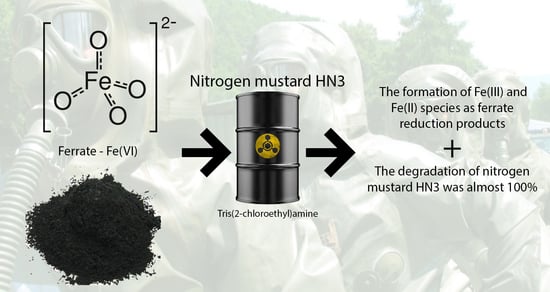
Degradation of Chemical Warfare Agent Nitrogen Mustard Using Ferrate (VI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ferrate(VI)
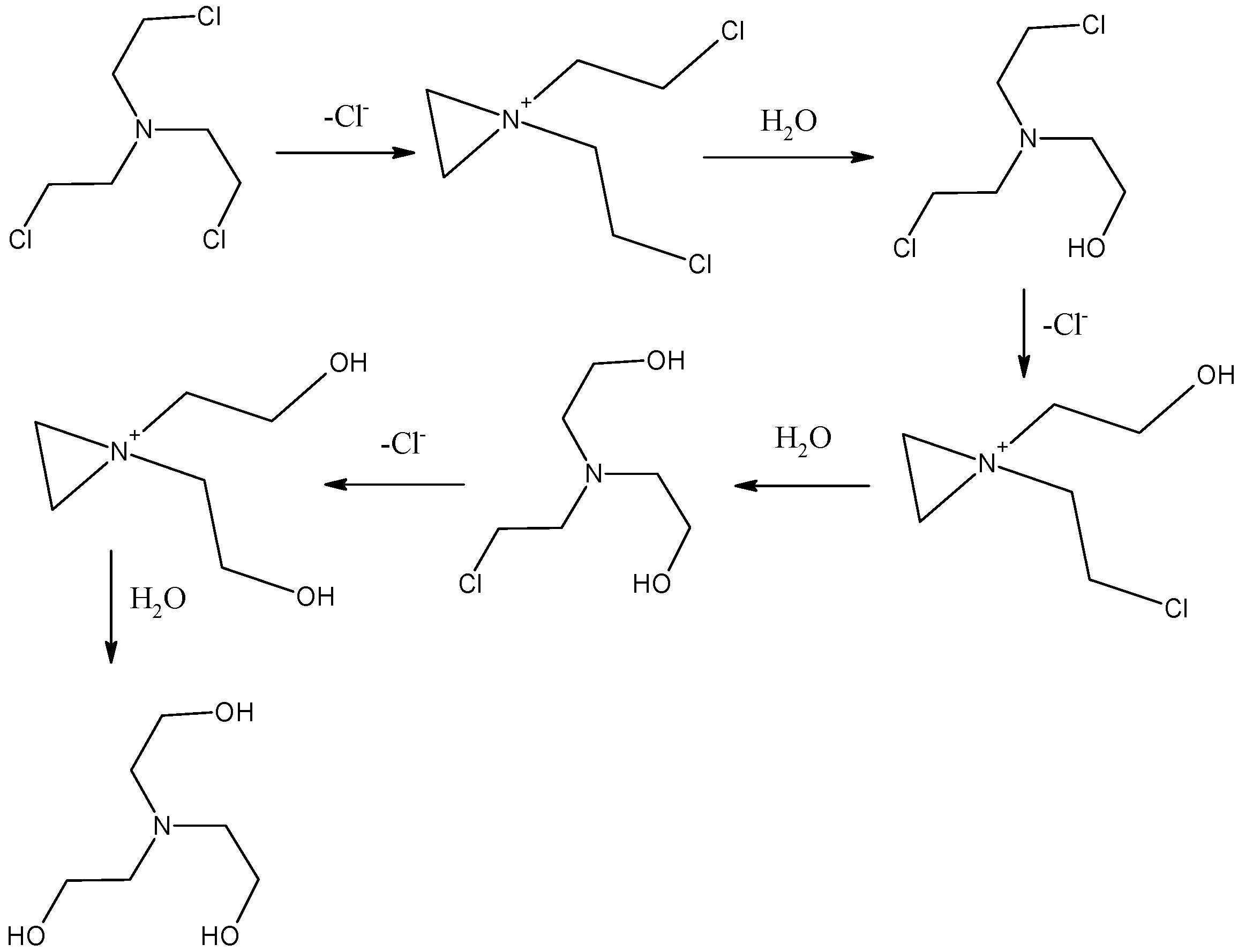
2.2. Preparation of Nitrogen Mustard HN3
2.3. Reaction of Ferrate Fe(VI) with Nitrogen Mustard HN3
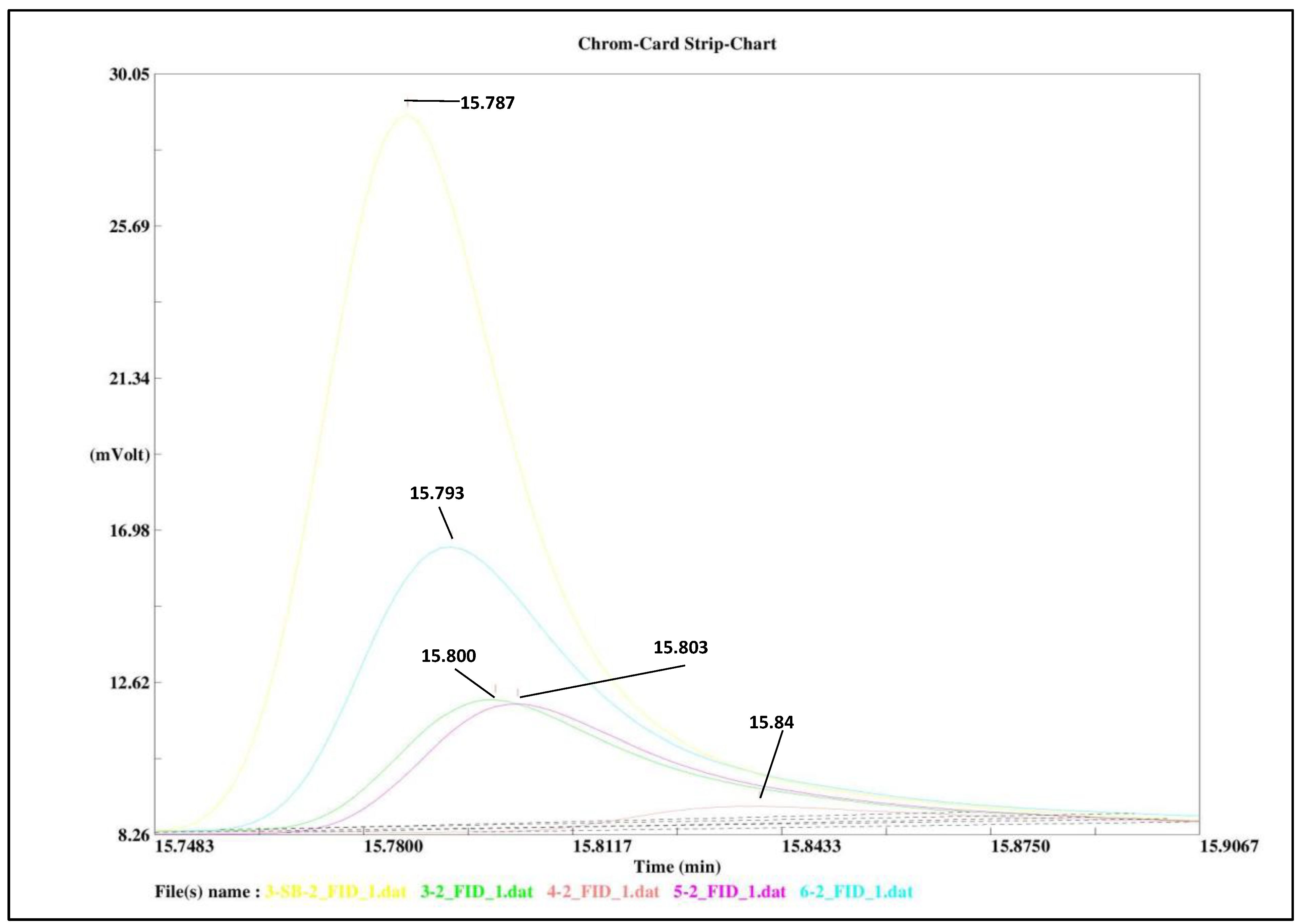
3. Results and Discussion
Degradation of Nitrogen Mustard
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Prasad, G.K.; Pandey, K.S.; Danikhel, R.K.; Vijayaraghavan, R. Decontamination of Chemical Warfare Agents. Def. Sci. J. 2010, 60, 428. [Google Scholar] [CrossRef]
- Chauhan, S.; Chauhan, S.; D’Cruz, R.; Faruqi, S.; Singh, K.K.; Varma, S.; Singh, M.; Karthik, V. Chemical warfare agents. Environ. Toxicol. Pharmacol. 2008, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Raza, S.K.; Vijayaraghavan, R. Chemical warfare agents. J. Pharm. Bioallied Sci. 2010, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Szinicz, L. History of chemical and biological warfare agents. Toxicology 2005, 214, 3. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Handbook of Toxicology of Chemical Warfare Agents; Academic Press: Cambridge, MA, USA, 2015; no. BN. [Google Scholar]
- Marrs, T.T.; Maynard, R.L.; Sidell, F. (Eds.) Chemical Warfare Agents: Toxicology and Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lukey, B.J.; Romano, J.A., Jr.; Romano, J.A.; Salem, H.; Lukey, B.J.; Salem, H. (Eds.) Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Munro, N.B. The Sources, Fate, and Toxicity of Chemical Warfare Agent Degradation Products. Environ. Health Perspect. 1999, 107, 933. [Google Scholar] [CrossRef]
