Short- and Medium-Chain Chlorinated Paraffins in the Sediment of the East China Sea and Yellow Sea: Distribution, Composition, and Ecological Risks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Chemical Standards
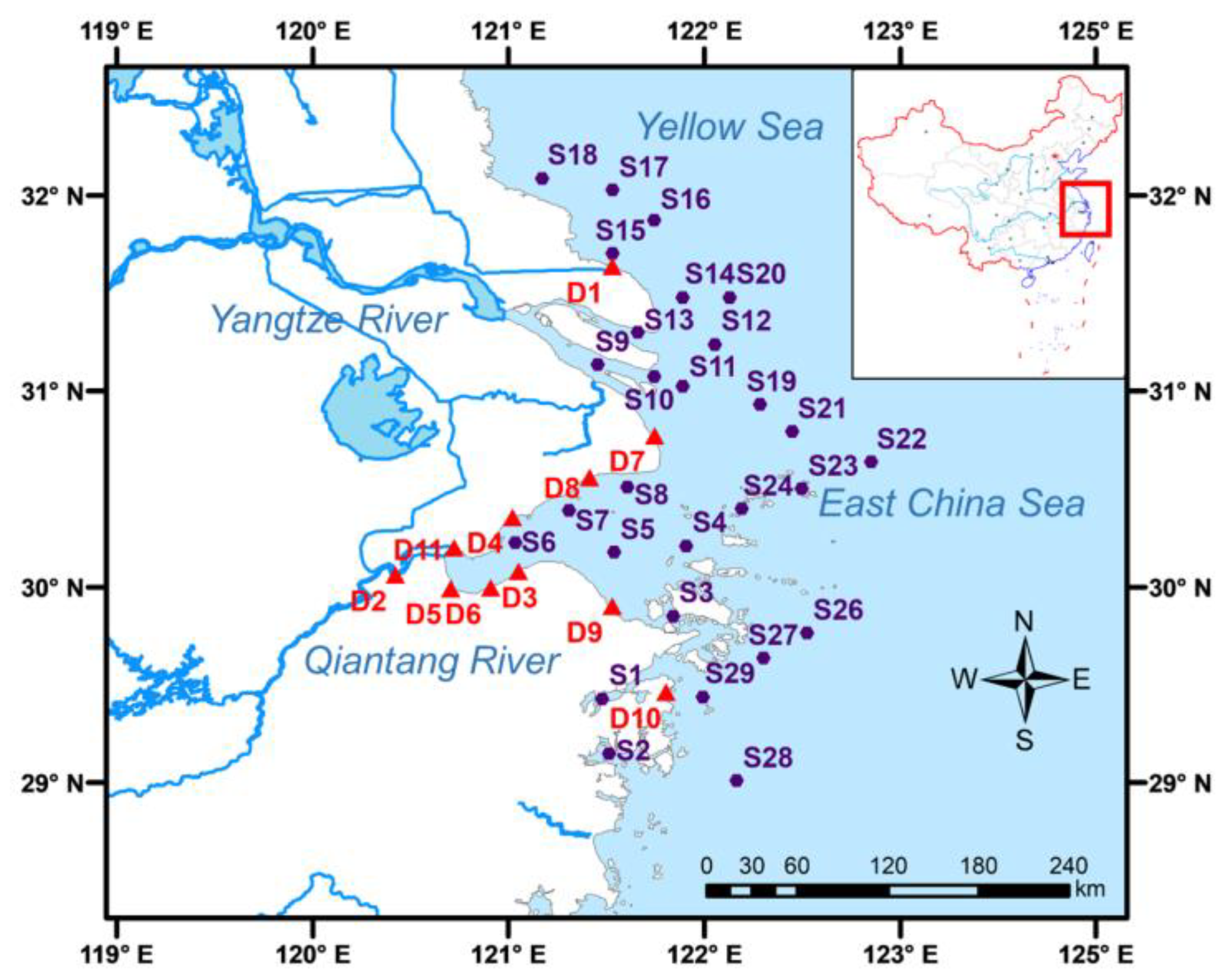
2.2. Studied Area and Sample Collection
2.3. Sample Extraction and Cleanup
2.4. Instrumental Analysis
2.5. Quality Assurance and Quality Control
2.6. Data Analysis
3. Results and Discussion
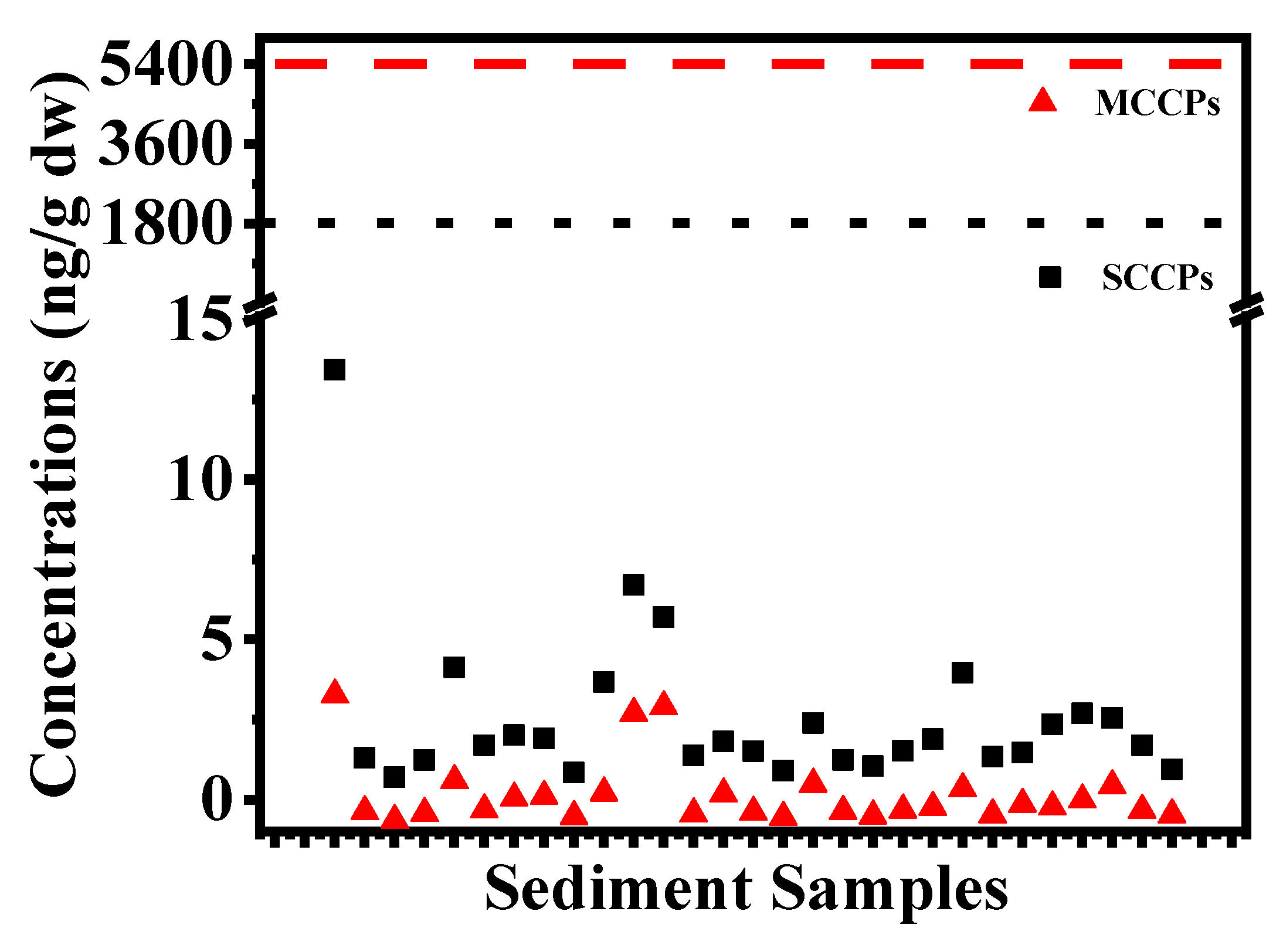
3.1. Concentrations and Profiles of SCCPs and MCCPs in Sediments
3.2. Congener Compositional Profiles, Sources, and Environmental Behaviors of SCCPs and MCCPs in Sediments
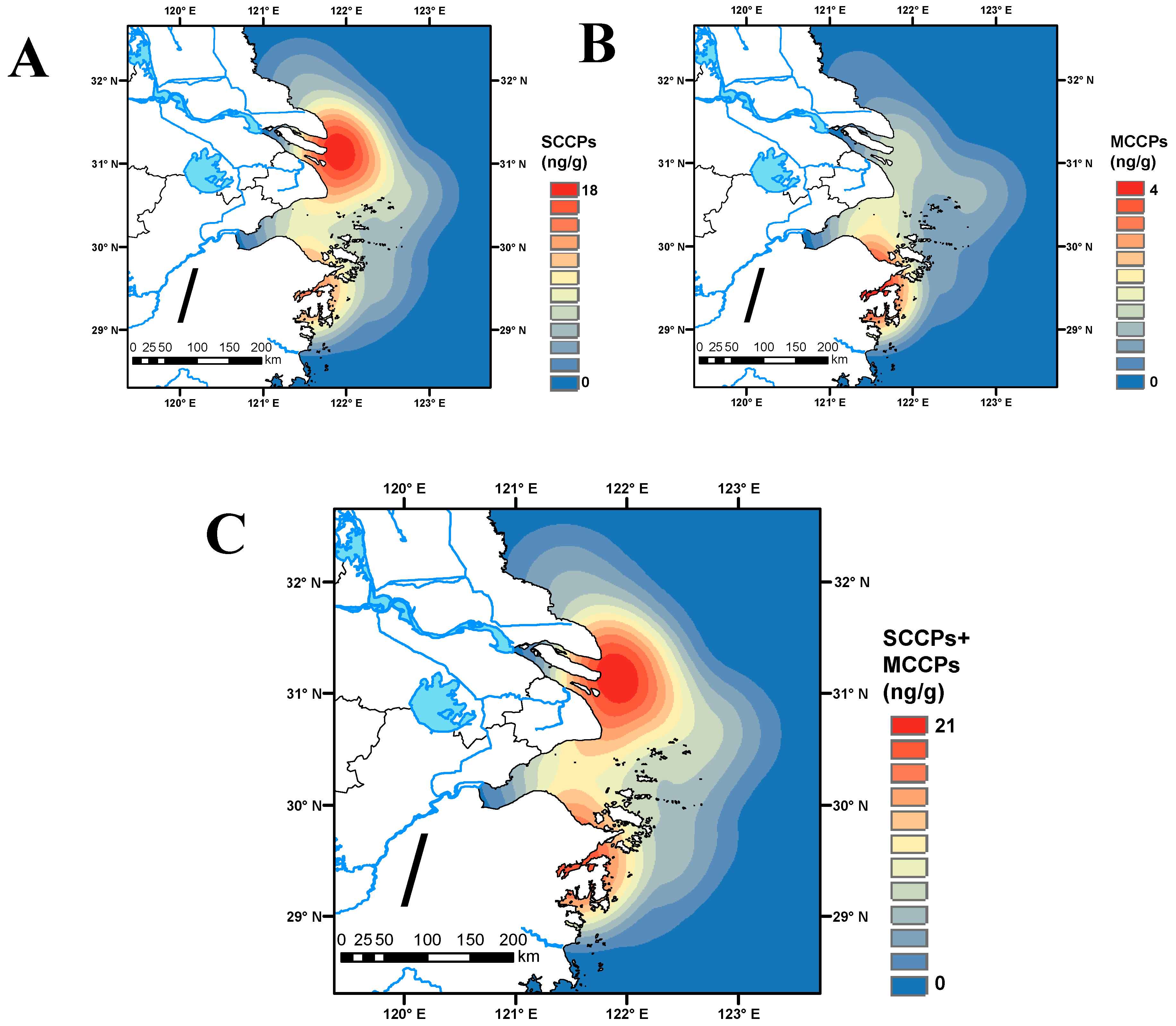
3.3. Spatial Distribution of SCCPs and MCCPs in the Sediments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bayen, S.; Obbard, J.P.; Thomas, G.O. Chlorinated paraffins: A review of analysis and environmental occurrence. Environ. Int. 2006, 32, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Feo, M.L.; Eljarrat, E.; Barcelo, D. Occurrence, fate and analysis of polychlorinated n-alkanes in the environment. Trac-Trends Anal. Chem. 2009, 28, 778–791. [Google Scholar] [CrossRef]
- Zhan, F.Q.; Zhang, H.J.; Wang, J.; Xu, J.Z.; Yuan, H.P.; Gao, Y.; Su, F.; Chen, J.P. Release and Gas-Particle Partitioning Behaviors of Short-Chain Chlorinated Paraffins (SCCPs) During the Thermal Treatment of Polyvinyl Chloride Flooring. Environ. Sci. Technol. 2017, 51, 9005–9012. [Google Scholar] [CrossRef] [PubMed]
