Bisphenols, Toxic Elements, and Potentially Toxic Elements in Ready-to-Eat Fish and Meat Foods and Their Associated Risks for Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standard Solutions
2.2. Sampling
2.3. Sample Preparation
2.4. Analysis by HPLC-MS/MS
2.5. Analysis by ICP-MS
2.6. Statistical Analysis
2.7. Consumer Health Risk Estimation
3. Results and Discussion
3.1. Occurrence of Bisphenols and Elements in Samples
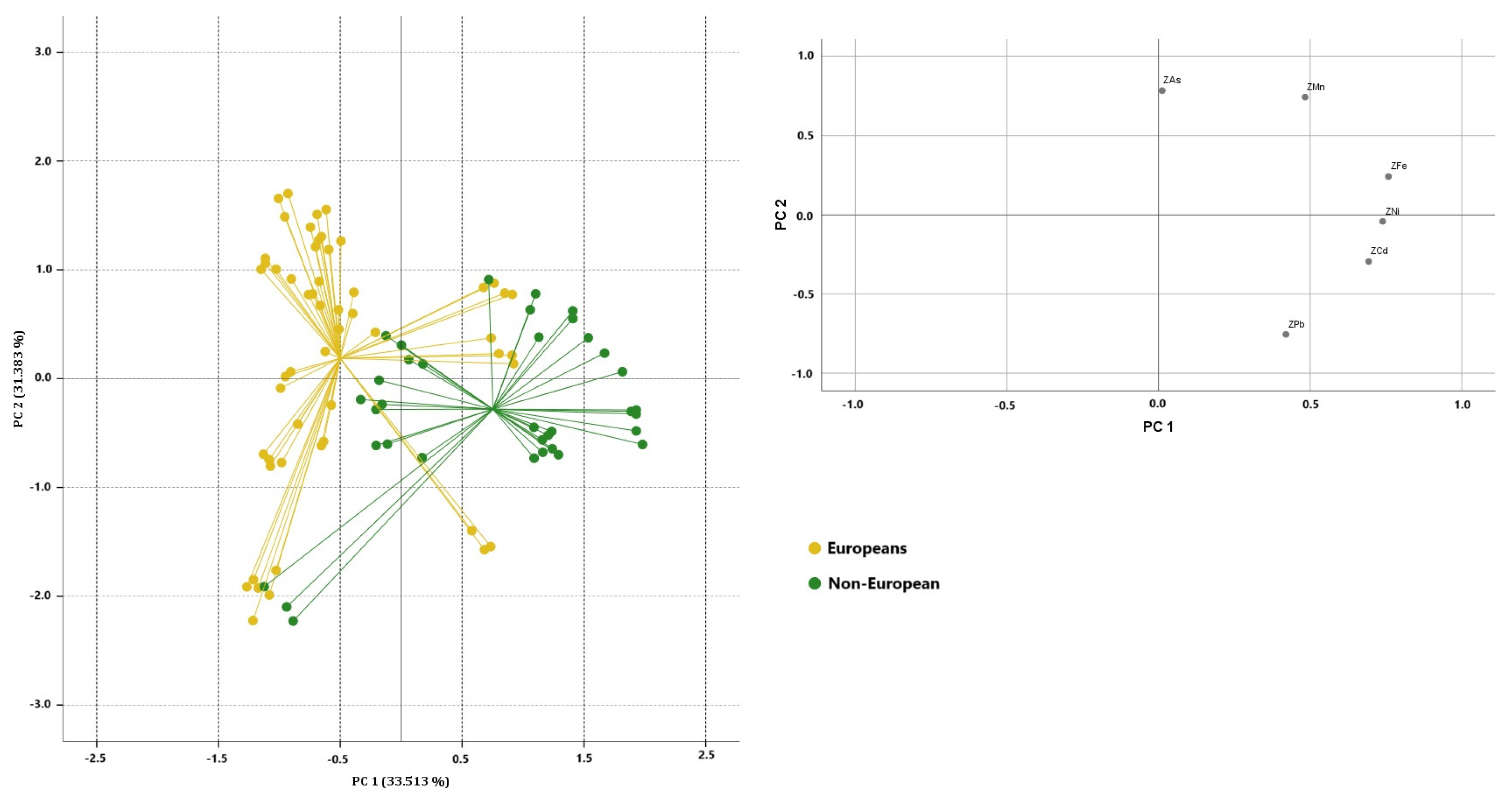
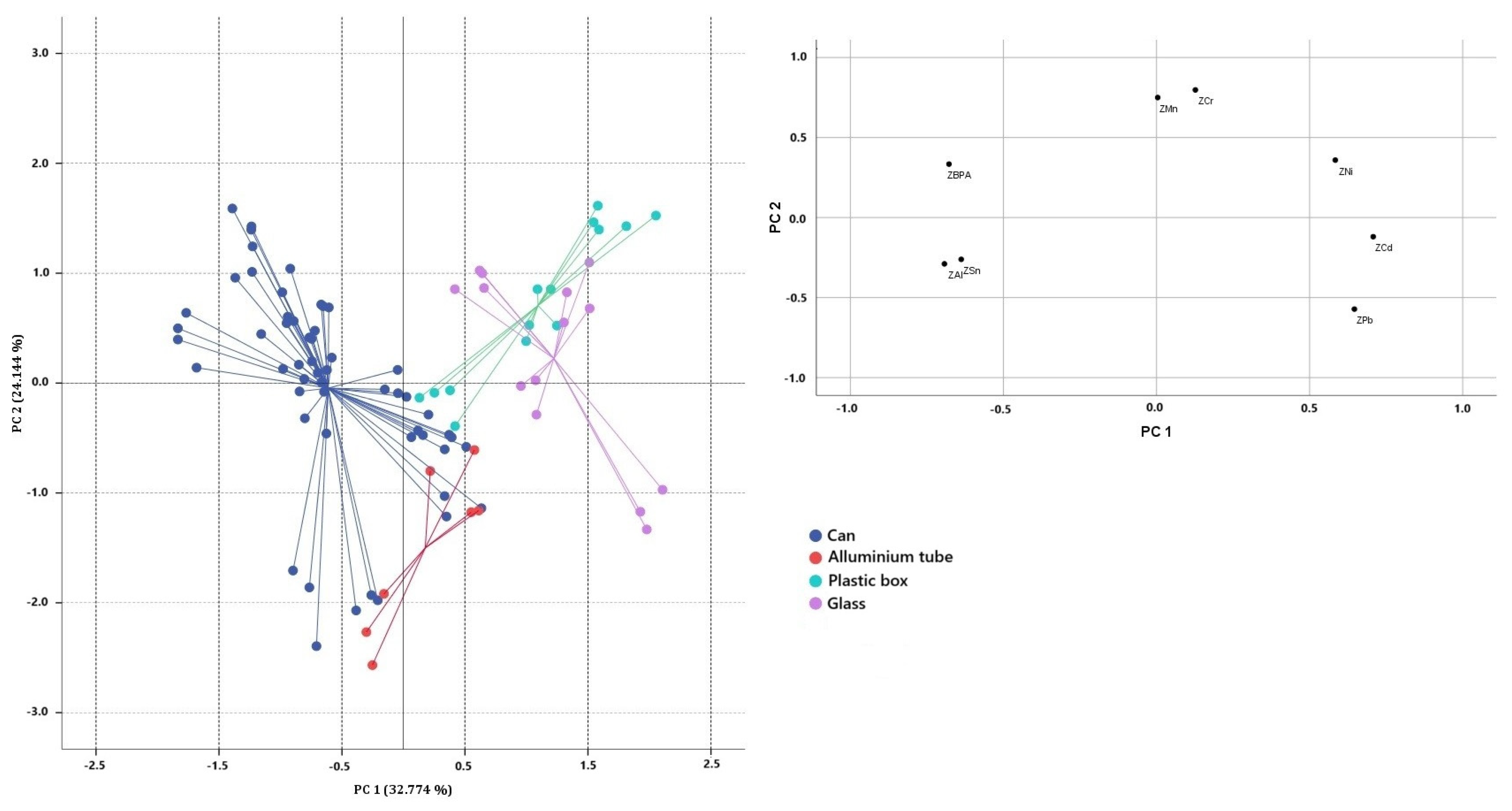
3.2. PCA Results
3.3. The Uptake of Bisphenols and Toxic/Potentially Toxic Elements from Processed Food
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.grandviewresearch.com/industry-analysis/canned-food-market-report (accessed on 22 May 2025).