- Wellert, S.; Imhof, H.; Dolle, M.; Altmann, H.-J.; Richardt, A.; Hellweg, T. Decontamination of chemical warfare agents using perchloroethylene–Marlowet IHF–H2O-based microemulsions: Wetting and extraction properties on realistic surfaces. Colloid Polym. Sci. 2008, 286, 417–426. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, H.; Zhu, H.; Wang, L.; Zhou, C. Soman hydrolysis catalysed by hypochlorite ions. E3S Web Conf. 2021, 267, 02043. [Google Scholar] [CrossRef]
- Groenewold, G.S. Degradation kinetics of VX. Main Group Chem. 2010, 9, 221–244. [Google Scholar] [CrossRef]
- Dubey, D.K.; Gupta, A.K.; Sharma, M.; Prabha, S.; Vaidyanathaswamy, R. Micellar Effects on Hypochlorite Catalyzed Decontamination of Toxic Phosphorus Esters. Langmuir 2002, 18, 10489–10492. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Begum, R.A.; Day, V.W.; Bowman-James, K. Sulfur, oxygen, and nitrogen mustards: Stability and reactivity. Org. Biomol. Chem. 2012, 10, 8786. [Google Scholar] [CrossRef]
- Chua, H.-C.; Lee, H.-S.; Sng, M.-T. Screening of nitrogen mustards and their degradation products in water and decontamination solution by liquid chromatography–mass spectrometry. J. Chromatogr. A 2006, 1102, 214–223. [Google Scholar] [CrossRef]
- Smolkin, B.; Levi, N.; Karton-Lifshin, N.; Yehezkel, L.; Zafrani, Y.; Columbus, I. Oxidative Detoxification of Sulfur-Containing Chemical Warfare Agents by Electrophilic Iodine. J. Org. Chem. 2018, 83, 13949–13955. [Google Scholar] [CrossRef]
- Snelson, A.; Taylor, K.; O’Neill, H.J. Reaction of CW agents simulants on surfaces in the presence of O3, UV and O3+UV. J. Environ. Sci. Health 1984, A19, 7. [Google Scholar] [CrossRef]
- Popiel, S.; Nalepa, T.; Dzierziak, D.; Stankiewicz, R.; Witkiewicz, Z. Rate of dibutylsulfide decomposition by ozonation and the O3/H2O2 advanced oxidation process. J. Hazard. Mater. 2009, 164, 1364–1371. [Google Scholar] [CrossRef]