- Gluge, J.; Schinkel, L.; Hungerbuhler, K.; Cariou, R.; Bogdal, C. Environmental Risks of Medium-Chain Chlorinated Paraffins (MCCPs): A Review. Environ. Sci. Technol. 2018, 52, 6743–6760. [Google Scholar] [CrossRef]
- Zhao, N.; Cui, Y.; Wang, P.W.; Li, S.S.; Jiang, W.; Luo, N.N.; Wang, Z.H.; Chen, X.F.; Ding, L. Short-chain chlorinated paraffins in soil, sediment, and seawater in the intertidal zone of Shandong Peninsula, China: Distribution and composition. Chemosphere 2019, 220, 452–458. [Google Scholar] [CrossRef]
- Hu, H.M.; Qu, J.L.; Zhao, M.R.; Wu, P.F.; Zhu, W.N.; Zhou, Y.D.; Jin, H.B. Bioaccumulation and trophic magnification of short chain chlorinated paraffins in marine organisms from East China Sea. Mar. Pollut. Bull. 2021, 173, 113049. [Google Scholar] [CrossRef]
- Zeng, L.X.; Chen, R.; Zhao, Z.S.; Wang, T.; Gao, Y.; Li, A.; Wang, Y.W.; Jiang, G.B.; Sun, L.G. Spatial Distributions and Deposition Chronology of Short Chain Chlorinated Paraffins in Marine Sediments across the Chinese Bohai and Yellow Seas. Environ. Sci. Technol. 2013, 47, 11449–11456. [Google Scholar] [CrossRef]
- Zeng, L.X.; Zhao, Z.S.; Li, H.J.; Wang, T.; Liu, Q.; Xiao, K.; Du, Y.G.; Wang, Y.W.; Jiang, G.B. Distribution of Short Chain Chlorinated Paraffins in Marine Sediments of the East China Sea: Influencing Factors, Transport and Implications. Environ. Sci. Technol. 2012, 46, 9898–9906. [Google Scholar] [CrossRef]
- Zeng, L.X.; Wang, T.; Wang, P.; Liu, Q.; Han, S.L.; Yuan, B.; Zhu, N.L.; Wang, Y.W.; Jiang, G.B. Distribution and Trophic Transfer of Short-Chain Chlorinated Paraffins in an Aquatic Ecosystem Receiving Effluents from a Sewage Treatment Plant. Environ. Sci. Technol. 2011, 45, 5529–5535. [Google Scholar] [CrossRef]
