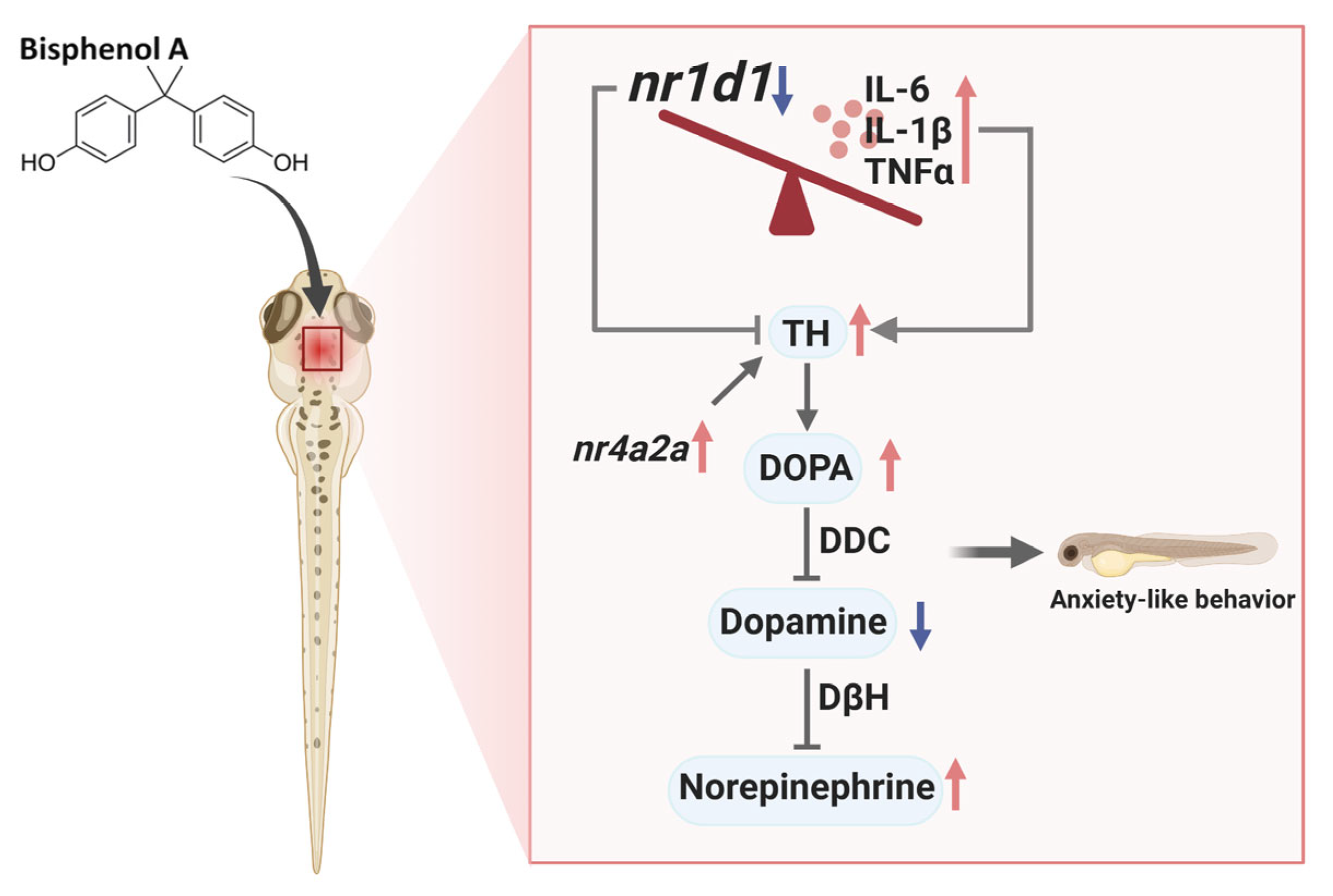
Role of NR1D1 in Bisphenol A-Induced Anxiety-like Behavior and Inflammation in Zebrafish Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagent Preparation
2.2. BPA, GSK, and BPA + GSK Exposure of Zebrafish Embryos
2.3. Locomotor Behavioral Analysis
2.4. Transcriptomic Analysis
2.5. RT-qPCR
2.6. Astrocyte Function Detection
2.7. Inflammation-Related Factor Detection
2.8. Dopamine, DOPA, and Norepinephrine Content Detection
2.9. Data Analysis
3. Results
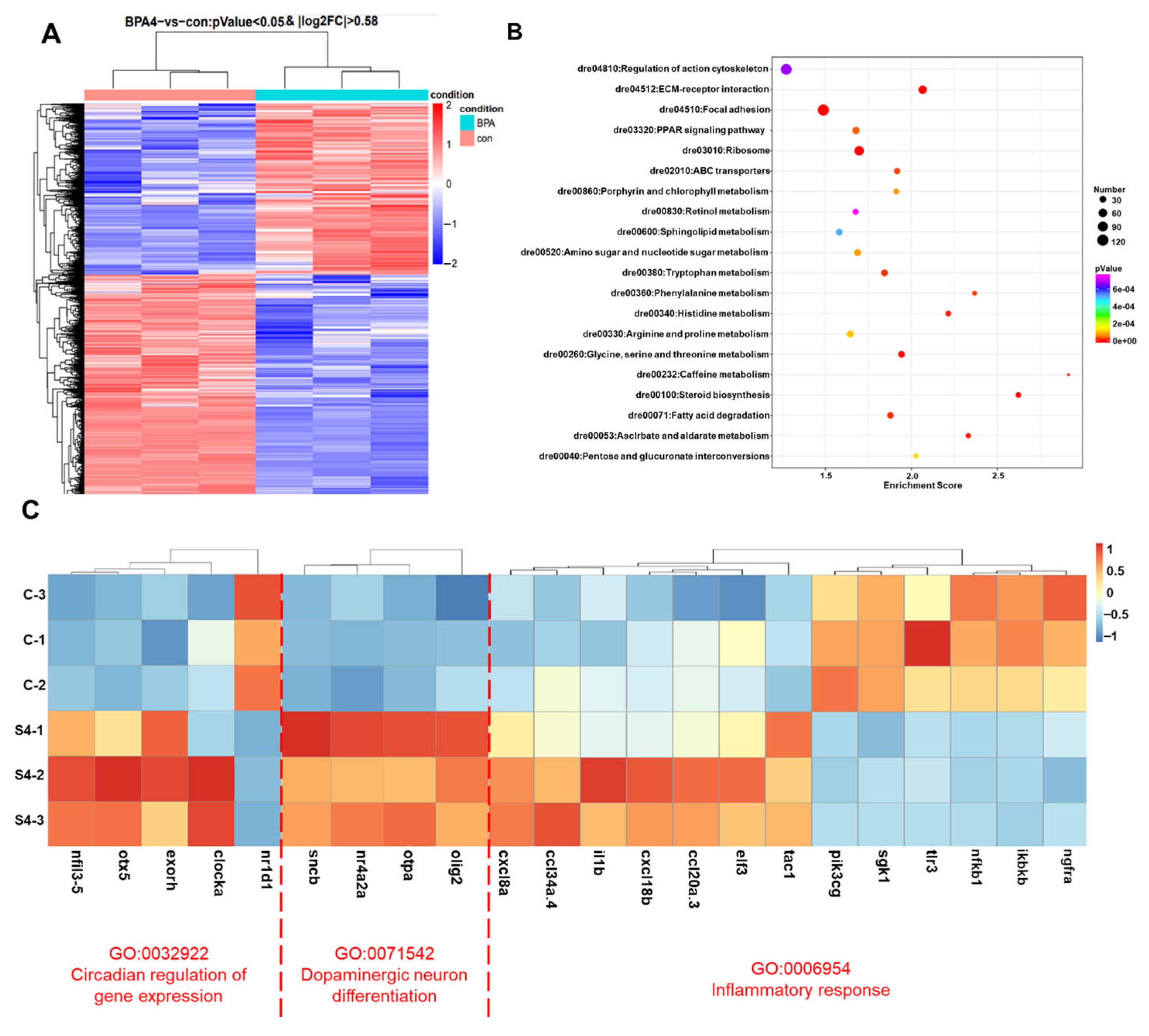
3.1. Transcriptomic Profiling
3.2. BPA Alters the Thigmotactic Behavior in Zebrafish Larvae
3.3. BPA Disrupts Spontaneous Locomotor Rhythms in Zebrafish Larvae
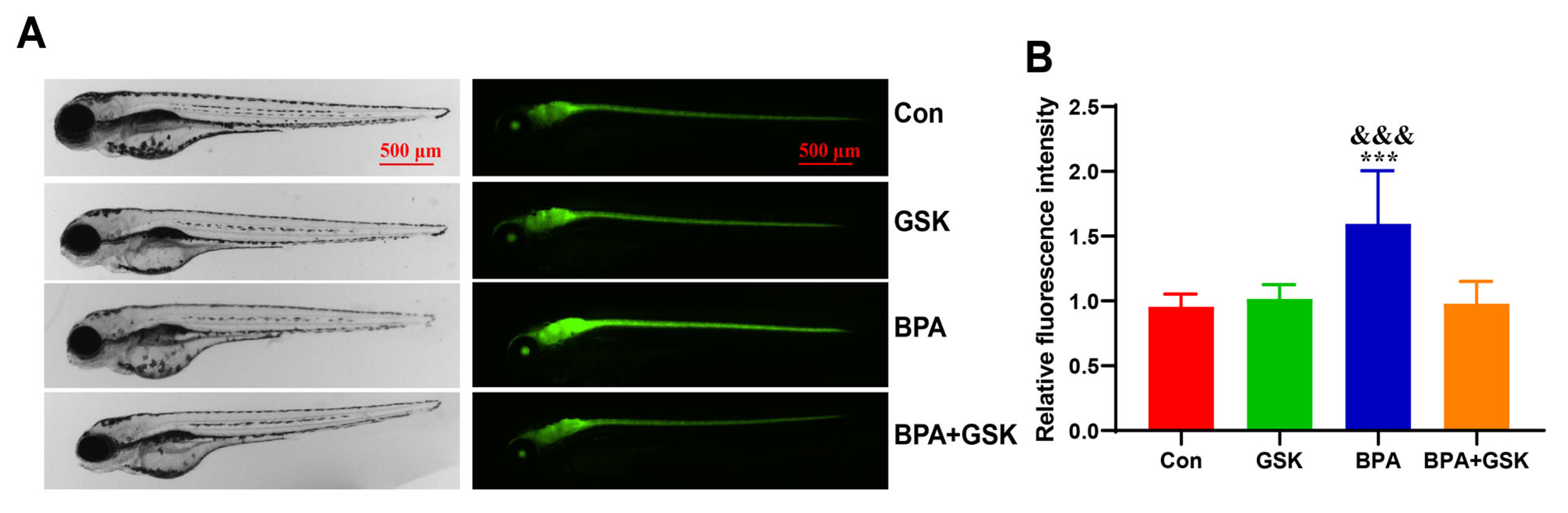
3.4. BPA Exacerbates Astrocyte Activation in the Brain of Zebrafish Larvae
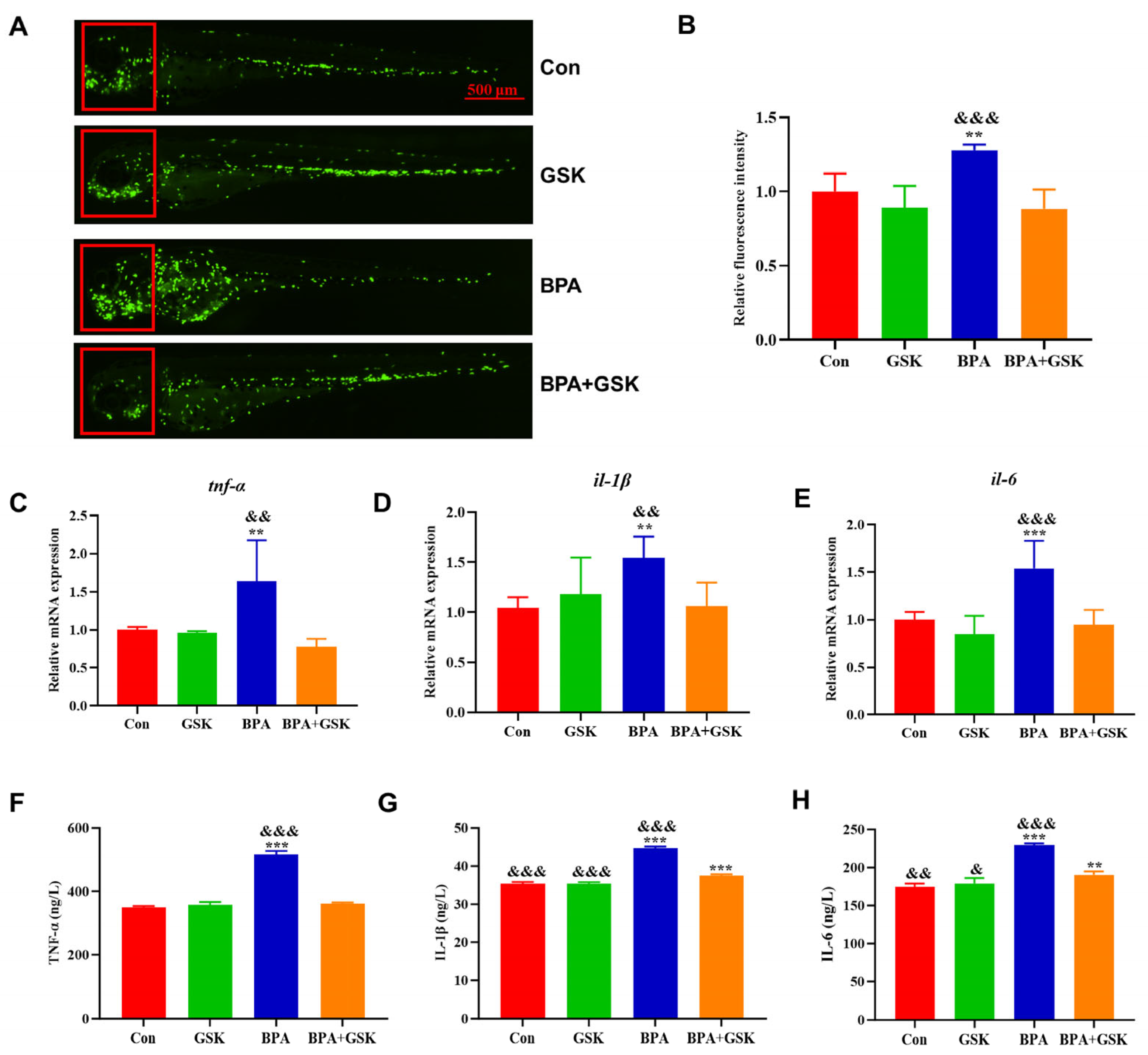
3.5. BPA Elevates Inflammatory Cytokine Levels
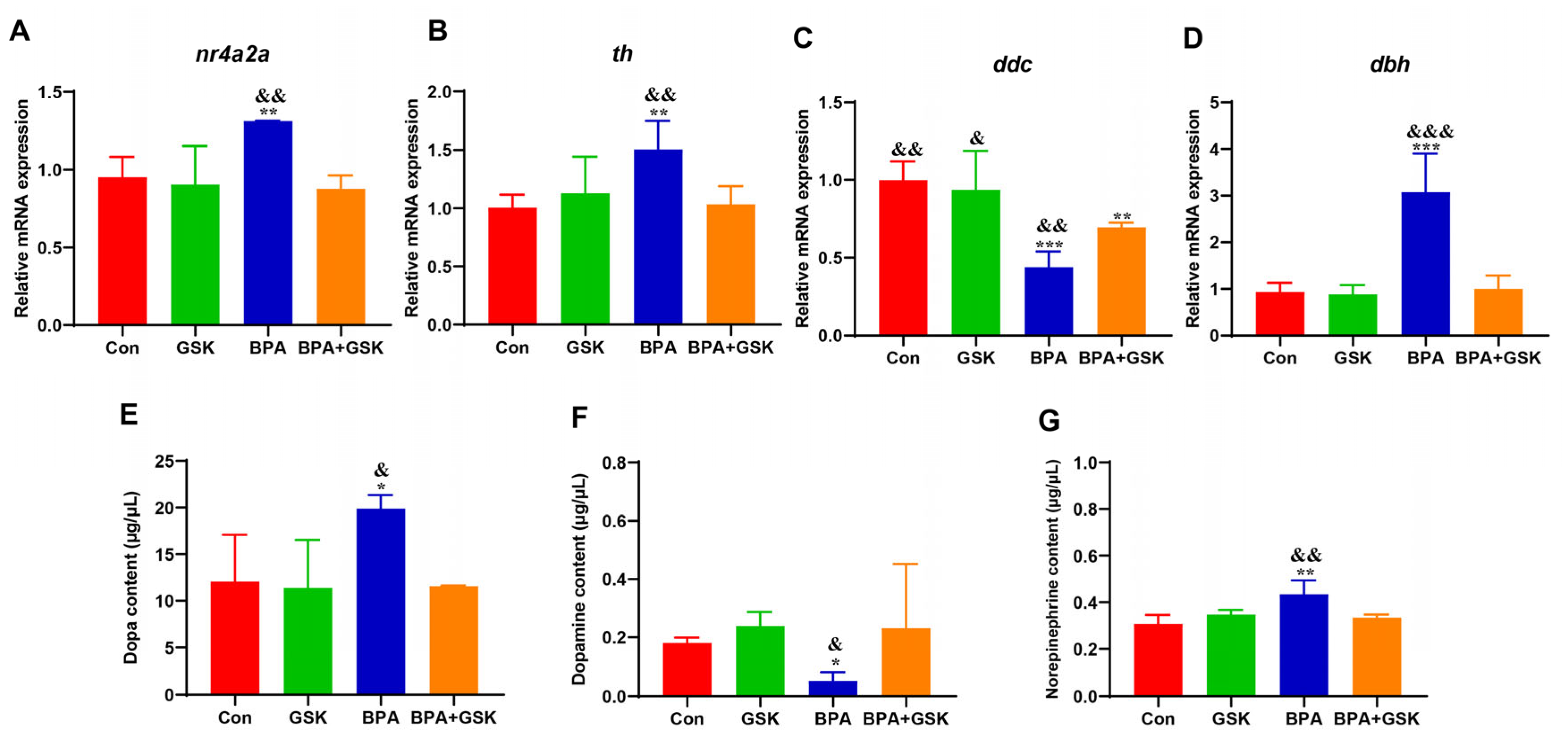
3.6. BPA Disrupts Dopamine System in Zebrafish Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response Publ. Int. Hormesis Soc. 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Fischnaller, M.; Bakry, R.; Bonn, G.K. A simple method for the enrichment of bisphenols using boron nitride. Food Chem. 2016, 194, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Aguilar-Gaxiola, S.; Alonso, J.; Chatterji, S.; Lee, S.; Ormel, J.; Ustün, T.B.; Wang, P.S. The global burden of mental disorders: An update from the WHO World Mental Health (WMH) surveys. Epidemiol. Psichiatr. Soc. 2009, 18, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, W.; Gong, L.; Zhang, J.; Zhang, Y.; Chen, J.; Yu, J.; Xu, J. Subchronic nonylphenol exposure induced anxiety-like behavior and decreased expressions of regulators of synaptic plasticity in rats. Chemosphere 2021, 282, 130994. [Google Scholar] [CrossRef]
- Castillo, L.Y.; Ríos-Carrillo, J.; González-Orozco, J.C.; Camacho-Arroyo, I.; Morin, J.P.; Zepeda, R.C.; Roldán-Roldán, G. Juvenile Exposure to BPA Alters the Estrous Cycle and Differentially Increases Anxiety-like Behavior and Brain Gene Expression in Adult Male and Female Rats. Toxics 2022, 10, 513. [Google Scholar] [CrossRef]
- Li, F.; Yang, F.; Li, D.K.; Tian, Y.; Miao, M.; Zhang, Y.; Ji, H.; Yuan, W.; Liang, H. Prenatal bisphenol A exposure, fetal thyroid hormones and neurobehavioral development in children at 2 and 4 years: A prospective cohort study. Sci. Total Environ. 2020, 722, 137887. [Google Scholar] [CrossRef]
- Raja, G.L.; Lite, C.; Subhashree, K.D.; Santosh, W.; Barathi, S. Prenatal bisphenol-A exposure altered exploratory and anxiety-like behaviour and induced non-monotonic, sex-specific changes in the cortical expression of CYP19A1, BDNF and intracellular signaling proteins in F1 rats. Food Chem. Toxicol. 2020, 142, 111442. [Google Scholar] [CrossRef]
- Ene, M.; Savuca, A.; Ciobica, A.S.; Jijie, R.; Gurzu, I.L.; Hritcu, L.D.; Chelaru, I.A.; Plavan, G.I.; Nicoara, M.N.; Gurzu, B. The Neurobehavioral Impact of Zinc Chloride Exposure in Zebrafish: Evaluating Cognitive Deficits and Probiotic