- Popiel, S.; Witkiewicz, Z.; Chrzanowski, M. Sulfur mustard destruction using ozone, UV, hydrogen peroxide and their combination. J. Hazard. Mater. 2007, 153, 37–43. [Google Scholar] [CrossRef]
- Gil-San-Millan, R.; López-Maya, E.; Platero-Prats, A.E.; Torres-Pérez, V.; Delgado, P.; Augustyniak, A.W.; Kim, M.K.; Lee, H.W.; Ryu, S.G.; Navarro, J.A. Magnesium exchanged zirconium metal-organic frameworks with improved detoxification properties of nerve agents. J. Am. Chem. Soc. 2019, 141, 11801–11805. [Google Scholar] [CrossRef]
- Ma, K.; Wasson, M.C.; Wang, X.; Zhang, X.; Idrees, K.B.; Chen, Z.; Wu, Y.; Lee, S.-J.; Cao, R.; Chen, Y.; et al. Near-instantaneous catalytic hydrolysis of organophosphorus nerve agents with zirconium-based MOF/hydrogel composites. Chem Catal. 2021, 1, 721–733. [Google Scholar] [CrossRef]
- Moon, S.; Proussaloglou, E.; Peterson, G.W.; DeCoste, J.B.; Hall, M.G.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Detoxification of Chemical Warfare Agents Using a Zr6-Based Metal–Organic Framework/Polymer Mixture. Chem. Eur. J. 2016, 22, 14864–14868. [Google Scholar] [CrossRef]
- Peterson, G.W.; Wagner, G.W. Detoxification of chemical warfare agents by CuBTC. J. Porous Mater. 2014, 21, 121–126. [Google Scholar] [CrossRef]
- Štengl, V.; Henych, J.; Janoš, P.; Skoumal, M. Nanostructured Metal Oxides for Stoichiometric Degradation of Chemical Warfare Agents. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2016; Volume 236, pp. 239–258. [Google Scholar] [CrossRef]
- Štengl, V.; Houšková, V.; Bakardjieva, S.; Murafa, N.; Maříková, M.; Opluštil, F.; Němec, T. Zirconium doped nano-dispersed oxides of Fe, Al and Zn for destruction of warfare agents. Mater. Charact. 2010, 61, 1080–1088. [Google Scholar] [CrossRef]