- Wang, H.P.; Chang, H.; Zhang, C.X.; Wu, F.C. Occurrence and mass balance of medium- and long-chain chlorinated paraffins in a municipal sewage treatment plant: Comparison to short-chain compounds. Environ. Int. 2019, 133, 105273. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.J.; Su, F.; Tian, Y.Z.; Chen, J.P. Environmental Occurrence and Distribution of Short Chain Chlorinated Paraffins in Sediments and Soils from the Liaohe River Basin, P.R. China. Environ. Sci. Technol. 2012, 46, 3771–3778. [Google Scholar] [CrossRef]
- Pan, X.H.; Tang, J.H.; Tian, C.G.; Li, J.; Zhang, G. Short- and medium-chain chlorinated paraffins in sediments from the Laizhou Bay area, North China: Implications for transportation from rivers to marine environment. Environ. Pollut. 2018, 243, 1460–1468. [Google Scholar] [CrossRef]
- Ji, B.J.; Wu, Y.; Liang, Y.; Gao, S.T.; Zeng, X.Y.; Yao, P.; Yu, Z.Q. Occurrence, congener patterns, and potential ecological risk of chlorinated paraffins in sediments of Yangtze River Estuary and adjacent East China Sea. Environ. Monit. Assess. 2022, 194, 329. [Google Scholar] [CrossRef] [PubMed]
- Hussy, I.; Webster, L.; Russell, M.; Moffat, C. Determination of chlorinated paraffins in sediments from the Firth of Clyde by gas chromatography with electron capture negative ionisation mass spectrometry and carbon skeleton analysis by gas chromatography with flame ionisation detection. Chemosphere 2012, 88, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, X.; Liang, B.W.; Deng, M.; Du, B.B.; Zeng, L.X. Spatial distribution, homologue patterns and ecological risks of chlorinated paraffins in mangrove sediments along the South China Coast. Environ. Pollut. 