Modulation. Toxics 2025, 13, 193. [Google Scholar] [CrossRef]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Zhuchen, H.Y.; Wang, J.Y.; Liu, X.S.; Shi, Y.W. Research Progress on Neurodevelopmental Toxicity in Offspring After Indirect Exposure to PFASs in Early Life. Toxics 2023, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N. Links Between Circadian Rhythms and Psychiatric Disease. Front. Behav. Neurosci. 2014, 8, 162. [Google Scholar] [CrossRef]
- Lamont, E.W.; Legault-Coutu, D.; Cermakian, N.; Boivin, D.B. The role of circadian clock genes in mental disorders. Dialogues Clin. Neurosci. 2007, 9, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, T.; Kananen, L.; Greco, D.; Donner, J.; Silander, K.; Terwilliger, J.D.; Auvinen, P.; Peltonen, L.; Lönnqvist, J.; Pirkola, S.; et al. An association analysis of circadian genes in anxiety disorders. Biol. Psychiatry 2010, 67, 1163–1170. [Google Scholar] [CrossRef]
- Xing, C.; Zhou, Y.; Xu, H.; Ding, M.; Zhang, Y.; Zhang, M.; Hu, M.; Huang, X.; Song, L. Sleep disturbance induces depressive behaviors and neuroinflammation by altering the circadian oscillations of clock genes in rats. Neurosci. Res. 2021, 171, 124–132. [Google Scholar] [CrossRef]
- Yin, L.; Lazar, M.A. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol. Endocrinol. 2005, 19, 1452–1459. [Google Scholar] [CrossRef]