- Prasad, G.K.; Mahato, T.H.; Singh, B.; Ganesan, K.; Pandey, P.; Sekhar, K. Detoxification reactions of sulphur mustard on the surface of zinc oxide nanosized rods. J. Hazard. Mater. 2007, 149, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.W.; Koper, O.B.; Lucas, E.; Decker, S.; Klabunde, K.J. Reactions of VX, GD, and HD with Nanosize CaO: Autocatalytic Dehydrohalogenation of HD. J. Phys. Chem. B 2000, 104, 5118–5123. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Guo, X.; Zhong, J. A flexible self-detoxifying Zr(OH)4-carbon fiber composite for decontamination of chemical warfare agent. J. Sol-Gel Sci. Technol. 2021, 97, 1–4. [Google Scholar] [CrossRef]
- Wen, X.; Ye, L.; Chen, L.; Kong, L.; Yuan, L.; Xi, H.; Zhong, J. Decontamination of Chemical Warfare Agents by Novel Oximated Acrylate Copolymer. Chem. Res. Chin. Univ. 2019, 35, 1095–1104. [Google Scholar] [CrossRef]
- Iwai, T.; Inoue, H.; Kakegawa, K.; Ohrui, Y.; Nagoya, T.; Nagashima, H.; Miyahara, H.; Chiba, K.; Seto, Y.; Okino, A. Development of a High Efficiency Decomposition Technology for Volatile Chemical Warfare Agent Sarin Using Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2020, 40, 907–920. [Google Scholar] [CrossRef]
- Ohmori, T.; Kawahara, K.; Nakayama, K.; Shioda, A.; Ishikawa, S.; Kanamori-Kataoka, M.; Kishi, S.; Komano, A.; Seto, Y. Decontamination of nerve agents by immobilized organophosphorus hydrolase. Forensic Toxicol. 2013, 31, 37–43. [Google Scholar] [CrossRef]
- Zboril, R.; Andrle, M.; Oplustil, F.; Machala, L.; Tucek, J.; Filip, J.; Marusak, Z.; Sharma, V.K. Treatment of chemical warfare agents by zero-valent iron nanoparticles and ferrate(VI)/(III) composite. J. Hazard. Mater. 2012, 211–212, 126–130. [Google Scholar] [CrossRef]