2022, 294, 118623. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Li, H.J.; Wang, Y.W.; Li, G.L.; Cao, Y.L.; Zeng, L.X.; Lan, J.; Wang, T.; Jiang, G.B. Source and Migration of Short-Chain Chlorinated Paraffins in the Coastal East China Sea Using Multiproxies of Marine Organic Geochemistry. Environ. Sci. Technol. 2013, 47, 5013–5022. [Google Scholar] [CrossRef]
- Hu, H.M.; Jin, H.B.; Li, T.J.; Guo, Y.M.; Wu, P.F.; Xu, K.D.; Zhu, W.B.; Zhou, Y.Q.; Zhao, M.R. Spatial distribution, partitioning, and ecological risk of short chain chlorinated paraffins in seawater and sediment from East China Sea. Sci. Total Environ. 2022, 811, 151932. [Google Scholar] [CrossRef]
- Chen, M.Y.; Luo, X.J.; Zhang, X.L.; He, M.J.; Chen, S.J.; Mi, B.X. Chlorinated Paraffins in Sediments from the Pearl River Delta, South China: Spatial and Temporal Distributions and Implication for Processes. Environ. Sci. Technol. 2011, 45, 9936–9943. [Google Scholar] [CrossRef] [PubMed]
- Canadian Environmental Protection Act. Federal Environmental Quality Guidelines Chlorinated Alkanes, May 2018; Canadian Environmental Protection Act: Toronto, ON, Canada, 1999. [Google Scholar]
- Ma, X.D.; Zhang, H.J.; Wang, Z.; Yao, Z.W.; Chen, J.W.; Chen, J.P. Bioaccumulation and Trophic Transfer of Short Chain Chlorinated Paraffins in a Marine Food Web from Liaodong Bay, North China. Environ. Sci. Technol. 2014, 48, 5964–5971. [Google Scholar] [CrossRef]
