gerofke, A.; Kolossa-Gehring, M.; Lignell, S.; Lindroos, A.K.; Fabelova, L.; Murinova, L.P.; et al. PFAS and phthalate/DINCH exposure in association with age at menarche in teenagers of the HBM4EU aligned studies. Toxics 2023, 11, 711. [Google Scholar] [CrossRef]
- Galán, I.; Díez-Gañán, L.; Gandarillas, A.; Mata, N.; Cantero, J.L.; Durbán, M. Effect of a smoking ban and school-based prevention and control policies on adolescent smoking in Spain: A multilevel analysis. Prev. Sci. 2012, 13, 574–583. [Google Scholar] [CrossRef]
- Hawkins, S.S.; Bach, N.; Baum, C.F. Impact of tobacco control policies on adolescent smoking. J. Adolesc. Health 2016, 58, 679–685. [Google Scholar] [CrossRef]
- Weschler, C.J.; Nazaroff, W.W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Tian, Z.; Yin, Y.; Wei, J.; Mu, Y.; Cai, J.; Song, Z.; Cen, K. Bioavailability-based risk assessment of various heavy metals via multi-exposure routes for children and teenagers in Beijing, China. Environ. Sci. Pollut. Res. 2023, 30, 114985–115002. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Human exposure to per-and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Weschler, C.J. Changes in indoor pollutants since the 1950s. Atmos. Environ. 2009, 43, 153–169. [Google Scholar] [CrossRef]
- Foley, J.M.; Kwiatkowski, C.F.; Rochester, J.R.; Neveux, I.; Dabe, S.; Lathrop, M.K.; Daza, E.J.; Grzymski, J.J.; Greenfield, B.K.; Hua, J. Associations Between Daily-Use Products and Urinary Biomarkers of Endocrine-Disrupting Chemicals in Adults of Reproductive Age. Int. J. Environ. Res. Public Health 2025, 22, 99. [Google Scholar] [CrossRef]
- Denson, K. Passive smoking in infants, children and adolescents. The effects of diet and socioeconomic factors. Int. Arch. Occup. Environ. Health 2001, 74, 525–532. [Google Scholar] [CrossRef]
| Exposure to Environmental Tobacco Smoking | p-Value * | |||
|---|---|---|---|---|
| No | Yes | |||
| Total | 634 (79.1) | 168 (20.9) | ||
| Gender | Male | 284 (44.8) | 84 (50.0) | 0.258 |
| Female | 350 (55.2) | 84 (50.0) | ||
| School year | 7th | 106 (16.7) | 19 (11.3) | 0.456 |
| 8th | 95 (15.0) | 24 (14.3) | ||
| 9th | 102 (16.1) | 25 (14.9) | ||
| 10th | 114 (18.0) | 31 (18.5) | ||
| 11th | 123 (19.4) | 32 (19.0) | ||
| 12th | 94 (14.8) | 37 (22.0) | ||
| Drinking | No | 447 (70.5) | 95 (56.5) | <0.0001 |
| Yes | 187 (29.5) | 73 (43.5) | ||
| Smoking | Never | 623 (98.3) | 155 (92.3) | <0.0001 |
| Ex-smoker | 11 (1.7) | 13 (7.7) | ||
| Income a ($/month) | ≤1520 | 34 (5.4) | 18 (10.7) | 0.010 |
| 1520–3800 | 280 (44.1) | 87 (51.8) | ||
| 3800≤ | 285 (45.0) | 54 (32.1) | ||
| No response | 35 (5.5) | 9 (5.4) | ||
