Metal Exposure, Bioaccumulation, and Toxicity Assessment in Sediments from the St. Lawrence River Before and After Remediation Using a Resuspension Technique
Abstract
1. Introduction
2. Materials and Methods
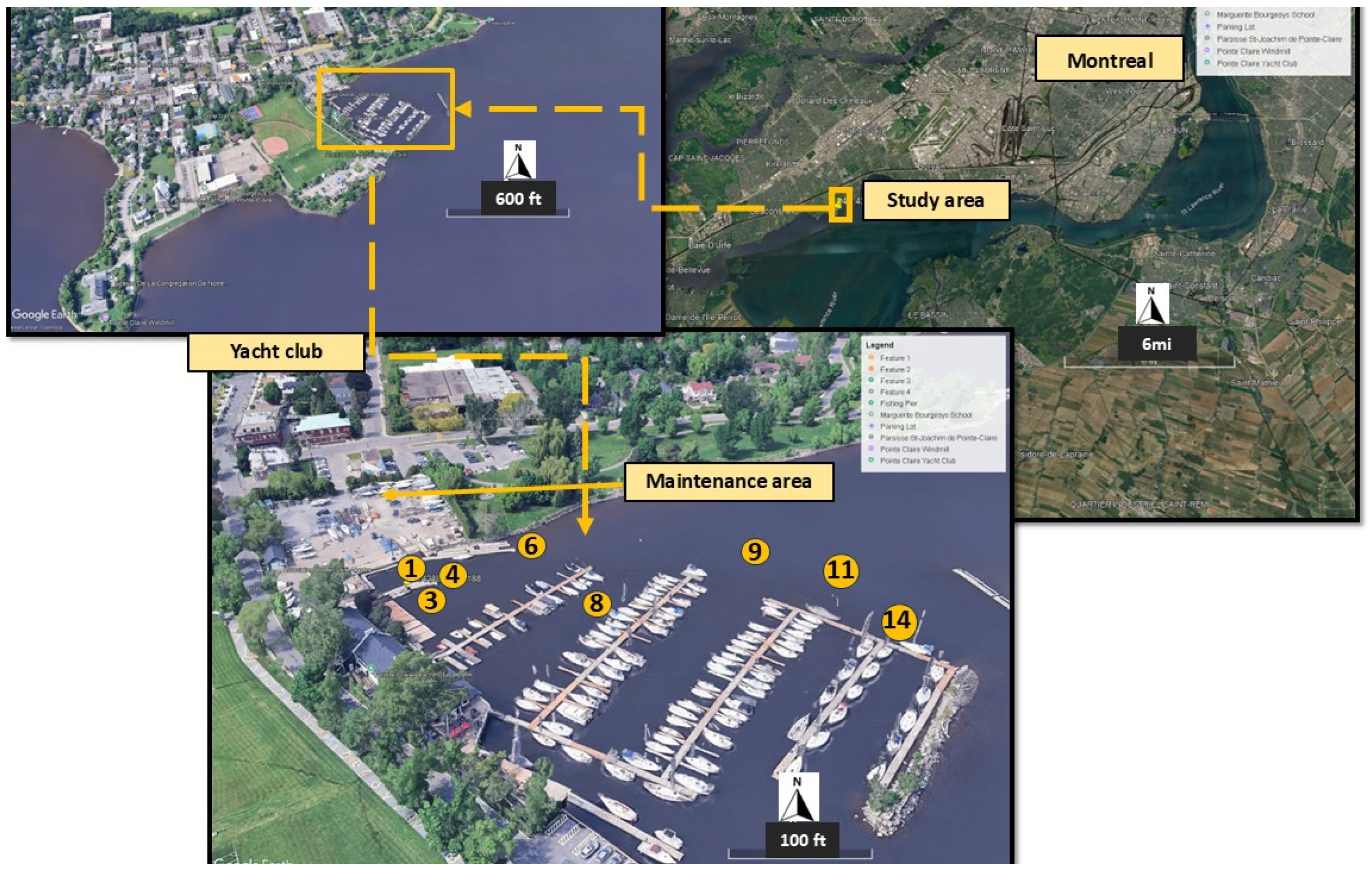
2.1. Study Area Characteristics and Source of Contamination
2.2. Sediment Sampling
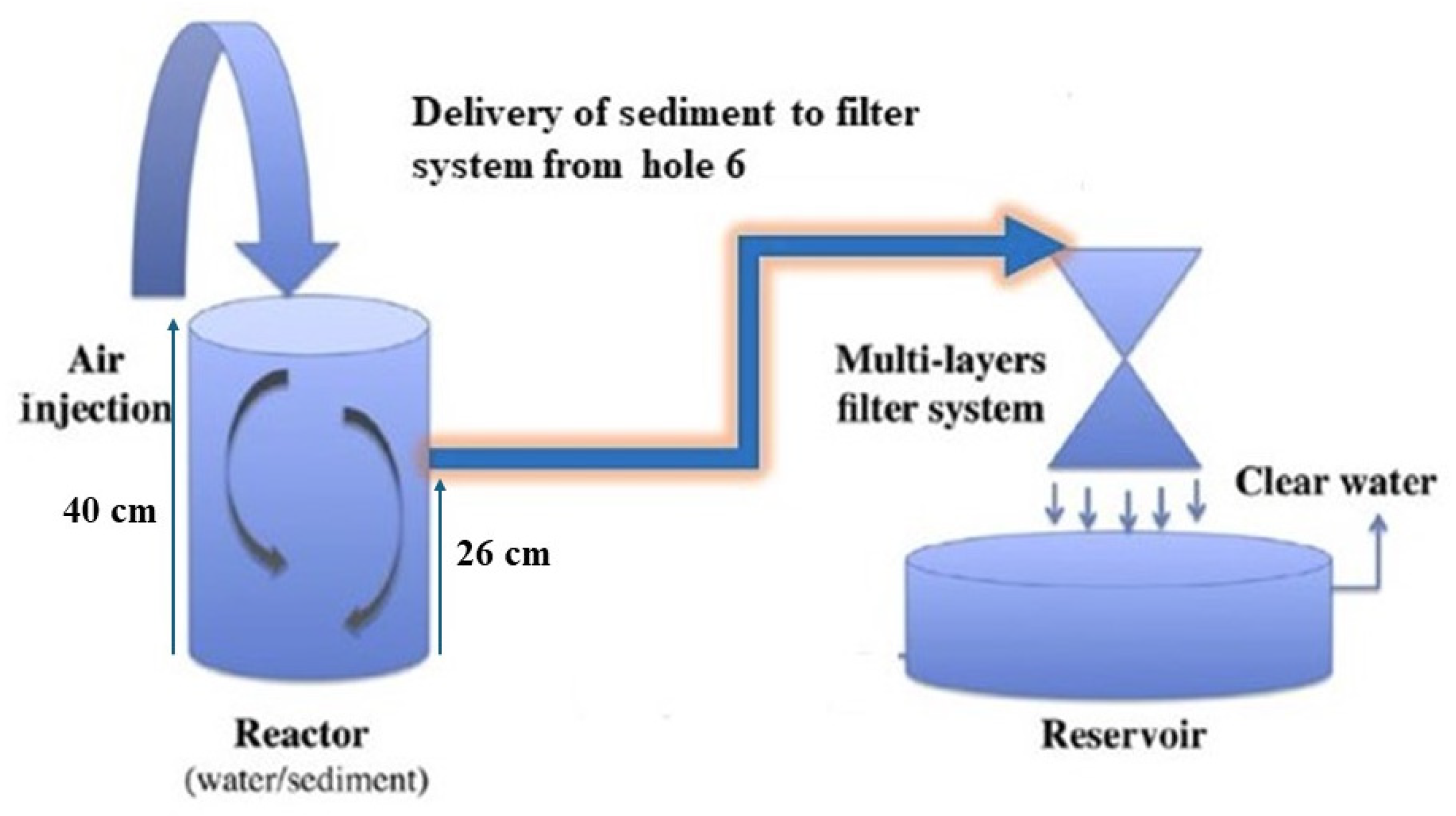
2.3. Experimental Setup of Remediation Technique
2.4. Analytical Parameters
2.4.1. Grain Size
2.4.2. Total Concentration of Heavy Metals
2.4.3. Sequential Extraction Method
2.4.4. Toxicity Tests
10 Days Survival/Growth Test of Chironomus riparius
14 Days Survival/Growth Test of Hyalella azteca Tests
2.4.5. Growth Parameters
2.4.6. Length Measurements
2.4.7. Bioaccumulation Analysis
2.4.8. Quality Assurance
2.5. Data Analysis
3. Results and Discussion
3.1. Quality of the Sediment Before Resuspension
3.2. Resuspension Effects on the Quality of Contaminated Sediment
3.3. Survival and Growth (RGR) of Amphipod Hyalella azteca in Before-Remediation Sediment Samples
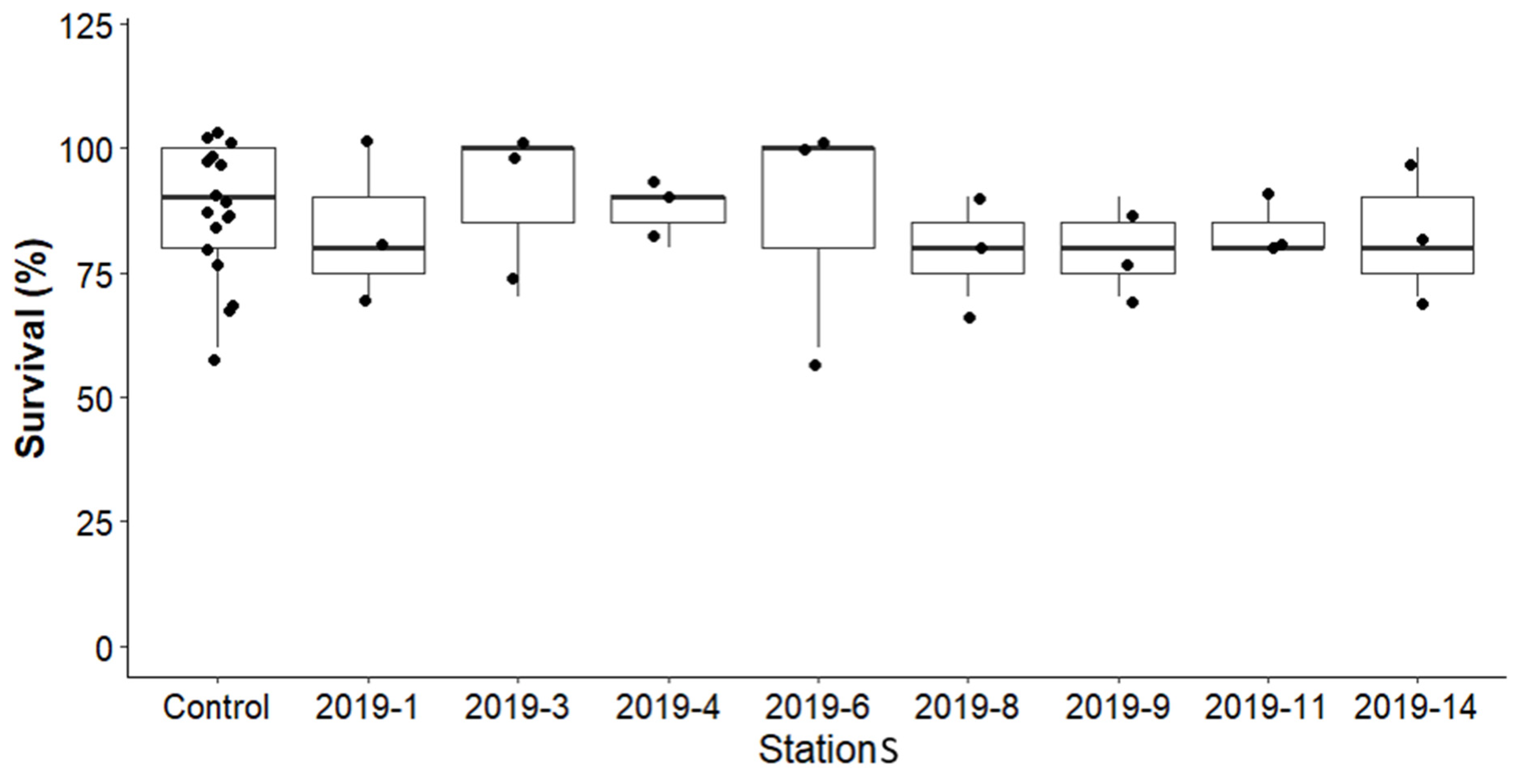
3.3.1. Survival of Amphipod Hyalella azteca in Before-Remediation Sediment Samples
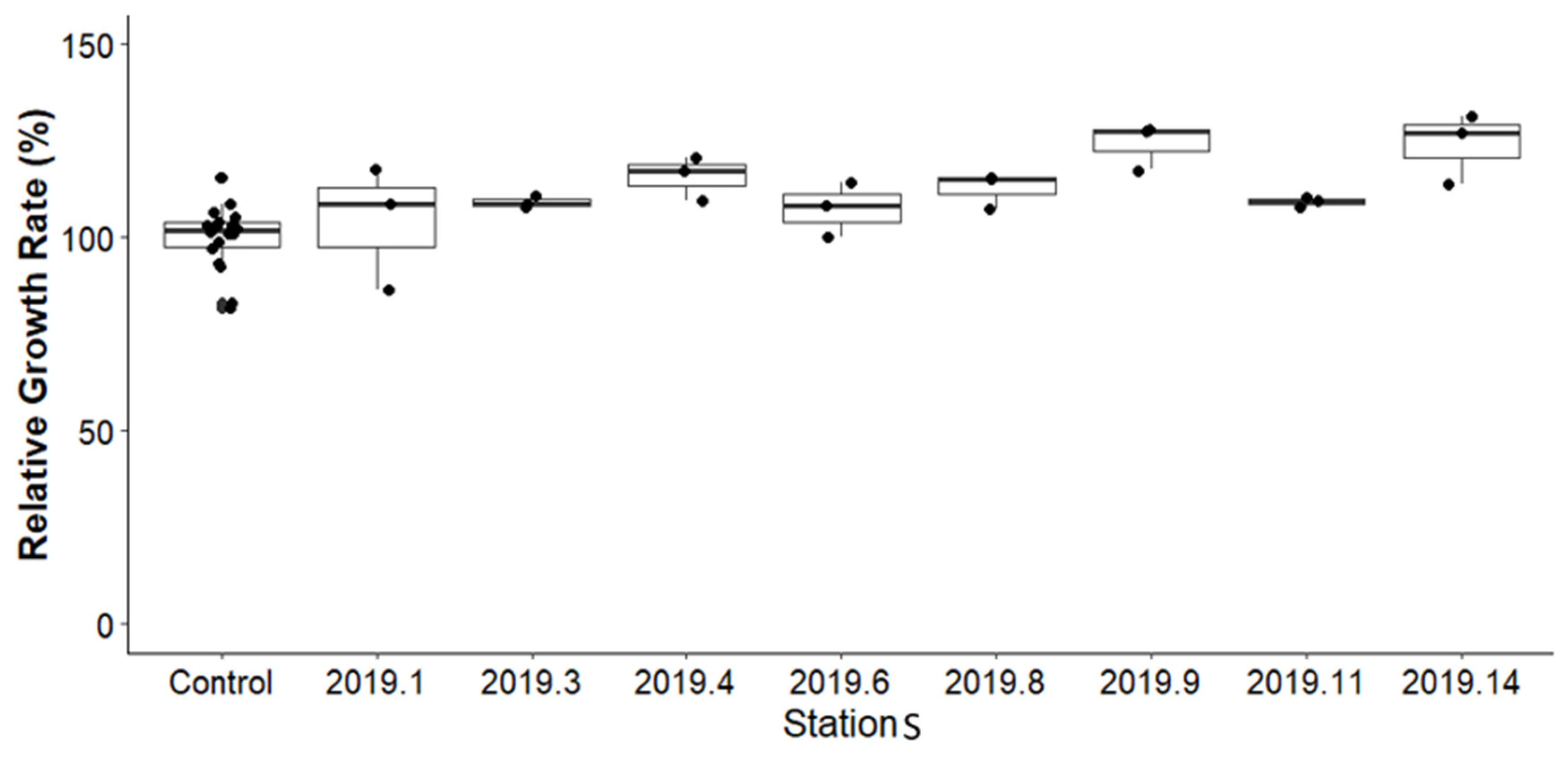
3.3.2. Growth of Amphipod Hyalella azteca in Before-Remediation Sediment Samples
3.4. Survival and Growth (RGR and Weight) of Amphipod Hyalella azteca in After Remediation Sediment Samples
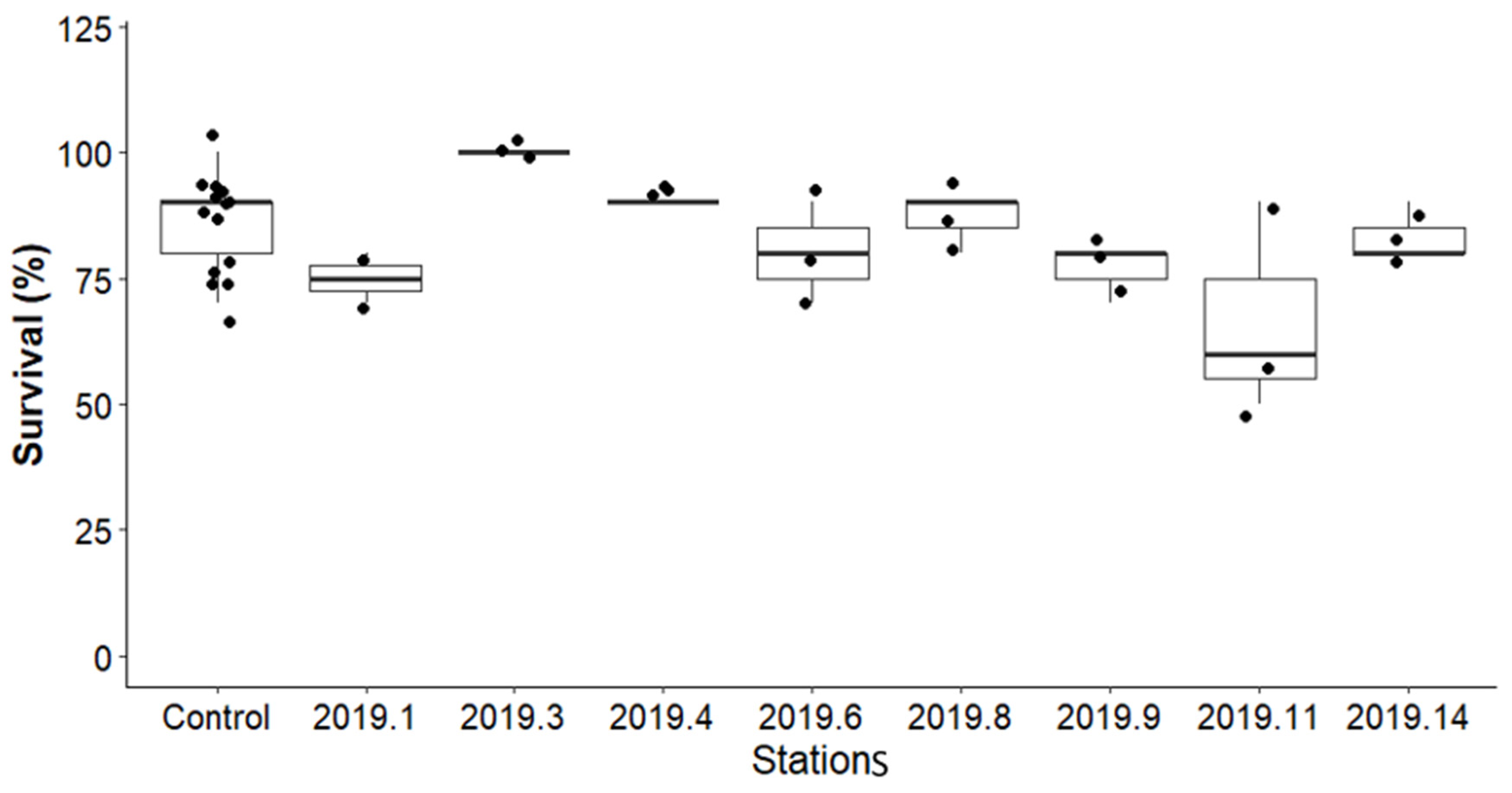
3.4.1. Survival of Amphipod Hyalella azteca in After Remediation Sediment Samples