- Qiao, L.; Xia, D.; Gao, L.R.; Huang, H.T.; Zheng, M.H. Occurrences, sources and risk assessment of short- and medium-chain chlorinated paraffins in sediments from the middle reaches of the Yellow River, China. Environ. Pollut. 2016, 219, 483–489. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Zhou, Y.; Wu, C.; Kanchanopas-Barnette, P. Distribution and source apportionment of polycyclic aromatic hydrocarbons in surface sediments from Zhoushan Archipelago and Xiangshan Harbor, East China Sea. Mar. Pollut. Bull. 2015, 101, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H.; Zhou, Y.; Wu, C.; Kanchanopas-Barnette, P. Spatial distribution and sources of polychlorinated biphenyls in surface sediments from the Zhoushan Archipelago and Xiangshan Harbor, East China Sea. Mar. Pollut. Bull. 2016, 105, 385–392. [Google Scholar] [CrossRef] [PubMed]
| Samples | SCCPs (ng/g dw) | C10 (%) | C11 (%) | C12 (%) | C13 (%) | MCCPs (ng/g dw) | C14 (%) | C15 (%) | C16 (%) | C17 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 13.4 | 29.2 | 22.4 | 23.5 | 24.9 | 4.19 | 42.8 | 27.5 | 15.1 | 14.5 |
| S2 | 1.30 | 44.3 | 19.0 | 17.6 | 19.1 | 0.297 | 45.0 | 29.7 | 13.0 | 12.3 |
| S3 | 0.703 | 45.6 | 19.3 | 16.6 | 18.4 | 0.0936 | 43.4 | 31.9 | 12.9 | 11.7 |
| S4 | 1.24 | 42.0 | 19.2 | 18.5 | 20.3 | 0.152 | 56.7 | 24.4 | 10.3 | 8.62 |
| S5 | 4.10 | 35.4 | 14.0 | 17.5 | 33.1 | 1.75 | 69.1 | 20.7 | 4.64 | 5.51 |
| S6 | 1.70 | 36.9 | 28.4 | 18.0 | 16.6 | 0.333 | 43.9 | 24.3 | 16.6 | 15.3 |
| S7 | 2.01 | 47.8 | 21.2 | 15.3 | 15.6 | 0.253 | 52.4 | 24.9 | 11.3 | 11.4 |