- Žabka, D.; Konečná, B.; Celec, P.; Janíková, M.; Ivašková, N.; Tóthová, Ľ.; Tamáš, M.; Škulcová, A.B.; Púček Belišová, N.; Horáková, I.; et al. Ferrate (VI), Fenton Reaction and Its Modification: An Effective Method of Removing SARS-CoV-2 RNA from Hospital Wastewater. Pathogens 2022, 11, 450. [Google Scholar] [CrossRef]
- Híveš, J.; Benová, M.; Bouzek, K.; Sitek, J.; Sharma, V.K. The cyclic voltammetric study of ferrate(VI) formation in a molten Na/K hydroxide mixture. Electrochim. Acta 2008, 54, 203–208. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Ross, S.D.; Swain, C.G. Kinetics and Mechanism of the Reactions of Tertiary β-Chloroethylamines in Solution. III. β-Chloroethyldiethylamine and tris-β-Chloroethylamine. J. Am. Chem. Soc 1947, 71, 4. [Google Scholar] [CrossRef]
- Sharma, V.K. Oxidation of nitrogen-containing pollutants by novel ferrate (VI) technology: A review. J. Environ. Sci. Health Part A 2010, 45, 645–667. [Google Scholar] [CrossRef]
- Sharma, V.K. Ferrate(VI) and ferrate(V) oxidation of organic compounds: Kinetics and mechanism. Coord. Chem. Rev. 2013, 257, 495–510. [Google Scholar] [CrossRef]
- Ramacharyulu, P.V.R.K.; Prasad, G.K.; Ganesan, K.; Singh, B. Photocatalytic decontamination of sulfur mustard using titania nanomaterials. J. Mol. Catal. A Chem. 2012, 353–354, 132–137. [Google Scholar] [CrossRef]
- Ramacharyulu, P.V.R.K.; Praveen Kumar, J.; Prasad, G.K.; Sreedhar, B. Sulphur doped nano TiO2: Synthesis, characterization and photocatalytic degradation of a toxic chemical in presence of sunlight. Mater. Chem. Phys. 2014, 148, 692–698. [Google Scholar] [CrossRef]