3.4.2. Growth of Amphipod Hyalella azteca in After Remediation Sediment Samples
3.5. Survival and Growth (RGR and Weight) of Amphipod Hyalella azteca in SPM Sediment Samples
3.5.1. Survival Amphipod Hyalella azteca in SPM Sediment
3.5.2. Growth of Amphipod Hyalella azteca in SPM Sediment
3.6. Survival and Growth (RGR and Weight) of Chironomus riparius for Before-Remediation Sediment Samples
3.6.1. Survival of Chironomus riparius for Before-Remediation Sediment Samples
3.6.2. Growth of Chironomus riparius for Before-Remediation Sediment Samples
3.7. Survival and Growth (RGR) of Chironomus riparius in After Remediation Sediment Samples
3.8. Impact of Geochemical Fractions of Metals on Their Bioaccumulation
3.9. Comparison of Heavy Metal Bioaccumulation in Hyalella azteca and Chironomus riparius with Controls
3.10. Comparison of Results for Total Toxicity Tests and Bioaccumulation for Before Remediation, After Remediation, and SPM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Löser, B.C.; Zehnsdorf, A.; Görsch, K.; Seidel, H. Bioleaching of Heavy Metal Polluted Sediment: Influence of Temperature and Oxygen (Part 1). Eng. Life Sci. 2006, 6, 355–363. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Kong, L.; Liu, E.; Wang, L.; Zhu, J. Spatial distribution, ecological risk assessment and source identification for heavy metals in surface sediments from Dongping Lake, Shandong, East China. Catena 2015, 125, 200–205. [Google Scholar] [CrossRef]
- Peng, J.-F.; Song, Y.-H.; Yuan, P.; Cui, X.-Y.; Qiu, G.-L. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef]