- Nesan, D.; Feighan, K.M.; Antle, M.C.; Kurrasch, D.M. Gestational low-dose BPA exposure impacts suprachiasmatic nucleus neurogenesis and circadian activity with transgenerational effects. Sci. Adv. 2021, 7, eabd1159. [Google Scholar] [CrossRef]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Maphanga, V.B.; Skalicka-Woźniak, K.; Budzynska, B.; Enslin, G.M.; Viljoen, A.M. Screening selected medicinal plants for potential anxiolytic activity using an in vivo zebrafish model. Psychopharmacology 2020, 237, 3641–3652. [Google Scholar] [CrossRef]
- Liu, T.; He, W.; Zhong, Z.; Lu, C.; Wu, L.; Wang, Z.; Smith, W.K.; Shi, Q.; Long, Q.; Wang, H. The circadian clock orchestrates spermatogonial differentiation and fertilization by regulating retinoic acid signaling in vertebrates. Natl. Sci. Rev. 2024, 12, nwae456. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, T.; Moore, A.M.; Troup, E.; Halliday, K.J.; Millar, A.J. Strengths and Limitations of Period Estimation Methods for Circadian Data. PLoS ONE 2014, 9, e96462. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lama, A.; Del Piano, F.; Annunziata, C.; Comella, F.; Opallo, N.; Melini, S.; Grumetto, L.; Pirozzi, C.; Mattace Raso, G.; Meli, R.; et al. Bisphenol A exacerbates anxiety-like behavior and neuroinflammation in prefrontal cortex of adult obese mice. Life Sci. 2023, 313, 121301. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Puar, P.; JavadiEsfahani, R.; Kwong, R.W.M. Early developmental exposure to bisphenol A and bisphenol S disrupts socio-cognitive function, isotocin equilibrium, and excitation-inhibition balance in developing zebrafish. Neurotoxicology 2022, 88, 144–154. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Cheng, W.; Rolls, E.T.; Huang, P.; Ma, N.; Liu, Y.; Zhang, Y.; Guan, X.; Guo, T.; et al. Dopamine depletion and subcortical dysfunction disrupt cortical synchronization and metastability affecting cognitive function in Parkinson’s disease. Human Brain Mapp. 2022, 43, 1598–1610. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, Y.; Dang, Y.; Zhu, X.; Li, Z.; Chen, H.; Xiang, M.; Li, Z.; Hu, G. Exposure of adult zebrafish (Danio rerio) to Tetrabromobisphenol A causes neurotoxicity in larval offspring, an adverse transgenerational effect. J. Hazard. Mater. 2021, 414, 125408. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Yang, J.; Wang, F.; Sima, Y.; Zhong, Z.M.; Wang, H.; Hu, L.F.; Liu, C.F. Parkinson’s disease-like motor and non-motor symptoms in rotenone-treated zebrafish. Neurotoxicology 2017, 58, 103–109. [Google Scholar] [CrossRef]
- Saucedo-Cardenas, O.; Quintana-Hau, J.D.; Le, W.D.; Smidt, M.P.; Cox, J.J.; De Mayo, F.; Burbach, J.P.; Conneely, O.M. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 4013–4018. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Shi, F.; Li, X.; Hu, Z.; Chu, J. Protective effect of surfactin on copper sulfate-induced inflammation, oxidative stress, and hepatic injury in zebrafish. Microbiol. Immunol. 2021, 65, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.H.; Noh, K.; Lee, B.H.; Barcelon, E.; Jun, S.B.; Park, H.Y.; Lee, S.J. Hippocampal astrocytes modulate anxiety-like behavior. Nat. Commun. 2022, 13, 6536. [Google Scholar] [CrossRef]
