Milk and Whole Blood Surveillance Following Lethal and Sublethal Lead Intoxication in a Michigan Dairy Herd
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Investigation and Workup
2.2. Laboratory Lead Analysis
2.3. Diagnostic Criteria and Decision Making
2.4. Statistics and Calculations
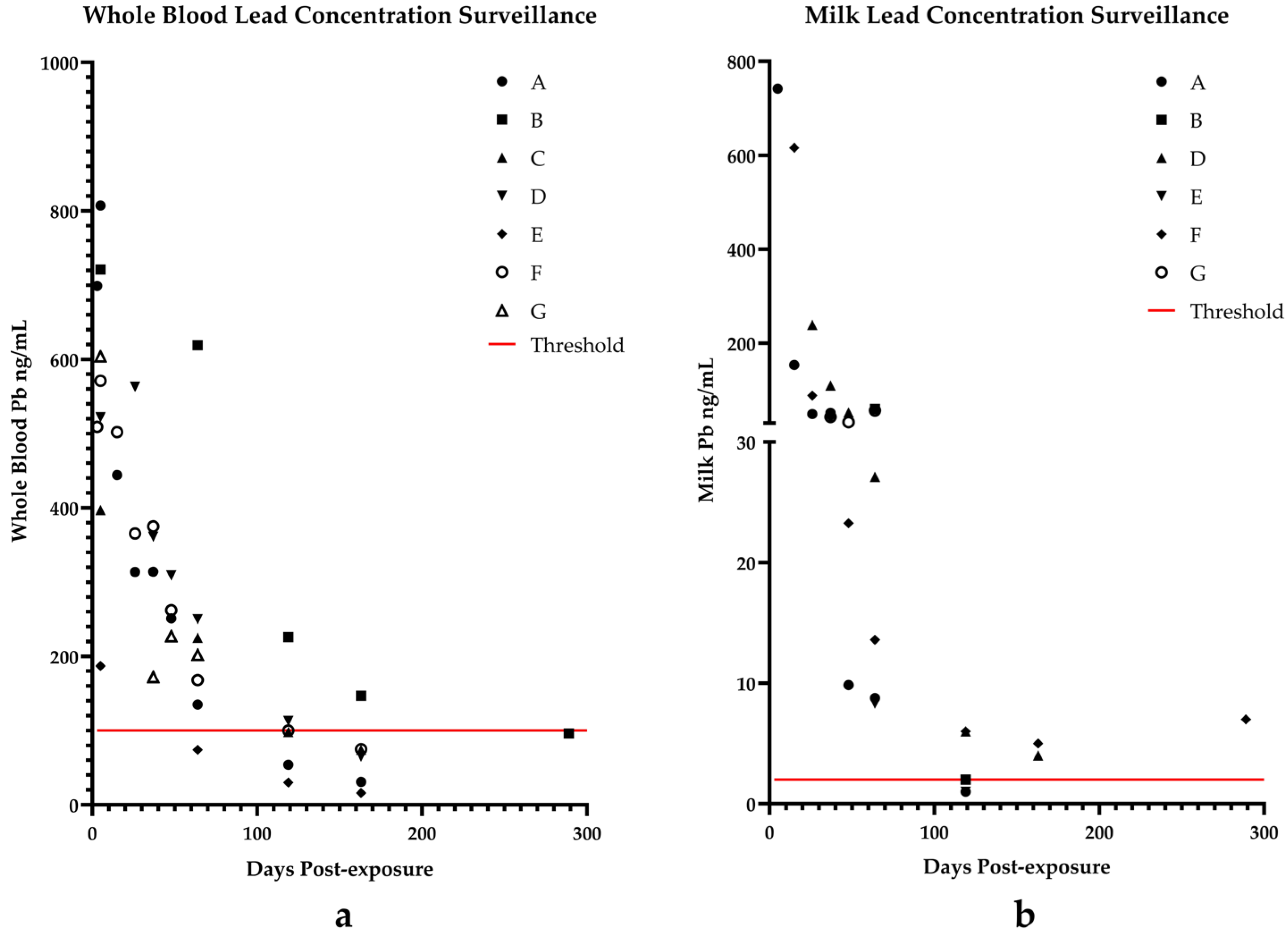
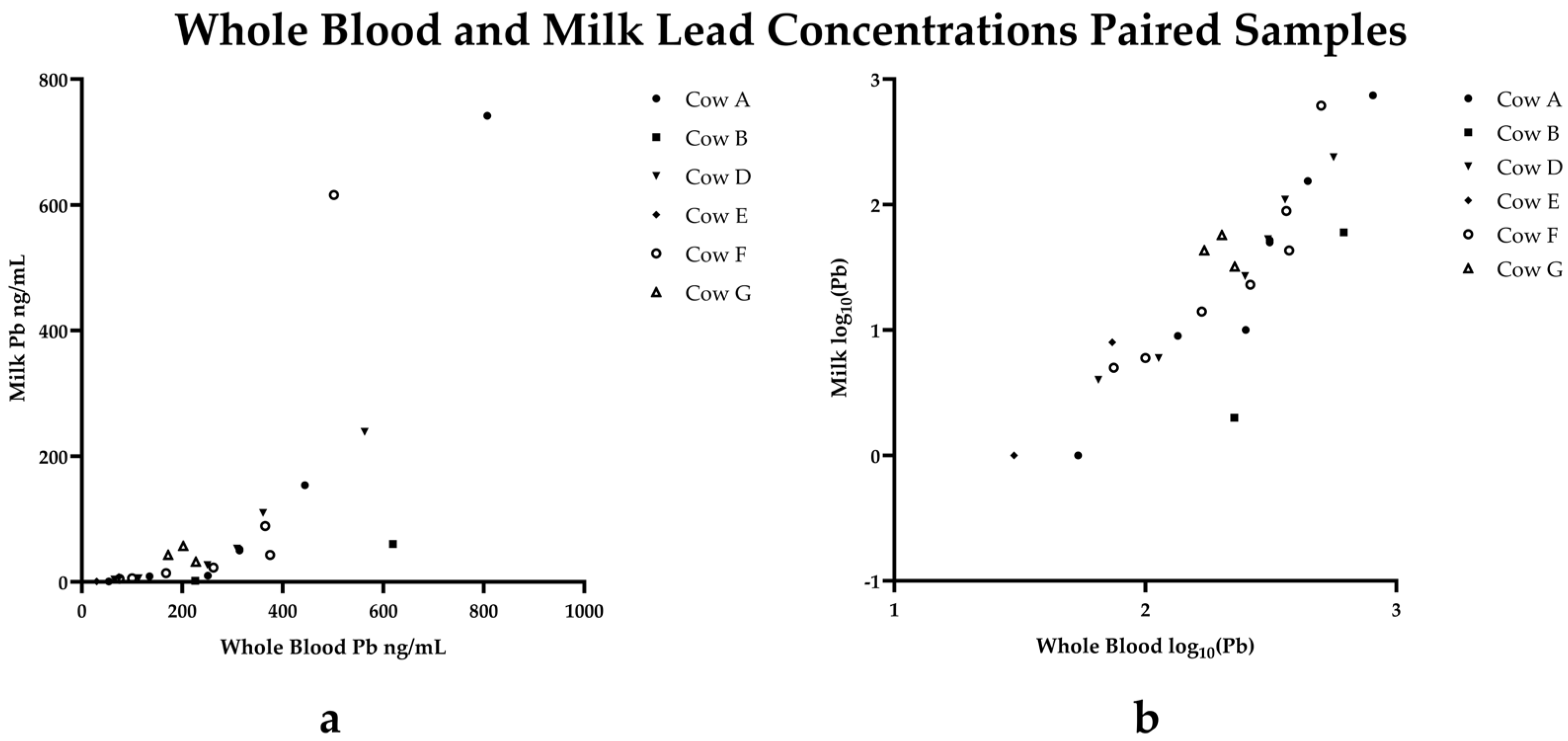
3. Results
Case Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDARD | Michigan Department of Agriculture and Rural Development |
| MSU VDL | Michigan State University Veterinary Diagnostic Laboratory |
| NHANES | National Health and Nutrition Examination Survey |
| CFSAN | FDA Center for Food Safety and Applied Nutrition |
| FOIA | Freedom of Information Act |
| CFR | Code of Federal Regulations |
| EDTA | Ethylenediaminetetraacetic acid |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| EU | European Union |
| USA | United States of America |
References
- Rumbold, P.; McCullogh, N.; Boldon, R.; Haskell-Ramsay, C.; James, L.; Stevenson, E.; Green, B. The Potential Nutrition-, Physical- and Health-Related Benefits of Cow’s Milk for Primary-School-Aged Children. Nutr. Res. Rev. 2022, 35, 50–69. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.A.R.; Vaz, J.S.; Maia, F.S.; Baker, P.; Gatica-Domínguez, G.; Piwoz, E.; Rollins, N.; Victora, C.G. Rates and Time Trends in the Consumption of Breastmilk, Formula, and Animal Milk by Children Younger than 2 Years from 2000 to 2019: Analysis of 113 Countries. Lancet Child Adolesc. Health 2021, 5, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Haile, B.; Headey, D. Growth in Milk Consumption and Reductions in Child Stunting: Historical Evidence from Cross-Country Panel Data. Food Policy 2023, 118, 102485. [Google Scholar] [CrossRef]