- Hosono, T.; Su, C.-C.; Delinom, R.; Umezawa, Y.; Toyota, T.; Kaneko, S.; Taniguchi, M. Decline in heavy metal contamination in marine sediments in Jakarta Bay, Indonesia due to increasing environmental regulations. Estuar. Coast. Shelf Sci. 2011, 92, 297–306. [Google Scholar] [CrossRef]
- Kalnejais, L.H.; Martin, W.R.; Signell, R.P.; Bothner, M.H. Role of Sediment Resuspension in the Remobilization of Particulate-Phase Metals from Coastal Sediments. Environ. Sci. Technol. 2007, 41, 2282–2288. [Google Scholar] [CrossRef]
- Santos, I.R.; Silva-Filho, E.V.; Schaefer, C.E.G.R.; Albuquerque-Filho, M.R.; Campos, L.S. Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar. Pollut. Bull. 2005, 50, 185–194. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Zeng, G.; Yuan, X.; Li, X.; Liang, J.; Wang, X.; Tang, X.; Bai, B. Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J. Geochem. Explor. 2013, 132, 75–83. [Google Scholar] [CrossRef]
- Roychowdhury, A.; Datta, R.; Sarkar, D. Chapter 3.10—Heavy Metal Pollution and Remediation. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–373. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Heavy metal removal from sediments by biosurfactants. J. Hazard. Mater. 2001, 15, 111–125. [Google Scholar] [CrossRef]
- Pascoe, D.; Williams, K.A.; Green, D.W.J. Chronic toxicity of cadmium to Chironomus riparius Meigen-effects upon larval development and adult emergence. Hydrobiologia 1989, 175, 109–115. [Google Scholar] [CrossRef]