- Nordin, Z.A.; Teo, S.S. Factors influencing purchase intention of packaged food among adults in Klang Valley, Malaysia. J. Tour. Hosp. Culin. Arts 2024, 16, 131–149. [Google Scholar]
- Deshwal, G.K.; Panjagari, N.R. Review on metal packaging: Materials, forms, food applications, safety and recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef]
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Migration phenomenon in food packaging. Food–package interactions, mechanisms, types of migrants, testing and relative legislation—A review. Food Bioprocess Technol. 2014, 7, 21–36. [Google Scholar] [CrossRef]
- Zhang, N.; Scarsella, J.B.; Hartman, T.G. Identification and quantitation studies of migrants from BPA alternative food-contact metal can coatings. Polymers 2020, 12, 2846. [Google Scholar] [CrossRef]
- Munguia-Lopez, E.M.; Soto-Valdez, H. Effect of heat processing and storage time on migration of bisphenol A (BPA) and bisphenol A−diglycidyl ether (BADGE) to aqueous food simulant from Mexican can coatings. J. Agric. Food Chem. 2001, 49, 3666–3671. [Google Scholar] [CrossRef]
- Zech, J.; Manowski, A.; Malchow, S.; Rettberg, N.; Garbe, L.A. Determination of bisphenols, bisphenol A diglycidyl ether (BADGE), BADGE chloro-hydrins and hydrates from canned beer by high-performance liquid chromatography-tandem mass spectrometry. Brew. Sci. 2015, 68, 102–109. [Google Scholar]
- EFSA Panel on Food Contact Materials; Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Van Loveren, H. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar]
- Commission Regulation (EU) 2024/3190 of 19 December 2024 on the Use of Bisphenol A (BPA) and Other Bisphenols and Bisphenol Derivatives with Harmonised Classification for Specific Hazardous Properties in Certain Materials and Articles Intended to Come into Contact with Food, Amending Regulation (EU) No 10/2011 and Repealing Regulation (EU) 2018/213. Available online: https://eur-lex.europa.eu/eli/reg/2024/3190 (accessed on 22 May 2025).
- European Commission. European Commission Commission Regulation (EC) No 1895/2005 of 18 November 2005 on the re-striction of use of certain epoxy derivatives in materials articles intended to come into contact with food. Off. J. Eur. Communities 2005, 302, 28–32. [Google Scholar]
- Koo, Y.J.; Pack, E.C.; Lee, Y.J.; Kim, H.S.; Jang, D.Y.; Lee, S.H.; Kim, Y.S.; Lim, K.M.; Choi, D.W. Determination of toxic metal release from metallic kitchen utensils and their health risks. Food Chem. Toxicol. 2020, 145, 111651. [Google Scholar] [CrossRef]
- Ikem, A.; Egiebor, N.O. Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America). J. Food Compos. Anal. 2005, 18, 771–787. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023R0915 (accessed on 22 May 2025).
- Shruti, V.C.; Kutralam-Muniasamy, G. Migration testing of microplastics in plastic food-contact materials: Release, characterization, pollution level, and influencing factors. TrAC Trends Anal. Chem. 2024, 170, 117421. [Google Scholar] [CrossRef]
- Noureddine El Moussawi, S.; Camel, V.; Cladière, M.; Lebbos, N.; Chébib, H.; Ouaini, R. Parameters influencing the migration of trace metals in uncoated fruit cans. J. Food Process. Preserv. 2020, 44, e14653. [Google Scholar] [CrossRef]
- Evans, T.J. Endocrine disruption. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2017; pp. 1091–1110. [Google Scholar]
- Kortenkamp, A.; Faust, M.; Scholze, M.; Backhaus, T. Low-level exposure to multiple chemicals: Reason for human health concerns? Environ. Health Perspect. 2007, 115 (Suppl. S1), 106–114. [Google Scholar] [CrossRef]
- Liotta, L.; Litrenta, F.; Turco, V.L.; Potortì, A.G.; Lopreiato, V.; Nava, V.; Bionda, A.; Di Bella, G. Evaluation of chemical contaminants in conventional and unconventional ragusana provola cheese. Foods 2022, 11, 3817. [Google Scholar] [CrossRef]
- Bruno, F.; Nava, V.; Fazio, F.; Sansotta, C.; Bruschetta, G.; Licata, P.; Parrino, V. Heavy Metals Bioaccumulation in Mytilus galloprovincialis and Tapes decussatus from Faro Lake (Messina), Italy. Biol. Trace Elem. Res. 2024, 202, 5762–5770. [Google Scholar] [CrossRef]
- Yonekubo, J.; Hayakawa, K.; Sajiki, J. Concentrations of bisphenol A, bisphenol A diglycidyl ether, and their derivatives in canned foods in Japanese markets. J. Agric. Food Chem. 2008, 56, 2041–2047. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary Exposure to Aluminium-Containing Food Additives; European Food Safety Authority (EFSA): Parma, Italy, 2013; Volume 10, p. 411E.