- Trejo, S.; Yeomans-Maldonado, G.; Jacob, B. The Effects of the Flint Water Crisis on the Educational Outcomes of School-Age Children. Sci. Adv. 2024, 10, eadk4737. [Google Scholar] [CrossRef]
- Schneider, J.S. Neurotoxicity and Outcomes from Developmental Lead Exposure: Persistent or Permanent? Environ. Health Perspect. 2023, 131, 85002. [Google Scholar] [CrossRef]
- Levin, R.; Zilli Vieira, C.L.; Rosenbaum, M.H.; Bischoff, K.; Mordarski, D.C.; Brown, M.J. The Urban Lead (Pb) Burden in Humans, Animals and the Natural Environment. Environ. Res. 2021, 193, 110377. [Google Scholar] [CrossRef]
- Bischoff, K.; Thompson, B.; Erb, H.N.; Higgins, W.P.; Ebel, J.G.; Hillebrandt, J.R. Declines in Blood Lead Concentrations in Clinically Affected and Unaffected Cattle Accidentally Exposed to Lead. J. Vet. Diagn. Investig. 2012, 24, 182–187. [Google Scholar] [CrossRef]
- Galey, F.D.; Slenning, B.D.; Anderson, M.L.; Breneman, P.C.; Littlefield, E.S.; Melton, L.A.; Tracy, M.L. Lead Concentrations in Blood and Milk from Periparturient Dairy Heifers Seven Months after an Episode of Acute Lead Toxicosis. J. Vet. Diagn. Investig. 1990, 2, 222–226. [Google Scholar] [CrossRef]
- Rumbeiha, W.K.; Braselton, W.E.; Donch, D. A Retrospective Study on the Disappearance of Blood Lead in Cattle with Accidental Lead Toxicosis. J. Vet. Diagn. Investig. 2001, 13, 373–378. [Google Scholar] [CrossRef]
- Wahlen, R.; Evans, L.; Turner, J.; Hearn, R. The Use of Collision/Reaction Cell ICP-MS for the Determination of Elements in Blood and Serum Samples. Spectroscopy 2005, 20, 84–89. [Google Scholar]
- State of Michigan Department of Agriculture and Rural Development. Lead Toxicity/Exposure Response Standard Operating Procedure; State of Michigan Department of Agriculture and Rural Development: Lansing, MI, USA, 2012.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Lüdecke, D.; Ben-Shachar, M.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. [Google Scholar]