| Obesity b (kg/m2) | Underweight | 86 (13.6) | 23 (13.7) | 0.782 |
| Normal weight | 428 (67.5) | 108 (64.3) | ||
| Overweight | 120 (18.9) | 37 (22.0) | ||
| Environmental Chemicals | Environmental Tobacco Smoking Exposure | p-Value ** | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n | GM (SE) * | n | GM (SE) | |||
| Nicotine a | COT (μg/g cr.) | 625 | 1.34 (1.05) | 166 | 1.81 (1.11) | <0.001 |
| Heavy metal b | BPb (μg/dL) | 631 | 0.80 (1.02) | 168 | 0.84 (1.05) | 0.318 |
| BHg (μg/L) | 631 | 1.38 (1.02) | 168 | 1.36 (1.05) | 0.687 | |
| Uhg (μg/g cr.) | 630 | 0.18 (1.03) | 166 | 0.18 (1.05) | 0.866 | |
| Ucd (μg/g cr.) | 630 | 0.08 (1.07) | 166 | 0.08 (1.12) | 0.616 | |
| PAHs c | OHP (μg/g cr.) | 618 | 0.06 (1.06) | 165 | 0.07 (1.09) | 0.187 |
| NAP (μg/g cr.) | 618 | 2.37 (1.06) | 165 | 2.35 (1.08) | 0.824 | |
| OHFlu (μg/g cr.) | 618 | 0.19 (1.06) | 165 | 0.19 (1.07) | 0.456 | |
| OHPhe (μg/g cr.) | 618 | 0.04 (1.05) | 165 | 0.05 (1.10) | 0.309 | |
| Phthalates d | MEHHP (μg/g cr.) | 628 | 6.85 (1.12) | 166 | 6.67 (1.20) | 0.994 |
| MEOHP (μg/g cr.) | 628 | 3.76 (1.12) | 166 | 3.74 (1.20) | 0.829 | |
| MnBP (μg/g cr.) | 628 | 12.08 (1.14) | 166 | 10.95 (1.20) | 0.667 | |
| MECPP (μg/g cr.) | 628 | 12.32 (1.04) | 166 | 14.19 (1.05) | 0.003 | |
| MBzP (μg/g cr.) | 628 | 0.45 (1.12) | 166 | 0.46 (1.17) | 0.457 | |
| MCPP (μg/g cr.) | 628 | 0.16 (1.04) | 166 | 0.16 (1.07) | 0.855 | |
| MEP (μg/g cr.) | 628 | 4.28 (1.11) | 166 | 4.07 (1.16) | 0.556 | |
| MMP (μg/g cr.) | 628 | 2.27 (1.07) | 166 | 2.04 (1.09) | 0.249 | |
| Environmental phenols e | BPA (μg/g cr.) | 630 | 0.53 (1.08) | 166 | 0.60 (1.11) | 0.192 |
| BPF (μg/g cr.) | 630 | 0.16 (1.16) | 166 | 0.16 (1.25) | 0.533 | |
| BPS (μg/g cr.) | 630 | 0.08 (1.09) | 166 | 0.09 (1.15) | 0.109 | |
| TCS (μg/g cr.) | 630 | 0.12 (1.06) | 166 | 0.11 (1.07) | 0.121 | |
| MP (μg/g cr.) | 630 | 7.90 (1.08) | 166 | 8.31 (1.13) | 0.820 | |
| EP (μg/g cr.) | 630 | 32.72 (1.12) | 166 | 33.32 (1.17) | 0.971 | |
| PP (μg/g cr.) | 630 | 0.38 (1.10) | 166 | 0.35 (1.18) | 0.848 | |
| BP (μg/g cr.) | 630 | 0.45 (1.04) | 166 | 0.46 (1.06) | 0.740 | |
| BP_3 (μg/g cr.) | 630 | 0.55 (1.08) | 166 | 0.50 (1.14) | 0.417 | |
| VOCs f | t,t-MA (μg/g cr.) | 610 | 33.75 (1.06) | 163 | 37.15 (1.07) | 0.074 |
| BMA (μg/g cr.) | 610 | 2.97 (1.04) | 163 | 2.83 (1.06) | 0.105 | |
| PFAS g | PFOA (μg/L) | 631 | 3.62 (1.03) | 168 | 3.77 (1.05) | 0.505 |
| PFOS (μg/L) | 631 | 7.92 (1.04) | 168 | 8.05 (1.06) | 0.866 | |
| PFHxS (μg/L) | 631 | 2.54 (1.07) | 168 | 2.51 (1.14) | 0.972 | |
| PFNA (μg/L) | 631 | 0.91 (1.03) | 168 | 0.95 (1.05) | 0.194 | |