| S8 | 1.92 | 52.9 | 19.4 | 12.7 | 15.0 | 0.232 | 58.7 | 24.8 | 8.18 | 8.32 |
| S9 | 0.838 | 51.5 | 23.0 | 13.4 | 12.1 | 0.971 | 44.0 | 31.6 | 14.1 | 10.3 |
| S10 | 3.68 | 30.2 | 20.9 | 31.7 | 17.3 | 0.675 | 47.7 | 18.2 | 8.72 | 25.4 |
| S11 | 6.70 | 50.7 | 20.6 | 14.0 | 14.7 | 0.611 | 55.8 | 25.0 | 8.51 | 10.7 |
| S12 | 5.71 | 62.9 | 24.5 | 8.26 | 4.25 | 0.110 | 38.3 | 40.0 | 14.0 | 7.68 |
| S13 | 1.38 | 37.0 | 31.6 | 12.2 | 19.3 | 0.164 | 42.1 | 28.5 | 18.0 | 11.4 |
| S14 | 1.82 | 59.8 | 24.5 | 6.95 | 8.81 | 0.121 | 38.4 | 29.6 | 12.0 | 20.0 |
| S15 | 1.52 | 36.7 | 27.5 | 18.9 | 16.9 | 0.212 | 48.7 | 23.4 | 15.2 | 12.7 |
| S16 | 0.903 | 45.7 | 26.1 | 14.0 | 14.2 | 0.108 | 49.4 | 25.7 | 15.0 | 9.85 |
| S17 | 2.39 | 57.2 | 27.3 | 8.11 | 7.38 | 0.109 | 42.4 | 35.1 | 15.2 | 7.32 |
| S18 | 1.24 | 47.0 | 19.7 | 12.8 | 20.5 | 0.357 | 62.9 | 24.0 | 6.97 | 6.08 |
| S19 | 1.04 | 43.5 | 29.0 | 14.4 | 13.1 | 0.115 | 37.1 | 33.8 | 17.6 | 11.5 |
| S20 | 1.52 | 40.8 | 29.7 | 15.3 | 14.2 | 0.133 | 38.1 | 29.2 | 20.4 | 12.3 |
| S21 | 1.89 | 37.1 | 28.2 | 18.0 | 16.7 | 0.190 | 54.3 | 23.0 | 12.9 | 9.84 |
| S22 | 3.97 | 31.0 | 19.3 | 15.6 | 34.1 | 0.941 | 69.0 | 20.1 | 5.97 | 4.94 |
| S23 | 1.34 | 36.1 | 26.3 | 19.4 | 18.1 | 0.208 | 51.8 | 24.6 | 13.1 | 10.6 |
| S24 | 1.47 | 53.7 | 24.2 | 11.0 | 11.2 | 0.0960 | 48.8 | 29.2 | 11.7 | 10.3 |
| S25 | 2.35 | 30.1 | 13.0 | 14.3 | 42.7 | 0.873 | 73.4 | 18.2 | 4.07 | 4.35 |
| S26 | 2.69 | 33.7 | 25.5 | 19.0 | 21.8 | 0.536 | 63.3 | 19.6 | 9.45 | 7.60 |
| S27 | 2.56 | 51.6 | 25.4 | 10.9 | 12.0 | 0.227 | 58.7 | 23.1 | 10.5 | 7.66 |