- Kunz, N.; Camm, E.J.; Somm, E.; Lodygensky, G.; Darbre, S.; Aubert, M.L.; Hüppi, P.S.; Sizonenko, S.V.; Gruetter, R. Developmental and metabolic brain alterations in rats exposed to bisphenol A during gestation and lactation. Int. J. Dev. Neurosci. 2011, 29, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pourcet, B.; Zecchin, M.; Ferri, L.; Beauchamp, J.; Sitaula, S.; Billon, C.; Delhaye, S.; Vanhoutte, J.; Mayeuf-Louchart, A.; Thorel, Q.; et al. Nuclear Receptor Subfamily 1 Group D Member 1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce the Severity of Fulminant Hepatitis in Mice. Gastroenterology 2018, 154, 1449–1464.e1420. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sakurai, T.; Ogasawara, J.; Shirato, K.; Ishibashi, Y.; Oh-ishi, S.; Imaizumi, K.; Haga, S.; Hitomi, Y.; Izawa, T.; et al. Direct and indirect suppression of interleukin-6 gene expression in murine macrophages by nuclear orphan receptor REV-ERBα. Sci. World J. 2014, 2014, 685854. [Google Scholar] [CrossRef]
- Fornes, O.; Castro-Mondragon, J.A.; Khan, A.; van der Lee, R.; Zhang, X.; Richmond, P.A.; Modi, B.P.; Correard, S.; Gheorghe, M.; Baranašić, D.; et al. JASPAR 2020: Update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020, 48, D87–D92. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.-H.; Bi, N.; Gu, X.; Huang, C.; Zhou, R.; Liu, H.; Wang, H.-L. Dysfunction of the medial prefrontal cortex contributes to BPA-induced depression- and anxiety-like behavior in mice. Ecotoxicol. Environ. Saf. 2023, 259, 115034. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Loosli, F.; Conti, F.; Foulkes, N.S.; Bertolucci, C. Comparison of anxiety-like and social behaviour in medaka and zebrafish. Sci. Rep. 2022, 12, 10926. [Google Scholar] [CrossRef]