- Praveen Kumar, J.; Prasad, G.K.; Allen, J.A.; Ramacharyulu, P.V.R.K.; Kadirvelu, K.; Singh, B. Synthesis of mesoporous metal aluminate nanoparticles and studies on the decontamination of sulfur mustard. J. Alloys Compd. 2016, 662, 44–53. [Google Scholar] [CrossRef]
- Sarma, R. Studies of the di-iron (VI) intermediate in ferrate-dependent oxygen evolution from water. J. Am. Chem. Soc. 2012, 134, 15371–15386. [Google Scholar] [CrossRef]
- Luo, C.; Feng, M.; Sharma, V.K.; Huang, C.-H. Revelation of ferrate(VI) unimolecular decay under alkaline conditions: Investigation of involvement of Fe(IV) and Fe(V) species. Chem. Eng. J. 2020, 338, 124–134. [Google Scholar] [CrossRef]


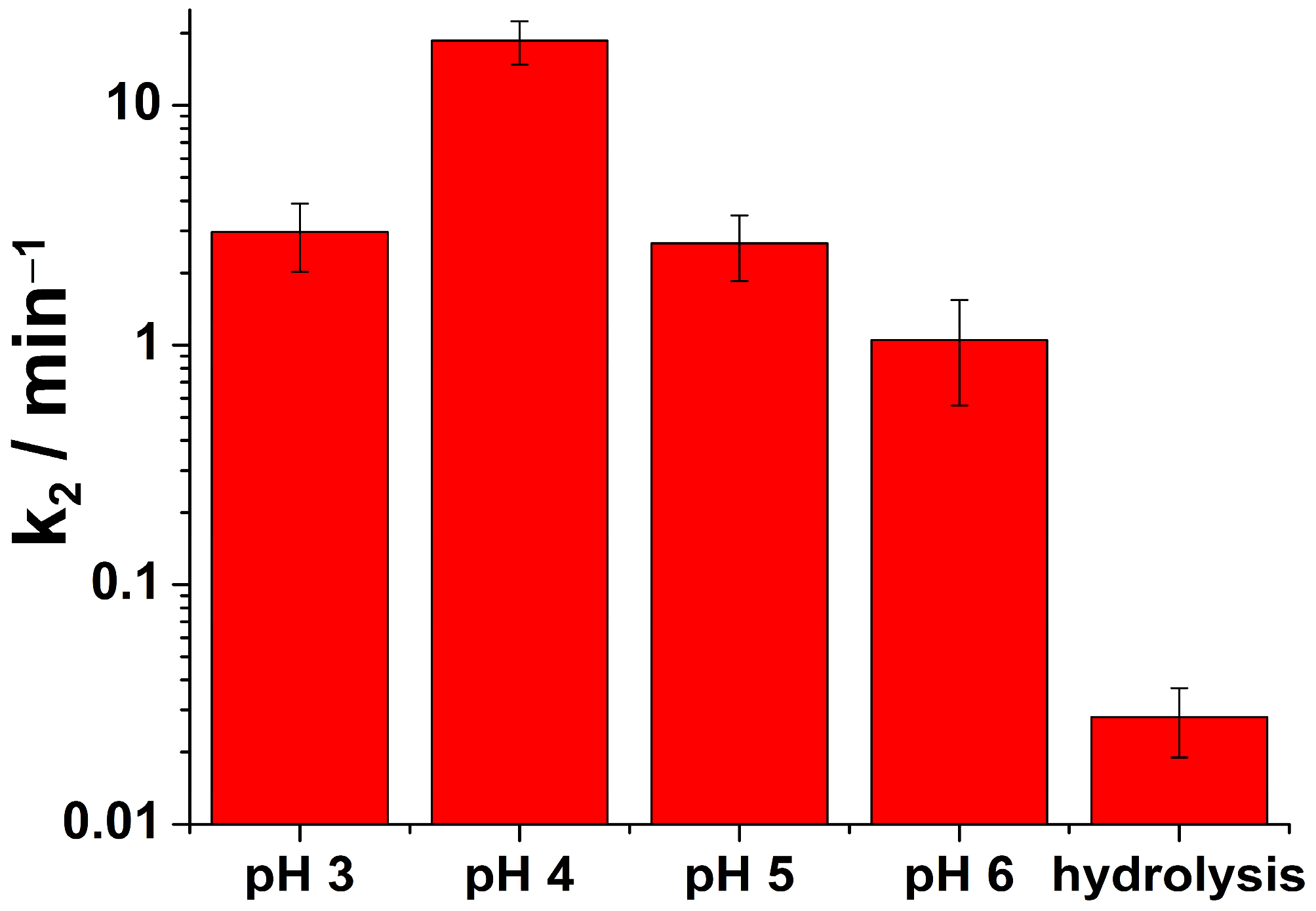
| pH | k2 (min−1) | χ2 | Adjusted R-Squared |
|---|---|---|---|
| 3 | 2.961 ± 0.942 | 6.48 × 10−4 | 0.997 |
| 4 | 18.596 ± 3.750 | 1.16 × 10−5 | 0.999 |
| 5 | 2.658 ± 0.808 | 6.87 × 10−4 | 0.996 |
| 6 | 1.054 ± 0.498 | 4.59 × 10−3 | 0.975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labaška, M.; Gál, M.; Mackuľak, T. Degradation of Chemical Warfare Agent Nitrogen Mustard Using Ferrate (VI). Toxics 2023, 11, 559. https://doi.org/10.3390/toxics11070559
Labaška M, Gál M, Mackuľak T. Degradation of Chemical Warfare Agent Nitrogen Mustard Using Ferrate (VI). Toxics. 2023; 11(7):559. https://doi.org/10.3390/toxics11070559
Chicago/Turabian StyleLabaška, Miroslav, Miroslav Gál, and Tomáš Mackuľak. 2023. "Degradation of Chemical Warfare Agent Nitrogen Mustard Using Ferrate (VI)" Toxics 11, no. 7: 559. https://doi.org/10.3390/toxics11070559
APA StyleLabaška, M., Gál, M., & Mackuľak, T. (2023). Degradation of Chemical Warfare Agent Nitrogen Mustard Using Ferrate (VI). Toxics, 11(7), 559. https://doi.org/10.3390/toxics11070559