- Cubadda, F.; Iacoponi, F.; Ferraris, F.; D'Amato, M.; Aureli, F.; Raggi, A.; Sette, S.; Turrini, A.; Mantovani, A. Dietary exposure of the Italian population to nickel: The national Total Diet Study. Food Chem. Toxicol. 2020, 146, 111813. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, F.J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 939–963. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar]
- Derrar, S.; Nava, V.; Ayad, M.A.; Saim, M.S.; Aggad, H.; Spanò, I.M.; Litrenta, F.; Leonardi, M.; Albergamo, A.; Turco, V.L.; et al. Safety Assessment of Honeys from Northern and Southern Algerian Regions. Agriculture 2024, 14, 1503. [Google Scholar] [CrossRef]
- El Moussawi, S.N.; Ouaini, R.; Matta, J.; Chébib, H.; Cladière, M.; Camel, V. Simultaneous migration of bisphenol compounds and trace metals in canned vegetable food. Food Chem. 2019, 288, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Noureddine El Moussawi, S.; Cladière, M.; Chébib, H.; Ouaini, R.; Camel, V. Empirical models to predict the effect of sterilisation and storage on bisphenols migration from metallic can coatings into food simulants. Food Addit. Contam. Part A 2019, 36, 1937–1949. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sendón, R.; Van der Kellen, A.; Vaz, M.F.; Silva, A.S. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019, 91, 33–65. [Google Scholar] [CrossRef]
- Choi, S.J.; Yun, E.S.; Shin, J.M.; Kim, Y.S.; Lee, J.S.; Lee, J.H.; Kim, D.G.; Oh, Y.H.; Jung, K.; Kim, G.H. Concentrations of bisphenols in canned foods and their risk assessment in Korea. J. Food Prot. 2018, 81, 903–916. [Google Scholar] [CrossRef]
- Cao, P.; Zhong, H.N.; Qiu, K.; Li, D.; Wu, G.; Sui, H.X.; Song, Y. Exposure to bisphenol A and its substitutes, bisphenol F and bisphenol S from canned foods and beverages on Chinese market. Food Control 2021, 120, 107502. [Google Scholar] [CrossRef]
- Cunha, S.C.; Inácio, T.; Almada, M.; Ferreira, R.; Fernandes, J.O. Gas chromatography–mass spectrometry analysis of nine bisphenols in canned meat products and human risk estimation. Food Res. Int. 2020, 135, 109293. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; de Quirós, A.R.B. Multi-analyte method for the quantification of bisphenol related compounds in canned food samples and exposure assessment of the Spanish adult population. Food Packag. Shelf Life 2021, 28, 100671. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, L.; Bayen, S. Chemical contaminants in canned food and can-packaged food: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 2687–2718. [Google Scholar] [CrossRef]
- Lau, O.W.; Wong, S.K. Contamination in food from packaging material. J. Chromatogr. A 2000, 882, 255–270. [Google Scholar] [CrossRef]
- Storelli, A.; Barone, G.; Dambrosio, A.; Garofalo, R.; Busco, A.; Storelli, M.M. Occurrence of trace metals in fish from South Italy: Assessment risk to consumer’s health. J. Food Compos. Anal. 2020, 90, 103487. [Google Scholar] [CrossRef]
| Sample | N° | Origin | Packaging Type | Protein (%) | Fat (%) | Ingredients | Manufacture Data/Expired Data |
|---|---|---|---|---|---|---|---|
| Canned mackerel fillets | 4 | Portugal | Can | 24 | 4.9 | Mackerel fillets, olive oil, salt. | */11 November 2026 |
| Canned horse mackerel | 4 | Chile | Can | 23 | 6.2 | Mackerel, water, salt. | */24 May 2025 |
| Grilled mackerel fillets in olive oil | 4 | Portugal | Can | 23 | 21 | Mackerel (87%), organic extra virgin olive oil, salt. | */October 2024 |
| Canned smoked mackerel fillets | 4 | Ireland | Can | 20 | 22 | Mackerel fillet (73.5%), sunflower oil, black pepper, salt. | */September 2024 |
| Natural grilled mackerel fillets | 3 | Portugal | Can | 20 | 9.4 | Mackerel (87%), water, salt. | */November 2024 |
| Mackerel in sos tomat | 3 | Romania | Can | 8.4 | 13.6 | Mackerel with skin, tomato sauce, water, tomato paste, sugar, rapeseed oil, potato flour, salt, dried onion, xanthan gum, dried parsnips, spices, spice extracts (celery, pepper), acetic acid. | */August 2024 |
| Canned Atlantic mackerel fillets | 3 | Morocco | Can | 25 | 3 | Mackerel, Olive Oil, Salt. | */November 2024 |