- Lüdecke, D.; Patil, I.; Ben-Shachar, M.; Wiernik, B.; Waggoner, P.; Makowski, D. See: An R Package for Visualizing Statistical Models. J. Open Source Softw. 2021, 6, 3393. [Google Scholar] [CrossRef]
- Wickham, H.; Bryan, J. _readxl: Read Excel Files_. R Package Version 1.4.5. 2025. Available online: https://CRAN.R-project.org/package=readxl (accessed on 11 May 2025).
- Hoffman-Pennesi, D.; Winfield, S.; Gavelek, A.; Santillana Farakos, S.M.; Spungen, J. Infants’ and Young Children’s Dietary Exposures to Lead and Cadmium: FDA Total Diet Study 2018–2020. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2024, 41, 1454–1479. [Google Scholar] [CrossRef]
- Bischoff, K.; Higgins, W.; Thompson, B.; Ebel, J.G. Lead Excretion in Milk of Accidentally Exposed Dairy Cattle. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 839–844. [Google Scholar] [CrossRef]
- Bischoff, K.; Hillebrandt, J.; Erb, H.N.; Thompson, B.; Johns, S. Comparison of Blood and Tissue Lead Concentrations from Cattle with Known Lead Exposure. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1563–1569. [Google Scholar] [CrossRef]
- Oskarsson, A.; Jorhem, L.; Sundberg, J.; Nilsson, N.G.; Albanus, L. Lead Poisoning in Cattle—Transfer of Lead to Milk. Sci. Total Environ. 1992, 111, 83–94. [Google Scholar] [CrossRef]
- Food and Drug Administration. Action Levels for Lead in Processed Food Intended for Babies and Young Children: Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2025.
- U.S. Centers for Disease Control and Prevention. What We Eat in America, DHHS-USDA Dietary Survey Integration WWEIA/NHANES Overview. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/ (accessed on 23 February 2025).