- Song, B.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Liu, Z.; Zhang, Y.; Zhang, C.; Cheng, M.; Liu, Y.; et al. Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ. Int. 2017, 105, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hope, B.K. An examination of ecological risk assessment and management practices. Environ. Int. 2006, 32, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Pardo, T.; Clemente, R.; Alvarenga, P.; Bernal, M.P. Efficiency of soil organic and inorganic amendments on the remediation of a contaminated mine soil: II. Biological and ecotoxicological evaluation. Chemosphere 2014, 107, 101–108. [Google Scholar] [CrossRef]
- Rodriguez-Iruretagoiena, A.; Rementeria, A.; Zaldibar, B.; de Vallejuelo, S.F.; Gredilla, A.; Arana, G.; de Diego, A. Is there a direct relationship between stress biomarkers in oysters and the amount of metals in the sediments where they inhabit ? Mar. Pollut. Bull. 2016, 111, 95–105. [Google Scholar] [CrossRef]
- Diepens, N.J.; Arts, G.H.P.; Brock, T.C.M.; Smidt, H.; Van Den Brink, P.J.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Sediment Toxicity Testing of Organic Chemicals in the Context of Prospective Risk Assessment: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 255–302. [Google Scholar] [CrossRef]
- Lim, D.-I.; Choi, J.-W.; Shin, H.H.; Jeong, D.H.; Jung, H.S. Toxicological impact assessment of heavy metal contamination on macrobenthic communities in southern coastal sediments of Korea. Mar. Pollut. Bull. 2013, 73, 362–368. [Google Scholar] [CrossRef]
