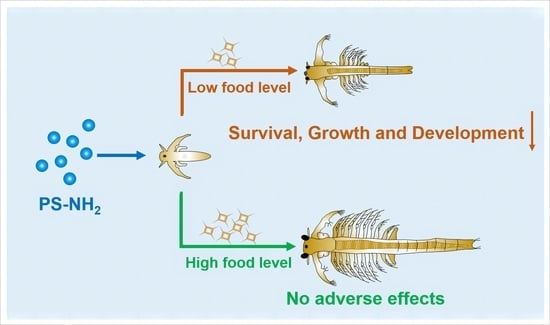
Long-Term Toxicity of 50-nm and 1-μm Surface-Charged Polystyrene Microbeads in the Brine Shrimp Artemia parthenogenetica and Role of Food Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization and Preparation of PS-NH2
2.2. Experimental Organisms
2.3. Experimental Design
2.4. Statistical Analyses
3. Results
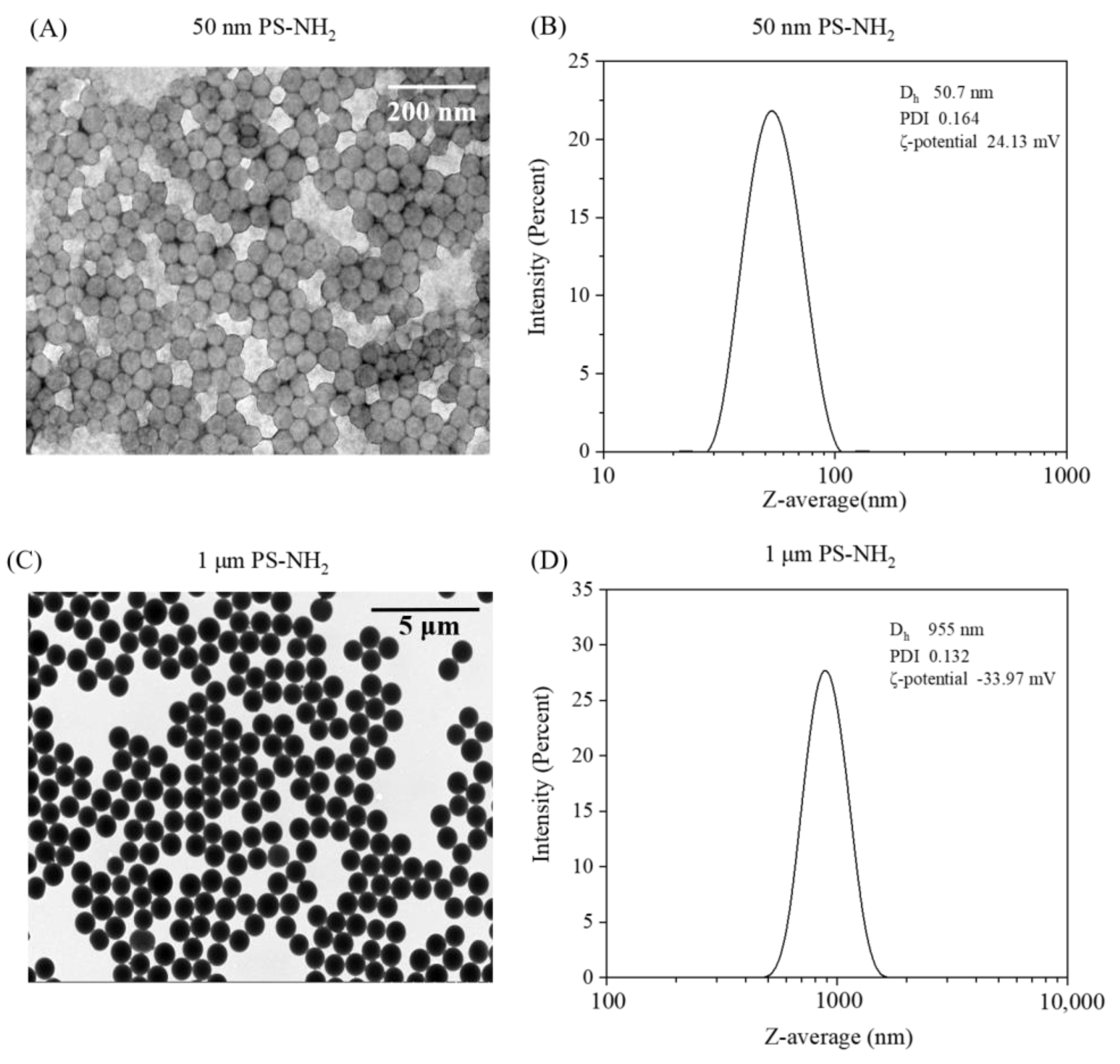
3.1. PS-NH2 Characterization
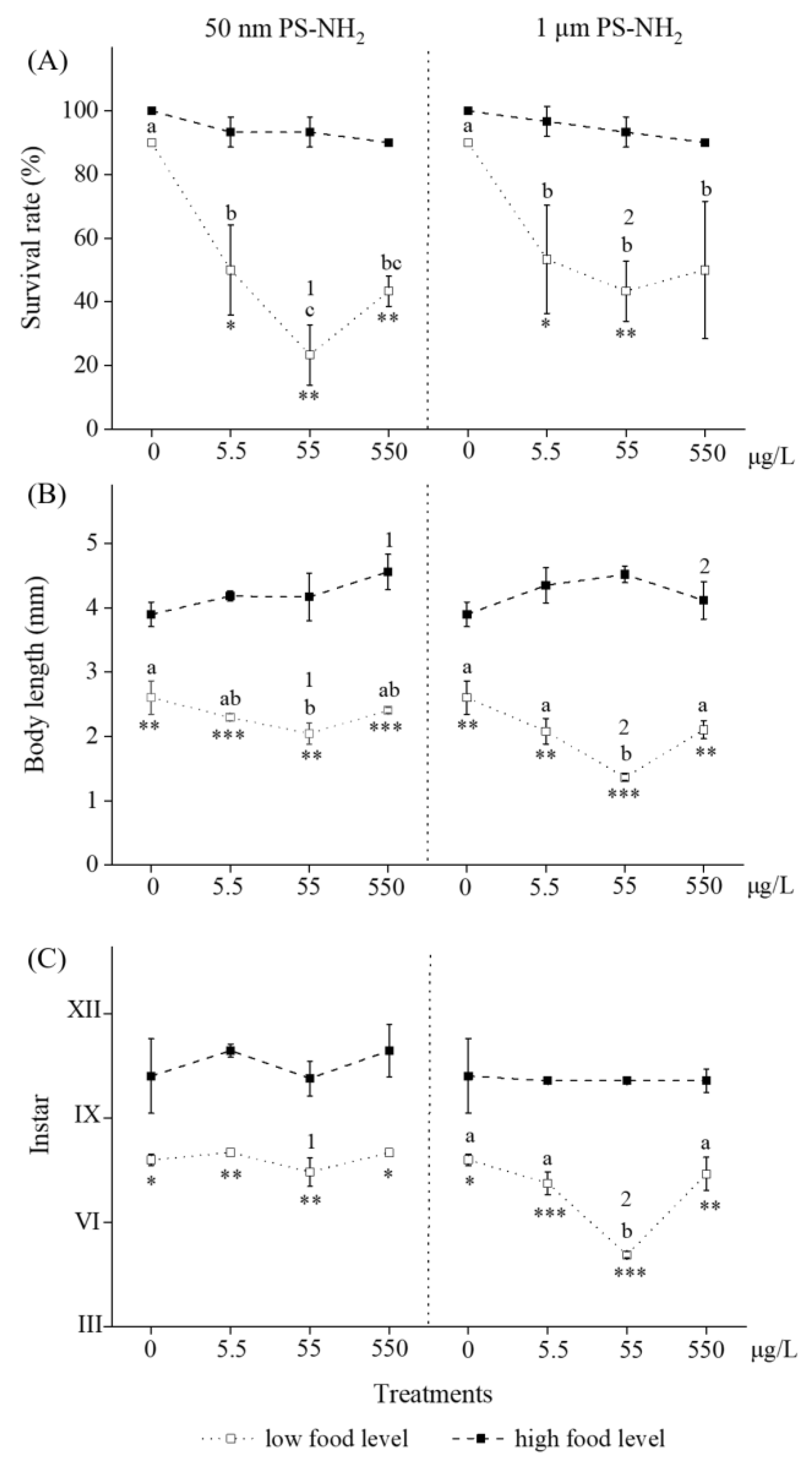