- Spungen, J.H. Children’s Exposures to Lead and Cadmium: FDA Total Diet Study 2014–16. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 893–903. [Google Scholar] [CrossRef]
- CREU. Commission Regulation (EU) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Official Journal of the European Union, 19 December 2006; p. 364. [Google Scholar]
- Zwierzchowski, G.; Ametaj, B.N. Minerals and Heavy Metals in the Whole Raw Milk of Dairy Cows from Different Management Systems and Countries of Origin: A Meta-Analytical Study. J. Agric. Food Chem. 2018, 66, 6877–6888. [Google Scholar] [CrossRef]
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Habila, S.; Boussadia, M.I.; Gueroui, Y. Heavy Metals Levels in Raw Cow Milk and Health Risk Assessment across the Globe: A Systematic Review. Sci. Total Environ. 2021, 751, 141830. [Google Scholar] [CrossRef]
- Denholm, S.J.; McNeilly, T.N.; Bashir, S.; Mitchell, M.C.; Wall, E.; Sneddon, A.A. Correlations of Milk and Serum Element Concentrations with Production and Management Traits in Dairy Cows. J. Dairy Sci. 2022, 105, 9726–9737. [Google Scholar] [CrossRef]
- Food and Drug Administration. 21 CFR Part 131 Milk and Cream; Department of Health and Human Services: Washington, DC, USA, 1977; pp. 474–490.
- Food and Agriculture Organization of the United Nations; World Health Organization. Code of Practice for the Prevention and Reduction of Lead Contamination in Foods; Codex Alimentarius Code of Practice, No. 56-2004; Codex Alimentarius Commission: Rome, Italy, 2004; pp. 1–6. [Google Scholar] [CrossRef]
| Days Since Exposure | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal ID | 3 | 5 | 7 | 15 | 26 | 37 | 48 | 64 | 65 | 119 | 163 | 289 |
| A | 699 | 807 | 444 | 314 | 314 | 251 | 135 | 54 | 31 | |||
| B | 721 | 619 | 226 | 147 | 96 | |||||||
| C *** | 397 | 225 | 98 | 74 | ||||||||
| D † | 522 | 563 | 361 | 309 | 250 | 113 | 65 | |||||
| E | 187 | 74 | 30 | 16 | ||||||||
| F | 509 | 571 | 502 | 365 | 375 | 262 | 168 | 100 | 75 | |||
| G ** | 604 | 172 | 227 | 202 | ||||||||
| H * | 2039 | |||||||||||
| I * | 1180 | |||||||||||
| J * | 869 | |||||||||||
| K * | 579 | |||||||||||
| L * | 2587 | |||||||||||
| M * | 930 | |||||||||||
| N * | 745 | |||||||||||
| O * | 735 | |||||||||||
| P * | 1055 | |||||||||||
| Q ‡ | 7 | |||||||||||
| A-Calf | 16 | |||||||||||
| B-Calf | 114 | 60 | ||||||||||
| E-Calf | 27 | |||||||||||
| F-Calf | 198 | 91 | ||||||||||
| G-Calf | 112 | 98 | ||||||||||
| Days Since Exposure | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal ID | 5 | 15 | 26 | 37 | 48 | 64 | 119 | 163 | 289 |
| A | 742 | 154 | 50 | 52 | 10 | 9 | <1 | ||
| B | 60 | 2 | |||||||
| D * | 239 | 110 | 53 | 27 | 6 | 4 | |||
| E | 8 | 1 | |||||||
| F | 616 | 89 | 43 | 23 | 14 | 6 | 5 | 7 | |
| G ** | 43 | 32 | 57 | ||||||
| Estimate | Std Error | df | t-Value | p-Value | |
|---|---|---|---|---|---|
| Intercept | 2.49 | 0.51 | 19.46 | 4.87 | 0.0001 |
| Days since exposure | −0.012 | 0.0035 | 22.35 | −3.49 | 0.0021 |
| Blood lead | 0.0057 | 0.00084 | 22.98 | 6.72 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheffler, R.; Rebolloso, S.; Scott, I.; Buchweitz, J.P.; Puschner, B. Milk and Whole Blood Surveillance Following Lethal and Sublethal Lead Intoxication in a Michigan Dairy Herd. Toxics 2025, 13, 445. https://doi.org/10.3390/toxics13060445
Sheffler R, Rebolloso S, Scott I, Buchweitz JP, Puschner B. Milk and Whole Blood Surveillance Following Lethal and Sublethal Lead Intoxication in a Michigan Dairy Herd. Toxics. 2025; 13(6):445. https://doi.org/10.3390/toxics13060445
Chicago/Turabian StyleSheffler, Rachel, Sarah Rebolloso, Isaiah Scott, John P. Buchweitz, and Birgit Puschner. 2025. "Milk and Whole Blood Surveillance Following Lethal and Sublethal Lead Intoxication in a Michigan Dairy Herd" Toxics 13, no. 6: 445. https://doi.org/10.3390/toxics13060445
APA StyleSheffler, R., Rebolloso, S., Scott, I., Buchweitz, J. P., & Puschner, B. (2025). Milk and Whole Blood Surveillance Following Lethal and Sublethal Lead Intoxication in a Michigan Dairy Herd. Toxics, 13(6), 445. https://doi.org/10.3390/toxics13060445