| Canned tuna in olive oil | 4 | Italy | Can | 22 | 2.4 | Tuna, olive oil, salt. | */September 2025 |
| Canned natural tuna | 4 | Italy | Can | 24.4 | 1.2 | Tuna, water, salt. | */March 2024 |
| Canned tuna pâté | 4 | Italy | Can | 13 | 15 | Tuna (37%), water, corn seed oil, green olives, soya protein, modified maize starch, capers, salt, oregano, natural flavouring nutmeg with other natural flavourings. | */February 2025 |
| Natural Alaskan salmon | 3 | Denmark | Can | 22.7 | 2.3 | Salmon, water, salt. | */31 December 2025 |
| Canned pink salmon | 3 | USA | Can | 19 | 7 | Salmon, water, salt. | */December 2024 |
| Canned sardines in olive oil | 4 | Morocco | Can | 16 | 9 | Sardines, olive oil, salt. | 7 February 2019/April 2024 |
| Canned sardines in vegetable oil | 4 | Morocco | Can | 23.9 | 17 | Sardines, vegetable oil, salt. | */September 2024 |
| Canned crab meat in brine | 4 | Indonesia | Can | 12 | 10 | Crab meat, water, salt, sugar, acidifier (E330), sodium metabisulphite, (E385). | */March 2024 |
| Salmon pâté | 3 | Italy | Aluminium collapsible tube | 18.5 | 13.5 | Pink salmon (46%), water, corn oil, rice starch, flavourings, salt, paprika extract. | */15 March 2026 |
| Surimi sticks | 4 | Estonia | Plastic box | 7 | 4.3 | Surimi 40%, water, potato starch, egg white, rapeseed oil, salt, sugar, flavours, soy, carrageenan, monosodium glutamate, titanium dioxide, paprika oleoresin, carmine. | */20 July 2025 |
| Anchovies paste in olive oil | 4 | USA | Aluminium collapsible tube | 17.7 | 11 | Anchovies, salt, olive oil, palm oil, sugar, spices, sodium benzoate. | */September 2024 |
| Shelled clams | 4 | Italy | Glass | 10 | 1.5 | Clams and their natural broth, water, salt, citric acid. | */June 2025 |
| Natural shrimp | 4 | Italy | Glass | 8 | 1.5 | Peeled and pre-cooked shrimps, water, salt, sugar, citric acid. May contain traces of shellfish and fish. | */February 2025 |
| Lumpfish eggs | 3 | Denmark | Glass | 11 | 3.3 | Lumpfish eggs (90%), water, salt, stabiliser (415), colouring agents (150d, 151, 110), acidity regulator. | */February 2025 |
| Cuttlefish Ink | 3 | Spain | Glass | 7.3 | 0.1 | Cuttlefish ink, water, salt, thickener: carboxymethylcellulose. | */16 March 2025 |
| Dried sardines | 5 | Argentina | Plastic box | 7 | 5.1 | Sardines. | */October 2024 |
| Dried shrimp | 5 | Argentina | Plastic box | 6 | 4.5 | Shrimp. | */December 2024 |
| Total | 90 |
| Sample | N° | Origin | Packaging Type | Protein (%) | Fat (%) | Ingredients | Manufacture Data |
|---|---|---|---|---|---|---|---|
| Canned light chicken | 3 | Italy | Can | 11 | 13.5 | Chicken, salt. | */20 February 2025 |
| Canned halal chicken | 3 | Poland | Can | 13 | 4.7 | Chicken, salt (2%), sugar, cooked chicken powder, sodium phosphates, spices, sodium erythorbate, sodium nitrite, natural flavouring. | */March 2025 |
| Canned meat broth | 3 | Italy | Can | 11 | 1.2 | Beef stock, salt, natural flavouring, yeast extract, Mirepoix (carrots, celery, onions), brown sugar. | 23 March 2020/February 2024 |
| Canned beef and pork pâté | 3 | Italy | Can | 11 | 5.2 | Pork, beef, stock, salt, spices. | */October 2025 |
| Canned chicken breast with jelly | 3 | Italy | Can | 11 | 19 | Cooked chicken breast stock 38%, agar-agar gelling agent, locust bean gum, monosodium glutamate, water, salt, flavourings. | */September 2024 |
| Canned luncheon chicken meat | 3 | Philippines | Can | 12 | 10 | Chicken, salt. | */June 2026 |
| Canned cooked ham | 3 | Italy | Can | 9 | 26 | Cooked ham (37%), water, salt, lactose, maize seed oil, soya protein, maize starch, locust bean gum, flavourings. | 12 January 2023/February 2024 |
| Canned beef with gelatine | 3 | Italy | Can | 11 | 1.5 | Cooked beef, beef, water, salt, sugar, sodium nitrite. | 20 September 2023/April 2024 |
| Canned beef and pork pâté | 3 | Italy | Can | 11 | 14 | Pork, beef, stock, salt, spices. | */February 2026 |
| Canned minced pork and ham with bacon | 3 | Denmark | Can | 15 | 22 | Pork (75%), smoked bacon, salt, water, antioxidants (E301), preservatives (E251), starch, ham 2%, sugar, stabilisers (E450), spice extract. | */October 2026 |
| Total | 30 |
| Samples | BPA | BPF | BPB | BADGE | BADGE∙ 2H2O | BADGE∙HCl∙ H2O | BADGE∙ 2HCl |