| PFDeA (μg/L) | 631 | 0.44 (1.02) | 168 | 0.47 (1.04) | 0.169 | |
| Pesticide h | PBA | 617 | 0.37 (1.06) | 164 | 0.41 (1.13) | 0.274 |
| Environmental Chemicals | Crude | Adjusted ** | |||
|---|---|---|---|---|---|
| r | p-Value * | r | p-Value * | ||
| Heavy metal a | BPb | 0.051 | 0.155 | 0.023 | 0.521 |
| BHg | 0.031 | 0.387 | 0.010 | 0.790 | |
| Uhg | 0.008 | 0.832 | 0.005 | 0.889 | |
| Ucd | 0.000 | 0.996 | 0.006 | 0.879 | |
| PAHs b | OHP | 0.043 | 0.232 | 0.040 | 0.271 |
| NAP | 0.100 | 0.005 | 0.090 | 0.013 | |
| OHFlu | 0.135 | <0.001 | 0.145 | <0.0001 | |
| OHPhe | 0.061 | 0.087 | 0.063 | 0.083 | |
| Phthalates c | MEHHP | −0.105 | 0.003 | −0.087 | 0.016 |
| MEOHP | −0.072 | 0.043 | −0.055 | 0.127 | |
| MnBP | −0.092 | 0.010 | −0.073 | 0.044 | |
| MECPP | 0.017 | 0.627 | 0.038 | 0.289 | |
| MBzP | −0.053 | 0.135 | −0.050 | 0.164 | |
| MCPP | −0.004 | 0.915 | 0.025 | 0.493 | |
| MEP | 0.011 | 0.762 | 0.020 | 0.586 | |
| MMP | 0.080 | 0.024 | 0.094 | 0.009 | |
| Environmental phenols d | BPA | 0.078 | 0.028 | 0.082 | 0.023 |
| BPF | 0.025 | 0.490 | 0.022 | 0.547 | |
| BPS | 0.098 | 0.006 | 0.098 | 0.007 | |
| TCS | −0.032 | 0.375 | −0.022 | 0.537 | |
| MP | 0.002 | 0.951 | 0.024 | 0.515 | |
| EP | 0.034 | 0.347 | 0.031 | 0.387 | |
| PP | −0.019 | 0.594 | −0.001 | 0.979 | |
| BP | 0.068 | 0.055 | 0.083 | 0.022 | |
| BP_3 | −0.012 | 0.744 | 0.004 | 0.905 | |
| VOCs e | t,t-MA | 0.116 | 0.001 | 0.126 | 0.001 |
| BMA | −0.019 | 0.596 | 0.008 | 0.833 | |
| PFAS f | PFOA | −0.091 | 0.011 | −0.091 | 0.012 |
| PFOS | −0.103 | 0.004 | −0.114 | 0.002 | |
| PFHxS | −0.076 | 0.032 | −0.076 | 0.034 | |
| PFNA | −0.073 | 0.041 | −0.084 | 0.020 | |
| PFDeA | −0.079 | 0.027 | −0.084 | 0.019 | |
| Pesticide g | PBA | 0.055 | 0.126 | 0.052 | 0.151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Jo, A.-R.; Lee, S.; Lee, W. Exposure to Environmental Chemicals from Environmental Tobacco Smoking in Korean Adolescents. Toxics 2025, 13, 546. https://doi.org/10.3390/toxics13070546
Lee J-E, Jo A-R, Lee S, Lee W. Exposure to Environmental Chemicals from Environmental Tobacco Smoking in Korean Adolescents. Toxics. 2025; 13(7):546. https://doi.org/10.3390/toxics13070546
Chicago/Turabian StyleLee, Jung-Eum, Ah-Reum Jo, Sunho Lee, and Wanhyung Lee. 2025. "Exposure to Environmental Chemicals from Environmental Tobacco Smoking in Korean Adolescents" Toxics 13, no. 7: 546. https://doi.org/10.3390/toxics13070546
APA StyleLee, J.-E., Jo, A.-R., Lee, S., & Lee, W. (2025). Exposure to Environmental Chemicals from Environmental Tobacco Smoking in Korean Adolescents. Toxics, 13(7), 546. https://doi.org/10.3390/toxics13070546