- Nebeker, A.V.; Cairns, M.A.; Gakstatter, J.H.; Malueg, K.W.; Schuytema, G.S.; Krawczyk, D.F. Biological methods for determining toxicity of contaminated freshwater sediments to invertebrates. Environ. Toxicol. Chem. 1984, 3, 617–630. [Google Scholar] [CrossRef]
- De Cooman, W.; Blaise, C.; Janssen, C.; Detemmerman, L.; Elst, R.; Persoone, G. History and sensitivity comparison of two standard whole-sediment toxicity tests with crustaceans: The amphipod Hyalella azteca and the ostracod Heterocypris incongruens microbiotest. Knowl. Managt. Aquat. Ecosyst. 2015, 416, 15. [Google Scholar] [CrossRef]
- Pourabadehei, M. Resuspension of Sediment, a New Technique For Remediation of Contaminated Sediment in Shallow Harbours. Ph.D. Thesis, Concordia University, Montreal, QC, Canada, 2017. [Google Scholar]
- Yong, N.; Galvez-Cloutier, R.; Phadungchewit, Y. Selective sequential extraction analysis of heavy-metal retention in soil. Can. Geotech. 1993, 30, 834–847. [Google Scholar] [CrossRef]
- Environment Canada. Biological test method. In Test for Survival and Growth in Sediment Using Larvae of Freshwater Midges (Chironomus Tentans or Chironomus Riparius), (Report: EPS 1/RM/32); Environment Canada: Ottawa, ON, Canada, 1997. [Google Scholar]
- OECD Guidelines for the Testing of Chemicals. Test Guideline No. 218, Sediment-Water Chironomid Toxicity Using Spiked Sediment; OECD: Paris, France, 2023. [Google Scholar]
- Environment Canada. Biological Test Method: Test for Survival, Growth and Reproduction in Sediment and Water Using the Freshwater Amphipod Hyalella azteca, Report RM/33; Environment Canada: Ottawa, ON, Canada, 2017.