|---|---|---|---|---|---|---|---|
| Canned mackerel fillets | 20.36 ± 0.82 c | - | - | 20.37 ± 0.94 a | 39.76 ± 1.08 b | 34.31 ± 1.00 b,c | 9.99 ± 0.68 a |
| Canned horse mackerel | 4.36 ± 0.36 a | 70.39 ± 0.66 c | - | 100.03 ± 1.03 c | - | - | - |
| Grilled mackerel fillets in olive oil | 34.02 ± 0.73 c,d | - | - | 30.30 ± 0.83 a | 20.81 ± 0.50 a | 40.05 ± 0.48 c | - |
| Canned smoked mackerel fillets | 31.85 ± 0.86 c,d | - | 9.58 ± 0.04 | 102.13 ± 2.91 c | 50.92 ± 0.30 b | - | - |
| Natural grilled mackerel fillets | 9.31 ± 0.13 b | - | - | 20.61 ± 0.33 a | 15.93 ± 0.18 a | 6.44 ± 0.42 a | 78.70 ± 0.97 c |
| Mackerel in sos tomat | 12.09 ± 0.16 b | 24.06 ± 0.57 b | 36.16 ± 0.70 | 361.54 ± 36.47 d | 199.66 ± 1.70 c | 20.86 ± 0.55 b | 17.25 ± 0.07 b |
| Canned Atlantic mackerel fillets | - | - | - | - | - | - | - |
| Canned tuna in olive oil | 25.33 ± 1.64 c | - | - | - | - | - | - |
| Canned natural tuna | 38.90 ± 1.79 d | - | - | - | 13.53 ± 0.21 a | 10.01 ± 0.10 a | - |
| Canned tuna pâté | 13.61 ± 0.58 b | 7.76 ± 0.25 a | - | - | - | - | - |
| Natural Alaskan salmon | - | - | - | - | - | - | - |
| Canned pink salmon | 37.62 ± 0.48 d | 121.09 ± 0.77 c | 54.44 ± 0.47 b | 11.78 ± 0.39 a | 9.97 ± 0.31 a | ||
| Canned sardines in olive oil | 2.85 ± 0.50 a | - | - | - | - | - | - |
| Canned sardines in vegetable oil | 7.60 ± 0.39 a,b | - | - | - | - | - | - |
| Canned crab meat in brine | 47.69 ± 0.58 d | 10.09 ± 0.19 a | - | 28.70 ± 0.36 a | 12.44 ± 0.31 a | 37.14 ± 0.42 b,c | 15.15 ± 0.18 b |
| Salmon pâté | - | - | - | - | - | - | - |
| Surimi sticks | 20.75 ± 0.68 c | - | - | 55.85 ± 0.35 b | 12.88 ± 0.24 a | 36.71 ± 0.36 c | 15.52 ± 0.35 b |
| Anchovy paste in olive oil | - | - | - | - | - | - | - |
| Shelled clams | - | - | - | - | - | - | - |
| Natural shrimp | - | - | - | - | - | - | - |
| Lumpfish eggs | - | - | - | - | - | - | - |
| Cuttlefish Ink | - | - | - | - | - | - | - |
| Dried sardines | 3.36 ± 0.39 a | - | - | 90.27 ± 0.34 b,c | - | - | - |
| Dried shrimp | 2.68 ± 0.13 a | - | - | - | - | - | - |
| p-value | 0.000 | 0.000 | - | 0.000 | 0.000 | 0.000 | 0.000 |
| Samples | BPA | BPF | BPB | BADGE | BADGE∙ 2H2O | BADGE∙HCl∙ H2O | BADGE∙ 2HCl |
|---|---|---|---|---|---|---|---|
| Canned light chicken | 20.52 ± 0.64 b | 10.35 ± 0.56 a | 3.34 ± 0.69 | - | - | - | - |
| Canned halal chicken | - | - | - | - | - | - | - |
| Canned meat broth | 4.30 ± 0.04 a | 6.49 ± 0.08 a | - | 16.72 ± 0.31 a | 9.35 ± 0.08 a | - | - |
| Canned beef and pork pâté | - | - | - | - | - | - | - |
| Canned chicken breast with jelly | 29.57 ± 1.09 c | 11.74 ± 0.29 a | - | 23.42 ± 0.27 a | - | - | - |
| Canned luncheon chicken meat | 25.12 ± 0.12 b | - | - | 100.62 ± 0.43 b | 9.94 ± 0.35 a | 10.75 ± 0.22 | 22.86 ± 0.65 |
| Canned cooked ham | 3.28 ± 0.05 a | - | - | - | - | - | - |
| Canned beef with gelatine | 0.52 ± 0.04 a | 20.72 ± 0.52 b | - | - | - | - | - |
| Canned beef and pork pâté | 2.75 ± 0.05 a | 13.21 ± 0.07 a | 10.95 ± 0.09 a | ||||
| Canned minced pork and ham with bacon | 9.17 ± 0.26 a,b | - | 2.34 ± 0.10 | 151.02 ± 0.57 b | 201.90 ± 0.15 b | 23.14 ± 0.15 | 13.81 ± 0.19 |
| p-value | 0.000 | 0.000 | - | 0.000 | 0.000 | - | 0.000 |
| Sample | Zn | As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Sn | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Canned mackerel fillets | 9.17 ± 0.31 a | 0.50 ± 0.09 b | 0.02 ± 0.00 a | 0.08 ± 0.01 a | 1.59 ± 0.20 a,b | 8.87 ± 0.26 a | 1.03 ± 0.07 b | 0.08 ± 0.02 a | 0.07 ± 0.04 a | 0.18 ± 0.07 a | 4.11 ± 0.54 a,b |
| Canned horse mackerel | 9.48 ± 0.27 a | 0.35 ± 0.08 a,b | 0.05 ± 0.01 a | 0.13 ± 0.02 a,b | 1.24 ± 0.12 a,b | 8.51 ± 0.30 a | 0.97 ± 0.05 b | 0.10 ± 0.01 a | 0.13 ± 0.0 a,b | 0.09 ± 0.01 a | 6.57 ± 0.28 b |
| Grilled mackerel fillets in olive oil | 10.39 ± 0.32 a,b | 0.28 ± 0.05 a,b | 0.02 ± 0.00 a | 0.10 ± 0.01 a,b | 1.73 ± 0.18 a,b | 6.69 ± 0.41 a | 0.91 ± 0.04 b | 0.05 ± 0.01 a | 0.11 ± 0.03 a | 0.13 ± 0.03 a | 6.66 ± 0.23 b |
| Canned smoked mackerel fillets | 10.81 ± 0.35 a,b | 0.34 ± 0.08 a,b | 0.01 ± 0.00 a | 0.11 ± 0.01 a,b | 4.80 ± 0.28 b | 14.36 ± 0.99 b | 0.90 ± 0.05 b | 0.06 ± 0.02 a | 0.04 ± 0.01 a | 0.59 ± 0.07 b | 6.29 ± 0.30 b |