- Grant, D.; Yin, L.; Collins, J.L.; Parks, D.J.; Orband-Miller, L.A.; Wisely, G.B.; Joshi, S.; Lazar, M.A.; Willson, T.M.; Zuercher, W.J. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbα. ACS Chem. Biol. 2010, 5, 925–932. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Chen, P.; Wang, Y.; Wang, X.; Bao, Y.; Fan, L.; Rao, Y.; Song, X.; Zhang, J. Role of NR1D1 in Bisphenol A-Induced Anxiety-like Behavior and Inflammation in Zebrafish Larvae. Toxics 2025, 13, 449. https://doi.org/10.3390/toxics13060449
Wu M, Chen P, Wang Y, Wang X, Bao Y, Fan L, Rao Y, Song X, Zhang J. Role of NR1D1 in Bisphenol A-Induced Anxiety-like Behavior and Inflammation in Zebrafish Larvae. Toxics. 2025; 13(6):449. https://doi.org/10.3390/toxics13060449
Chicago/Turabian StyleWu, Mingjun, Pinyi Chen, Yuting Wang, Xinwei Wang, Yuqianrui Bao, Liqiao Fan, Yuxiao Rao, Xiaoyao Song, and Jie Zhang. 2025. "Role of NR1D1 in Bisphenol A-Induced Anxiety-like Behavior and Inflammation in Zebrafish Larvae" Toxics 13, no. 6: 449. https://doi.org/10.3390/toxics13060449
APA StyleWu, M., Chen, P., Wang, Y., Wang, X., Bao, Y., Fan, L., Rao, Y., Song, X., & Zhang, J. (2025). Role of NR1D1 in Bisphenol A-Induced Anxiety-like Behavior and Inflammation in Zebrafish Larvae. Toxics, 13(6), 449. https://doi.org/10.3390/toxics13060449