- Crémazy, A.; Brix, K.V.; Wood, C.M. Chronic Toxicity of Binary Mixtures of Six Metals (Ag, Cd, Cu, Ni, Pb, and Zn) to the Great Pond Snail Lymnaea stagnalis. Environ. Sci. Technol. 2018, 52, 5979–5988. [Google Scholar] [CrossRef]
- Taddei, A.; Räsänen, K.; Burdon, F.J. Size-dependent sensitivity of stream amphipods indicates population-level responses to chemical pollution. Freshw. Biol. 2021, 66, 765–784. [Google Scholar] [CrossRef]
- Julien, L. Bioaccumulation et Répartition Subcellulaire D’éléments Traces Métalliques Chez L’amphipode Hyalella azteca Provenant de la Région de Yellowknife (Territoires du Nord-Ouest, Canada). Master’s Thesis, Université du Québec à Montréal, Montreal, QC, Canada, 2022. [Google Scholar]
- Sibley, P.K.; Benoit, D.A.; Ankley, G.T. The Significance of Growth in Chironomus Tentans Sediment Toxicity Tests: Relationship to Reproduction and Demographic Endpoints. Environ. Toxicol. Chem. 1997, 16, 336–345. [Google Scholar] [CrossRef]
- Rosabal, M.; Pierron, F.; Couture, P.; Baudrimont, M.; Hare, L.; Campbell, P.G.C. Subcellular partitioning of non-essential trace metals (Ag, As, Cd, Ni, Pb, and Tl) in livers of American (Anguilla rostrata) and European (Anguilla anguilla) yellow eels. Aquat. Toxicol. 2015, 160, 128–141. [Google Scholar] [CrossRef]
- Javid, M.; Mulligan, C.N. In-Situ Remediation of Heavy Metal–Contaminated Sediments Using the Resuspension Technique. Water 2025, 17, 376. [Google Scholar] [CrossRef]
- Song, L.; Vijver, M.G.; Peijnenburg, W.J.G.M. Comparative toxicity of copper nanoparticles across three Lemnaceae species. Sci. Total Environ. 2015, 518–519, 217–224. [Google Scholar] [CrossRef]
- Ball, A.L.; Borgmann, U.; Dixon, D.G. Toxicity of a cadmium-contaminated diet to Hyalella azteca. Environ. Toxicol. Chem. 2006, 25, 2526–2532. [Google Scholar] [CrossRef]
- Steevens, J.A.; Benson, W.H. Hyalella azteca 10-Day Sediment Toxicity Test: Comparison of Growth Measurement Endpoints. Environ. Toxicol. Water Qual. 1998, 13, 243–248. [Google Scholar] [CrossRef]
- Kemble, N.E.; Brumbaugh, W.G.; Brunson, E.L.; Dwyer, F.J.; Ingersoll, C.G.; Monda, D.P.; Woodward, D.F. Toxicity of metal-contaminated sediments from the upper Clark Fork River, Montana, to aquatic invertebrates and fish in laboratory exposures. Environ. Toxicol. Chem. 1994, 13, 1985–1997. [Google Scholar] [CrossRef]
- Kubitz, J.A.; Lewek, E.C.; Besser, J.M.; Iii, J.B.D.; Giesy, J.P. Effects of Copper-Contaminated Sediments on Hyalella azteca, Daphnia magna, and Ceriodaphnia dubia: Survival, Growth, and Enzyme inhibition. Arch. Environ. Contam. Toxicol. 1995, 29, 97–103. [Google Scholar] [CrossRef]
- Borrely, S.I.; Garcia, V.S.G.; Borrely, T.; Favaro, D.I.T. Metals, trace elements and ecotoxicity in sediments of the Cubatão River, Brazil. Ecotoxicol. Environ. Contam. 2018, 13, 49–61. [Google Scholar] [CrossRef]
- Liber, K.; Doig, L.E.; White-Sobey, S.L. Toxicity of uranium, molybdenum, nickel, and arsenic to Hyalella azteca and Chironomus dilutus in water-only and spiked-sediment toxicity tests. Ecotoxicol. Environ. Saf. 2011, 74, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Harrahy, E.A.; Clements, W.H. Toxicity and bioaccumulation of a mixture of heavy metals in Chironomus tentans (Diptera: Chironomidae) in synthetic sediment. Environ. Toxicol. Chem. 1997, 16, 317–327. [Google Scholar] [CrossRef]
- Arambourou, H.; Llorente, L.; Moreno-Ocio, I.; Herrero, Ó.; Barata, C.; Fuertes, I.; Delorme, N.; Méndez-Fernández, L.; Planelló, R. Exposure to heavy metal-contaminated sediments disrupts gene expression, lipid profile, and life history traits in the midge Chironomus riparius. Water Res. 2020, 168, 115165. [Google Scholar] [CrossRef]
- Amirah Hamzah, H.; Kadhum, S.A.; Zahmir Zulkifli, S.; Ali Abed, S.; Awad, A.; Al-Ansari, N. Heavy metal speciation in surface sediments and their impact on the bioaccumulation of green mussels (Perna viridis) from the eastern part of the Straits of Johor, Malaysia. Total Environ. Res. Themes 2023, 7, 100064. [Google Scholar] [CrossRef]
- Buhari, T.R.; Ismail, A. Correlations between Geo-Chemical Speciation of Heavy Metals (Cu, Zn, Pb, Cd and Ni) in Surface Sediments and Their Concentrations in Giant Mudskipper (Periophthalmodon schlosseri) Collected from the West Coast of Peninsular Malaysia. J. Geosci. Environ. Prot. 2016, 4, 28–36. [Google Scholar] [CrossRef][Green Version]