| Natural grilled mackerel fillets | 8.63 ± 0.29 a | 0.78 ± 0.05 b | 0.04 ± 0.01 a | 0.10 ± 0.01 a,b | 1.73 ± 0.15 a,b | 8.56 ± 0.42 a | 0.96 ± 0.06 b | 0.05 ± 0.01 a | 0.06 ± 0.01 a | 0.09 ± 0.02 a | 8.54 ± 0.31 b |
| Mackerel in sos tomat | 15.33 ± 0.40 b | 1.16 ± 0.05 c | 0.01 ± 0.00 a | 0.13 ± 0.03 a,b | 3.91 ± 0.25 b | 14.16 ± 0.41 b | 1.06 ± 0.06 b | 0.09 ± 0.01 a | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 5.58 ± 0.33 a,b |
| Canned Atlantic mackerel fillets | 6.72 ± 0.34 a | 0.26 ± 0.04 a,b | 0.07 ± 0.03 a | 0.07 ± 0.01 a | 1.28 ± 0.14 a,b | 8.56 ± 0.20 a | 1.06 ± 0.05 b | 0.06 ± 0.02 a | 0.21 ± 0.03 a,b | 0.09 ± 0.01 a | 7.91 ± 0.14 b |
| Canned tuna in olive oil | 16.30 ± 0.37 b | 0.51 ± 0.05 b | 0.02 ± 0.01 a | 0.14 ± 0.03 a,b | 1.41 ± 0.15 a,b | 6.29 ± 0.23 a | 2.00 ± 0.08 c | 0.24 ± 0.04 a | 0.09 ± 0.02 a | 0.47 ± 0.04 a,b | 5.47 ± 0.19 a,b |
| Canned natural tuna | 13.81 ± 0.51 b | 0.66 ± 0.08 b | 0.03 ± 0.01 a | 0.16 ± 0.02 a,b | 1.58 ± 0.08 a,b | 5.74 ± 0.21 a | 1.97 ± 0.07 b | 0.29 ± 0.05 a | 0.03 ± 0.01 a | 0.41 ± 0.05 a,b | 4.71 ± 0.15 a,b |
| Canned tuna pâté | 19.47 ± 0.53 b,c | 0.84 ± 0.06 b | 0.02 ± 0.01 a | 0.14 ± 0.03 a,b | 1.52 ± 0.12 a,b | 5.44 ± 0.38 a | 2.10 ± 0.07 c | 0.27 ± 0.04 a | 0.04 ± 0.01 a | 0.44 ± 0.03 a,b | 5.15 ± 0.08 a,b |
| Natural Alaskan salmon | 6.29 ± 0.27 a | 0.07 ± 0.01 a | 0.02 ± 0.01 a | 0.05 ± 0.01 a | 1.70 ± 0.04 a,b | 5.66 ± 0.45 a | 0.06 ± 0.01 a | 0.20 ± 0.02 a | 0.11 ± 0.01 a,b | 0.84 ± 0.04 b | 3.10 ± 0.12 a,b |
| Canned pink salmon | 6.74 ± 0.29 a | 0.09 ± 0.01 a | 0.02 ± 0.01 a | 0.05 ± 0.02 a | 1.71 ± 0.06 a,b | 7.10 ± 0.22 a | 0.05 ± 0.01 a | 0.23 ± 0.02 a | 0.18 ± 0.02 a,b | 0.82 ± 0.03 b | 3.12 ± 0.10 a,b |
| Canned sardines in olive oil | 26.19 ± 1.05 c | 0.14 ± 0.01 a | 0.13 ± 0.02 b | 0.06 ± 0.01 a | 1.60 ± 0.22 a,b | 20.85 ± 1.19 b | 1.27 ± 0.17 b | 0.79 ± 0.08 b | 0.14 ± 0.03 a,b | 0.27 ± 0.02 a,b | 4.00 ± 0.09 a,b |
| Canned sardines in vegetable oil | 22.83 ± 0.51 c | 0.11 ± 0.02 a | 0.13 ± 0.02 b | 0.05 ± 0.01 a | 1.12 ± 0.10 a,b | 20.50 ± 1.01 b | 1.14 ± 0.09 b | 0.96 ± 0.06 b | 0.11 ± 0.02 a,b | 0.34 ± 0.02 a,b | 3.64 ± 0.18 a,b |
| Canned crab meat in brine | 10.58 ± 0.89 a,b | 0.30 ± 0.03 a,b | 0.05 ± 0.01 a | 0.10 ± 0.01 a,b | 0.46 ± 0.09 a | 9.63 ± 0.75 a,b | 1.55 ± 0.05 b | 0.21 ± 0.02 a | 0.08 ± 0.01 a | 0.75 ± 0.05 b | 1.97 ± 0.05 a |
| Salmon pâté | 7.33 ± 0.38 a | 0.07 ± 0.01 a | 0.01 ± 0.00 a | 0.05 ± 0.02 a | 1.86 ± 0.05 a,b | 5.71 ± 0.33 a | 0.08 ± 0.02 a | 0.17 ± 0.03 a | 0.21 ± 0.02 a,b | 0.76 ± 0.05 b | 3.37 ± 0.16 a,b |
| Surimi sticks | 4.36 ± 0.18 a | 0.41 ± 0.08 b | 0.08 ± 0.02 a | 0.13 ± 0.03 a,b | 0.36 ± 0.05 a | 3.90 ± 0.25 a | 0.16 ± 0.03 a | 0.07 ± 0.02 a | 0.10 ± 0.02 a | 0.08 ± 0.01 a | 0.52 ± 0.05 a |
| Anchovy paste in olive oil | 21.00 ± 1.15 c | 1.42 ± 0.24 c | 0.17 ± 0.04 b | 0.06 ± 0.01 a | 3.31 ± 0.19 b | 33.17 ± 1.98 c | 1.22 ± 0.06 b | 0.12 ± 0.03 a | 0.13 ± 0.04 a,b | 0.13 ± 0.02 a | 7.68 ± 0.23 b |
| Shelled clams | 20.27 ± 0.69 c | 0.85 ± 0.06 b | 0.03 ± 0.01 a | 0.39 ± 0.04 b | 0.68 ± 0.07 a | 70.41 ± 0.82 d | 0.68 ± 0.08 a | 0.25 ± 0.04 a | 0.09 ± 0.01 a | 0.12 ± 0.02 a | 1.61 ± 0.10 a |
| Natural shrimp | 4.36 ± 0.67 a | 0.34 ± 0.05 a,b | 0.03 ± 0.01 a | 0.12 ± 0.03 a,b | 8.35 ± 0.37 b | 15.98 ± 0.23 b | 1.06 ± 0.07 b | 3.48 ± 0.22 c | 0.09 ± 0.02 a | 0.04 ± 0.01 a | 0.55 ± 0.09 a |
| Lumpfish eggs | 11.32 ± 0.21 b | 0.23 ± 0.03 a | 0.04 ± 0.01 a | 0.10 ± 0.02 a,b | 0.86 ± 0.06 a | 4.76 ± 0.20 a | 0.36 ± 0.05 a | 0.20 ± 0.02 a | 0.08 ± 0.01 a | 0.05 ± 0.01 a | 0.37 ± 0.05 a |
| Cuttlefish ink | 17.89 ± 0.81 b | 0.49 ± 0.06 b | 0.91 ± 0.05 c | 0.10 ± 0.02 a,b | 4.58 ± 0.38 b | 5.45 ± 0.29 a | 0.31 ± 0.04 a | 0.14 ± 0.03 a | 0.31 ± 0.04 b | 0.05 ± 0.01 a | 1.11 ± 0.18 a |
| Dried sardines | 23.59 ± 1.43 c | 0.14 ± 0.02 a | 0.19 ± 0.03 b | 0.12 ± 0.03 a,b | 2.55 ± 0.23 b | 30.18 ± 1.84 c | 2.46 ± 0.28 c | 1.78 ± 0.18 b | 0.15 ± 0.02 a,b | 0.10 ± 0.02 a | 2.04 ± 0.13 a,b |