| S28 | 1.69 | 36.1 | 30.1 | 16.4 | 17.5 | 0.240 | 47.8 | 26.9 | 14.7 | 10.6 |
| S29 | 0.945 | 51.5 | 20.7 | 13.5 | 14.3 | 0.140 | 53.9 | 26.3 | 9.75 | 10.0 |
| Range | 0.703–13.4 | 29.2–62.9 | 13.0–31.6 | 6.95–31.7 | 4.25–42.7 | 0.0936–4.19 | 37.1–73.4 | 18.2–40.0 | 4.07–20.4 | 4.35–25.4 |
| Median | 1.70 | 43.5 | 24.2 | 15.3 | 16.7 | 0.212 | 48.8 | 25.0 | 12.9 | 10.3 |
| GM | 1.96 | 42.4 | 23.0 | 14.7 | 16.2 | 0.259 | 50.1 | 25.9 | 11.3 | 9.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Guo, H.; Hong, J.; Gao, Y.; Ma, X.; Chen, J. Short- and Medium-Chain Chlorinated Paraffins in the Sediment of the East China Sea and Yellow Sea: Distribution, Composition, and Ecological Risks. Toxics 2023, 11, 558. https://doi.org/10.3390/toxics11070558
Li X, Guo H, Hong J, Gao Y, Ma X, Chen J. Short- and Medium-Chain Chlorinated Paraffins in the Sediment of the East China Sea and Yellow Sea: Distribution, Composition, and Ecological Risks. Toxics. 2023; 11(7):558. https://doi.org/10.3390/toxics11070558
Chicago/Turabian StyleLi, Xiaoying, Haiqiang Guo, Jianyao Hong, Yuan Gao, Xindong Ma, and Jiping Chen. 2023. "Short- and Medium-Chain Chlorinated Paraffins in the Sediment of the East China Sea and Yellow Sea: Distribution, Composition, and Ecological Risks" Toxics 11, no. 7: 558. https://doi.org/10.3390/toxics11070558
APA StyleLi, X., Guo, H., Hong, J., Gao, Y., Ma, X., & Chen, J. (2023). Short- and Medium-Chain Chlorinated Paraffins in the Sediment of the East China Sea and Yellow Sea: Distribution, Composition, and Ecological Risks. Toxics, 11(7), 558. https://doi.org/10.3390/toxics11070558