- Faria, M.S.; Lopes, R.J.; Nogueira, A.J.A.; Soares, A.M.V.M. In situ and laboratory bioassays with Chironomus riparius larvae to assess toxicity of metal contamination in rivers: The relative toxic effect of sediment versus water contamination. Environ. Toxicol. Chem. 2007, 26, 1968–1977. [Google Scholar] [CrossRef]
- Bonnet, C. Développement de bioessais sur sédiments et applications à l’étude, en laboratoire, de la toxicité de sédiments dulçaquicoles contaminés. Ph.D. Thesis, Université de Metz, Metz, France, 2000. [Google Scholar]
- Halpern, M.; Gasith, A.; Broza, M. Does the tube of a benthic chironomid larva play a role in protecting its dweller against chemical toxicants? Hydrobiologia 2002, 470, 49–55. [Google Scholar] [CrossRef]
- Amiard-Triquet, C.; Berthet, B.; Metayer, C. Comparative study of the patterns of bioaccumulation of essential (Cu, Zn) and non-essential (Cd, Pb) trace metals in various estuarine and coastal organisms. Mar. Biol. Ecol. 1987, 106, 73–89. [Google Scholar] [CrossRef]
- Cummins, K.W.; Klug, M.J. Feeding Ecology of Stream Invertebrates. Annu. Rev. Ecol. Syst. 1979, 10, 147–172. [Google Scholar] [CrossRef]
- Borgmann, U.; Couillard, Y.; Doyle, P.; George Dixon, D. Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ. Toxicol. Chem. 2005, 24, 641–652. [Google Scholar] [CrossRef]
- Bourgoin, B.P.; Risk, M.J.; Evans, R.D.; Cornett, R.J. Relationships between the partitioning of lead in sediments and its accumulation in the marine Mussel, Mytilus Edulis near a lead smelter. Water Air Soil Pollut. 1991, 57–58, 377–386. [Google Scholar] [CrossRef]
- Bochenek, I.; Protasowicki, M.; Brucka-Jastrzƙbska, E. Concentrations of Cd, Pb, Zn, and Cu in roach, Rutilus rutilis (l.) from the lower reaches of the Oder River, and their correlation with concentrations of heavy metals in bottom sediments collected in the same area. Arch. Pol. Fish. 2008, 16, 21–36. [Google Scholar] [CrossRef]
- Fan, W.; Wang, W.X.; Chen, J. Geochemistry of Cd, Cr, and Zn in highly contaminated sediments and its influences on assimilation by marine bivalves. Environ. Sci. Technol. 2002, 36, 5164–5171. [Google Scholar] [CrossRef]
- Dhanakumar, S.; Solaraj, G.; Mohanraj, R. Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol. Environ. Saf. 2015, 113, 145–151. [Google Scholar] [CrossRef]
- Gust, K.A.; Fleeger, J.W. Exposure-related effects on Cd bioaccumulation explain toxicity of Cd-phenanthrene mixtures in Hyalella azteca. Environ. Toxicol. Chem. 2005, 24, 2918–2926. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Kraemer, L.D.; Giguère, A.; Hare, L.; Hontela, A. Subcellular distribution of cadmium and nickel in chronically exposed wild fish: Inferences regarding metal detoxification strategies and implications for setting water quality guidelines for dissolved metals. Hum. Ecol. Risk Assess. 2008, 14, 290–316. [Google Scholar] [CrossRef]
- Wang, F.; Goulet, R.R.; Chapman, P.M. Testing sediment biological effects with the freshwater amphipod Hyalella azteca: The gap between laboratory and nature. Chemosphere 2004, 57, 1713–1724. [Google Scholar] [CrossRef]
- Stephenson, M.; Turner, M.A. A Field Study of Cadmium Dynamics in Periphyton and in Hyalella azteca (CRUSTACEA: AMPHIPODA). Water Air Soil Pollut. 1993, 68, 341–361. [Google Scholar] [CrossRef]
- Warren, L.A.; Tessier, A.; Hare, L. Modelling cadmium accumulation by benthic invertebrates in situ: The relative contributions of sediment and overlying water reservoirs to organism cadmium concentrations. Limnol. Oceanogr. 1998, 43, 1442–1454. [Google Scholar] [CrossRef]
- Hare, L.; Tessier, A.; Warren, L. Cadmium accumulation by invertebrates living at the sediment-water interface. Environ. Toxicol. Chem. 2001, 20, 880–889. [Google Scholar] [CrossRef]
- Borgmann, U.; Nowierski, M.; Grapentine, L.C.; Dixon, D.G. Assessing the cause of impacts on benthic organisms near Rouyn-Noranda, Quebec. Environ. Pollut. 2004, 129, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Goulet, R.R.; Thompson, P. Bioaccumulation and toxicity of uranium, arsenic, and nickel to juvenile and adult Hyalella azteca in spiked sediment bioassays. Environ. Toxicol. Chem. 2018, 37, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]



| Extraction Steps | Cr | Ni | Cu | Zn | As | Cd | Pb |
|---|---|---|---|---|---|---|---|
| Exchangeable fraction (F1) | −0.68 ** | −0.51 | −0.72 ** | −0.60 * | −0.36 | −0.15 | 0.17 |
| Acid soluble fraction (F2) | −0.45 | −0.54 * | −0.36 | −0.39 | −0.71 ** | 0.50 | −0.50 |
| Reducible fraction (F3) | −0.49 | −0.36 | −0.37 | 0.34 | −0.39 | 0.29 | −0.48 |
| ∑bioavailable (F1 + F2 + F3) | −0.54 * | −0.64 * | −0.36 | −0.14 | −0.39 | −0.13 | −0.59 * |