| Dried shrimp | 9.54 ± 0.40 a | 0.63 ± 0.06 b | 0.07 ± 0.01 a | 0.20 ± 0.02 a,b | 13.46 ± 0.47 c | 20.69 ± 0.81 b | 1.84 ± 0.12 b | 4.22 ± 0.25 c | 0.14 ± 0.04 a,b | 0.10 ± 0.02 a | 0.10 ± 0.02 a |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sample | Zn | As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Sn | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Canned light chicken | 10.29 ± 0.14 a,b | - | 0.02 ± 0.00 a | 0.16 ± 0.02 a | 0.72 ± 0.06 a | 10.56 ± 0.33 a | 0.70 ± 0.03 b | 0.02 ± 0.01 a | 0.07 ± 0.01 a | 0.16 ± 0.02 a | 0.82 ± 0.06 a |
| Canned halal chicken | 9.59 ± 0.33 a | - | 0.03 ± 0.01 a | 0.19 ± 0.02 a | 0.55 ± 0.05 a | 11.23 ± 0.22 a | 0.77 ± 0.03 b | 0.04 ± 0.01 b | 0.16 ± 0.02 a | 0.51 ± 0.05 a,b | 3.59 ± 0.11 b,c |
| Canned meat broth | 19.54 ± 0.59 b | 0.02 ± 0.01 | 0.04 ± 0.01 b | 0.10 ± 0.02 a | 1.77 ± 0.21 b | 12.93 ± 0.34 a | 0.39 ± 0.03 a | 0.02 ± 0.01 a | 0.20 ± 0.02 a | 0.33 ± 0.03 a | 0.84 ± 0.06 a |
| Canned beef and pork pâté | 16.04 ± 0.67 b | - | 0.01 ± 0.00 a | 0.24 ± 0.02 b | 0.95 ± 0.05 a | 21.23 ± 0.33 a,b | 0.19 ± 0.01 a | 0.01 ± 0.00 a | 0.28 ± 0.02 b | 0.05 ± 0.01 a | 0.95 ± 0.07 a |
| Canned chicken breast with jelly | 11.87 ± 0.44 a,b | - | 0.05 ± 0.01 b | 0.25 ± 0.02 b | 0.79 ± 0.03 a | 12.54 ± 0.43 a | 0.94 ± 0.04 b | 0.05 ± 0.01 a | 0.08 ± 0.01 a | 0.35 ± 0.06 a | 2.03 ± 0.18 b |
| Canned luncheon chicken meat | 9.40 ± 0.36 a | 0.29 ± 0.02 | 0.05 ± 0.01 b | 0.20 ± 0.01 a | 0.60 ± 0.06 a | 17.24 ± 0.16 a,b | 0.65 ± 0.04 a,b | 0.02 ± 0.01 a | 0.20 ± 0.01 a | 1.69 ± 0.12 b | 4.90 ± 0.14 c |
| Canned cooked ham | 6.87 ± 0.19 a | 0.06 ± 0.01 | 0.04 ± 0.01 b | 0.13 ± 0.02 a | 1.23 ± 0.11 b | 13.45 ± 0.65 a | 0.54 ± 0.03 a | 0.02 ± 0.00 a | 0.20 ± 0.02 a | 0.24 ± 0.02 a | 0.61 ± 0.06 a |
| Canned beef with gelatine | 7.60 ± 0.06 a | 0.02 ± 0.01 | 0.03 ± 0.01 a | 0.30 ± 0.04 b | 0.81 ± 0.05 a | 27.30 ± 0.22 b | 0.23 ± 0.02 a | 0.05 ± 0.01 b | 0.20 ± 0.03 a | 0.22 ± 0.04 a | 0.54 ± 0.08 a |
| Canned beef and pork pâté | 16.38 ± 0.42 b | - | 0.02 ± 0.01 a | 0.27 ± 0.02 b | 1.04 ± 0.08 a,b | 21.95 ± 0.83 a,b | 0.22 ± 0.03 a | 0.02 ± 0.01 a | 0.15 ± 0.02 a | 0.90 ± 0.09 a,b | 1.05 ± 0.07 b |
| Canned minced pork and ham with bacon | 9.46 ± 0.31 a | - | 0.10 ± 0.02 a | 0.29 ± 0.03 b | 1.54 ± 0.23b | 13.11 ± 0.88 a | 0.31 ± 0.03 a | 0.01 ± 0.00 a | 0.09 ± 0.01 a | 0.22 ± 0.04 a | 2.14 ± 0.11 b |
| p-value | 0.000 | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sample | BPA | Zn | As | Cd | Cu | Mn | Ni | Pb | Al | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDI (ng/kg) | PTWI (µg/kg) | PTWI (µg/kg) | TWI (µg/kg) | PTWI (µg/kg) | PTWI (µg/kg) | TDI (µg/kg) | BMDL01 (µg/kg) | TWI (µg/kg) | ||||||||||
| EWI | HQ | EWI | HQ | EWI | HQ | EWI | HQ | EWI | HQ | EWI | HQ | EWI | HQ | EWI | HQ | EWI | HQ | |
| Canned mackerel fillets | 43.62 | 31.16 | 19.64 | <1 | 1.08 | <1 | 0.05 | <1 | 3.41 | <1 | 2.21 | <1 | 0.16 | <1 | 0.15 | <1 | 8.81 | <1 |
| Canned horse mackerel | 9.94 | 7.10 | 21.66 | <1 | 0.79 | <1 | 0.10 | <1 | 2.82 | <1 | 2.22 | <1 | 0.22 | <1 | 0.30 | <1 | 15.01 | <1 |
| Grilled mackerel fillets in olive oil | 116.62 | 83.30 | 35.63 | <1 | 0.97 | <1 | 0.08 | <1 | 5.93 | <1 | 3.12 | <1 | 0.17 | <1 | 0.36 | <1 | 22.83 | <1 |
| Canned smoked mackerel fillets | 72.80 | 52.00 | 24.70 | <1 | 0.77 | <1 | 0.02 | <1 | 10.97 | <1 | 2.05 | <1 | 0.14 | <1 | 0.08 | <1 | 14.37 | <1 |
| Natural grilled mackerel fillets | 26.61 | 19.01 | 24.67 | <1 | 2.23 | <1 | 0.12 | <1 | 4.94 | <1 | 2.74 | <1 | 0.13 | <1 | 0.17 | <1 | 24.40 | <1 |
| Mackerel in sos tomat | 27.64 | 19.74 | 35.03 | <1 | 2.65 | <1 | 0.02 | <1 | 8.93 | <1 | 2.42 | <1 | 0.21 | <1 | 0.14 | <1 | 12.76 | <1 |
| Canned Atlantic mackerel fillets | - | - | 15.35 | <1 | 0.59 | <1 | 0.17 | <1 | 2.93 | <1 | 2.42 | <1 | 0.13 | <1 | 0.47 | <1 | 18.07 | <1 |
| Canned tuna in olive oil | 57.90 | 41.36 | 37.26 | <1 | 1.17 | <1 | 0.05 | <1 | 3.21 | <1 | 4.57 | <1 | 0.55 | <1 | 0.21 | <1 | 12.50 | <1 |
| Canned natural tuna | 88.92 | 63.51 | 31.55 | <1 | 1.51 | <1 | 0.06 | <1 | 3.60 | <1 | 4.50 | <1 | 0.66 | <1 | 0.06 | <1 | 10.77 | <1 |
| Canned tuna pâté | 31.11 | 22.22 | 44.51 | <1 | 1.93 | <1 | 0.03 | <1 | 3.47 | <1 | 4.79 | <1 | 0.61 | <1 | 0.10 | <1 | 11.78 | <1 |
| Natural Alaskan salmon | - | - | 14.37 | <1 | 0.17 | <1 | 0.05 | <1 | 3.89 | <1 | 0.13 | <1 | 0.45 | <1 | 0.24 | <1 | 7.09 | <1 |
| Canned pink salmon | 85.98 | 61.41 | 15.41 | <1 | 0.21 | <1 | 0.04 | <1 | 3.90 | <1 | 0.11 | <1 | 0.52 | <1 | 0.42 | <1 | 7.12 | <1 |
| Canned sardines in olive oil | 7.33 | 5.23 | 67.33 | <1 | 0.36 | <1 | 0.33 | <1 | 4.11 | <1 | 3.26 | <1 | 2.04 | <1 | 0.36 | <1 | 10.29 | <1 |
| Canned sardines in vegetable oil | 17.38 | 12.41 | 52.18 | <1 | 0.24 | <1 | 0.29 | <1 | 2.55 | <1 | 2.61 | <1 | 2.20 | <1 | 0.24 | <1 | 8.33 | <1 |
| Canned crab meat in brine | 109.01 | 77.86 | 24.18 | <1 | 0.69 | <1 | 0.12 | <1 | 1.05 | <1 | 3.53 | <1 | 0.48 | <1 | 0.18 | <1 | 4.50 | <1 |
| Salmon pâté | - | - | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 |
| Surimi sticks | 59.28 | 42.34 | 12.44 | <1 | 1.18 | <1 | 0.23 | <1 | 1.04 | <1 | 0.44 | <1 | 0.19 | <1 | 0.27 | <1 | 1.49 | <1 |
| Anchovy paste in olive oil | - | - | 47.99 | <1 | 3.25 | <1 | 0.39 | <1 | 7.56 | <1 | 2.79 | <1 | 0.27 | <1 | 0.30 | <1 | 17.55 | <1 |
| Shelled clams | - | - | 46.34 | <1 | 1.93 | <1 | 0.07 | <1 | 1.55 | <1 | 1.55 | <1 | 0.57 | <1 | 0.20 | <1 | 3.67 | <1 |
| Natural shrimp | - | - | 9.97 | <1 | 0.78 | <1 | 0.07 | <1 | 19.09 | <1 | 2.42 | <1 | 7.96 | <1 | 0.20 | <1 | 1.25 | <1 |
| Lumpfish eggs | - | - | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 |
| Cuttlefish ink | - | - | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 | 0.00 | <1 |
| Dried sardines | 7.68 | 5.49 | 53.92 | <1 | 0.32 | <1 | 0.43 | <1 | 5.84 | <1 | 5.61 | <1 | 4.06 | <1 | 0.34 | <1 | 4.66 | <1 |
| Dried shrimp | 6.13 | 4.38 | 21.81 | <1 | 1.44 | <1 | 0.16 | <1 | 30.76 | <1 | 4.21 | <1 | 9.64 | <1 | 0.32 | <1 | 0.23 | <1 |
| Canned light chicken | 46.90 | 33.50 | 23.51 | <1 | - | - | 0.05 | <1 | 1.65 | <1 | 1.61 | <1 | 0.04 | <1 | 0.15 | <1 | 1.88 | <1 |
| Canned halal chicken | - | - | 21.91 | <1 | - | - | 0.08 | <1 | 1.26 | <1 | 1.77 | <1 | 0.08 | <1 | 0.37 | <1 | 8.21 | <1 |
| Canned meat broth | 9.82 | 7.01 | 44.66 | <1 | 0.04 | <1 | 0.10 | <1 | 4.05 | <1 | 0.89 | <1 | 0.04 | <1 | 0.46 | <1 | 1.92 | <1 |
| Canned beef and pork pâté | - | - | 36.66 | <1 | - | - | 0.02 | <1 | 2.17 | <1 | 0.44 | <1 | 0.02 | <1 | 0.64 | <1 | 2.17 | <1 |
| Canned chicken breast with jelly | 67.58 | 48.27 | 27.14 | <1 | - | - | 0.11 | <1 | 1.80 | <1 | 2.15 | <1 | 0.11 | <1 | 0.19 | <1 | 4.63 | <1 |
| Canned lunchon chicken meat | 57.42 | 41.02 | 21.49 | <1 | 0.66 | <1 | 0.11 | <1 | 1.38 | <1 | 1.49 | <1 | 0.04 | <1 | 0.46 | <1 | 11.21 | <1 |
| Canned cooked ham | 7.49 | 5.35 | 15.70 | <1 | 0.13 | <1 | 0.10 | <1 | 2.81 | <1 | 1.23 | <1 | 0.05 | <1 | 0.45 | <1 | 1.39 | <1 |
| Canned beef with gelatine | 1.20 | <1 | 17.37 | <1 | 0.05 | <1 | 0.06 | <1 | 1.85 | <1 | 0.52 | <1 | 0.11 | <1 | 0.46 | <1 | 1.23 | <1 |
| Canned beef and pork pâté | 6.29 | 4.50 | 37.43 | <1 | - | - | 0.04 | <1 | 2.37 | <1 | 0.50 | <1 | 0.04 | <1 | 0.35 | <1 | 2.40 | <1 |
| Canned minced pork and ham with bacon | 20.97 | 14.98 | 21.62 | <1 | - | - | 0.22 | <1 | 3.53 | <1 | 0.70 | <1 | 0.02 | <1 | 0.21 | <1 | 4.89 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litrenta, F.; Nava, V.; Potortì, A.G.; Lo Turco, V.; Sgrò, B.; Di Bella, G. Bisphenols, Toxic Elements, and Potentially Toxic Elements in Ready-to-Eat Fish and Meat Foods and Their Associated Risks for Human Health. Toxics 2025, 13, 433. https://doi.org/10.3390/toxics13060433
Litrenta F, Nava V, Potortì AG, Lo Turco V, Sgrò B, Di Bella G. Bisphenols, Toxic Elements, and Potentially Toxic Elements in Ready-to-Eat Fish and Meat Foods and Their Associated Risks for Human Health. Toxics. 2025; 13(6):433. https://doi.org/10.3390/toxics13060433
Chicago/Turabian StyleLitrenta, Federica, Vincenzo Nava, Angela Giorgia Potortì, Vincenzo Lo Turco, Benedetta Sgrò, and Giuseppa Di Bella. 2025. "Bisphenols, Toxic Elements, and Potentially Toxic Elements in Ready-to-Eat Fish and Meat Foods and Their Associated Risks for Human Health" Toxics 13, no. 6: 433. https://doi.org/10.3390/toxics13060433
APA StyleLitrenta, F., Nava, V., Potortì, A. G., Lo Turco, V., Sgrò, B., & Di Bella, G. (2025). Bisphenols, Toxic Elements, and Potentially Toxic Elements in Ready-to-Eat Fish and Meat Foods and Their Associated Risks for Human Health. Toxics, 13(6), 433. https://doi.org/10.3390/toxics13060433