| Oxidizable fraction (F4) | −0.67 ** | 0.37 | −0.51 | −0.49 | −0.13 | −0.26 | −0.62 * |
| Residual fraction (F5) | 0.23 | 0.46 | −0.31 | −0.27 | 0.42 | 0.29 | 0.61 * |
| ∑non-bioavailable (F4 + F5) | 0.12 | 0.45 | −0.51 | −0.58 * | 0.39 | 0.01 | −0.20 |
| ∑bioavailable + non-bioavailable (F1 + F2 + F3 + F4 + F5) | −0.29 | −0.67 ** | −0.43 | −0.47 | −0.16 | −0.12 | −0.51 |
| Total concentration | 0.49 | 0.32 | −0.21 | 0.26 | 0.74 ** | 0.37 | −0.07 |
| Extraction Steps | Cr | Ni | Cu | Zn | As | Cd | Pb |
|---|---|---|---|---|---|---|---|
| Exchangeable fraction (F1) | 0.17 | 0.23 | 0.36 | 0.16 | −0.30 | −0.15 | −0.04 |
| Acid soluble fraction (F2) | 0.46 * | 0.46 * | 0.59 ** | 0.38 | 0.54 ** | 0.28 | 0.10 |
| Reducible fraction (F3) | 0.51 * | 0.35 | 0.62 ** | 0.10 | 0.52 * | 0.14 | 0.33 |
| ∑bioavailable (F1 + F2 + F3) | 0.47 * | 0.42 * | 0.66 ** | 0.29 | 0.47 * | −0.07 | 0.22 |
| Oxidizable fraction (F4) | 0.11 | 0.10 | 0.06 | 0.35 | 0.38 | −0.23 | 0.28 |
| Residual fraction (F5) | 0.14 | 0.19 | 0.02 | −0.06 | 0.01 | 0.11 | 0.31 |
| ∑non-bioavailable (F4 + F5) | 0.17 | 0.17 | 0.06 | 0.04 | 0.05 | −0.22 | 0.34 |
| ∑bioavailable + non-bioavailable (F1 + F2 + F3 + F4 + F5) | 0.38 | 0.42 * | 0.38 | 0.45 * | 0.38 | −0.09 | 0.37 |
| Total concentration | 0.08 | 0.15 | 0.32 | −0.15 | −0.28 | −0.23 | 0.28 |
| Metal | Control | Station 1 | Station 3 | Station 4 | Station 14 |
|---|---|---|---|---|---|
| Cr | 0.81 | 0.88 | 1.18 | 0.49 | 0.84 |
| Ni | 1.93 | 0.73 | 0.98 | 17.54 | 0.72 |
| Cu | 76.16 | 130.61 | 99.35 | 124.20 | 82.79 |
| Zn | 119.28 | 60.85 | 95.98 | 136.90 | 139.48 |
| As | 0.62 | 0.63 | 0.42 | 0.57 | 0.32 |
| Cd | 0.59 | 0.95 | 0.82 | 0.40 | 0.50 |
| Pb | 0.59 | 1.00 | 1.10 | 0.60 | 0.48 |
| Metal | Control | Station 1 | Station 3 | Station 4 | Station 14 |
|---|---|---|---|---|---|
| Cr | 2.91 | 0.64 | 1.82 | 5.13 | 3.63 |
| Ni | 6.72 | 0.74 | 2.23 | 10.62 | 4.07 |
| Cu | 119.99 | 182.54 | 165.52 | 105.42 | 122.75 |
| Zn | 316.00 | 1176.38 | 317.08 | 178.68 | 114.69 |
| As | 1.50 | 0.97 | 0.72 | 0.73 | 0.69 |
| Cd | 1.08 | 1.78 | 1.63 | 1.23 | 1.82 |
| Pb | 1.95 | 0.812 | 0.94 | 0.72 | 1.37 |
| Metal | Control | Station 1 | Station 3 | Station 4 | Station 14 |
|---|---|---|---|---|---|
| Cr | 1.19 | 9.00 | 1.71 | 0.66 | 0.82 |
| Ni | 0.682 | 2.73 | 1.65 | 2.13 | 0.45 |
| Cu | 87.25 | 254.79 | 184.00 | 165.21 | 129.41 |
| Zn | 97.45 | 130.87 | 66.74 | 75.31 | 63.58 |
| As | 0.81 | 0.48 | 0.67 | 0.55 | 0.46 |
| Cd | 0.56 | 2.01 | 0.92 | 2.09 | 1.01 |
| Pb | 0.14 | 0.31 | 0.42 | 0.18 | 0.35 |
| Metal | Control | Station 1 | Station 3 | Station 14 |
|---|---|---|---|---|
| Cr | 1.31 | 2.56 | 1.63 | 2.62 |
| Ni | 1.66 | 1.73 | 1.78 | 2.52 |
| Cu | 31.39 | 50.00 | 35.94 | 69.28 |
| Zn | 133.78 | 260.48 | 279.20 | 234.63 |
| As | 0.38 | 0.08 | 0.19 | 0.47 |
| Cd | 0.79 | 1.16 | 1.52 | 2.47 |
| Pb | 0.82 | 1.72 | 0.85 | 1.74 |
| Metal | Control | Station 1 | Station 3 | Station 4 | Station 14 |
|---|---|---|---|---|---|
| Cr | 1.41 | 1.41 | 1.12 | 1.02 | 1.33 |
| Ni | 2.11 | 2.11 | 1.36 | 0.74 | 0.99 |
| Cu | 23.37 | 23.37 | 61.69 | 35.31 | 37.42 |
| Zn | 115.83 | 115.83 | 208.44 | 108.26 | 168.28 |
| As | 0.40 | 0.40 | 0.13 | 0.15 | 0.17 |
| Cd | 0.68 | 0.68 | 2.31 | 1.76 | 1.83 |
| Pb | 0.77 | 0.77 | 0.78 | 0.48 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javid, M.; Mulligan, C.N.; Lefranc, M.; Rosabal Rodriguez, M. Metal Exposure, Bioaccumulation, and Toxicity Assessment in Sediments from the St. Lawrence River Before and After Remediation Using a Resuspension Technique. Toxics 2025, 13, 432. https://doi.org/10.3390/toxics13060432
Javid M, Mulligan CN, Lefranc M, Rosabal Rodriguez M. Metal Exposure, Bioaccumulation, and Toxicity Assessment in Sediments from the St. Lawrence River Before and After Remediation Using a Resuspension Technique. Toxics. 2025; 13(6):432. https://doi.org/10.3390/toxics13060432
Chicago/Turabian StyleJavid, Masoumeh, Catherine N. Mulligan, Marie Lefranc, and Maikel Rosabal Rodriguez. 2025. "Metal Exposure, Bioaccumulation, and Toxicity Assessment in Sediments from the St. Lawrence River Before and After Remediation Using a Resuspension Technique" Toxics 13, no. 6: 432. https://doi.org/10.3390/toxics13060432
APA StyleJavid, M., Mulligan, C. N., Lefranc, M., & Rosabal Rodriguez, M. (2025). Metal Exposure, Bioaccumulation, and Toxicity Assessment in Sediments from the St. Lawrence River Before and After Remediation Using a Resuspension Technique. Toxics, 13(6), 432. https://doi.org/10.3390/toxics13060432