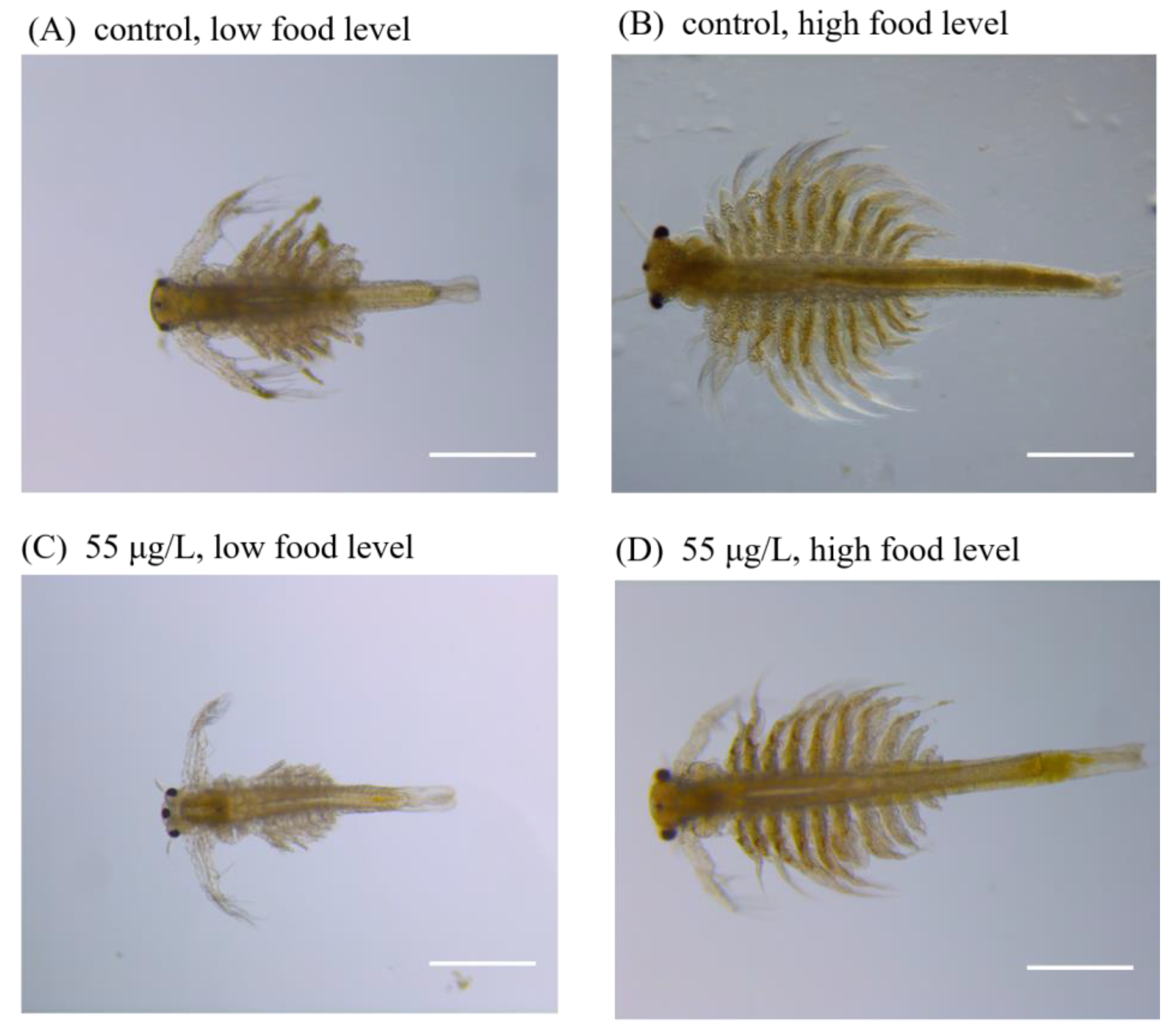
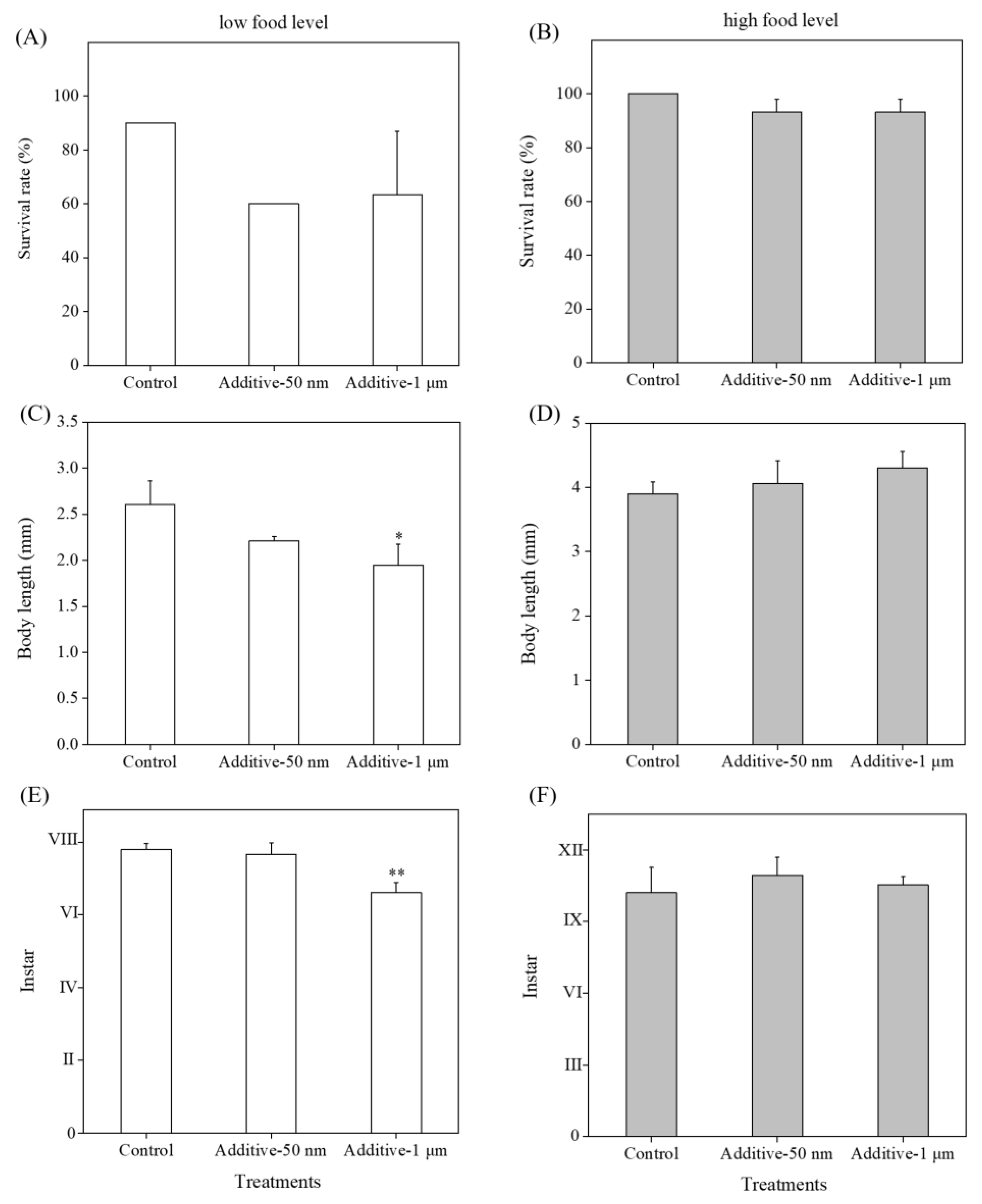
3.2. Long-Term Effects of 50-nm and 1-μm PS-NH2 on Survival, Growth and Development of Brine Shrimp by Providing Low Food Level
3.3. Long-Term Effects of 50-nm and 1-μm PS-NH2 on Survival, Growth and Development of Brine Shrimp by Providing High Food Level
3.4. Effects of Food Level on Long-Term Toxicity of Selected MNPs
3.5. Effects of Additives in Plastic Commercial Formulations on Toxicity
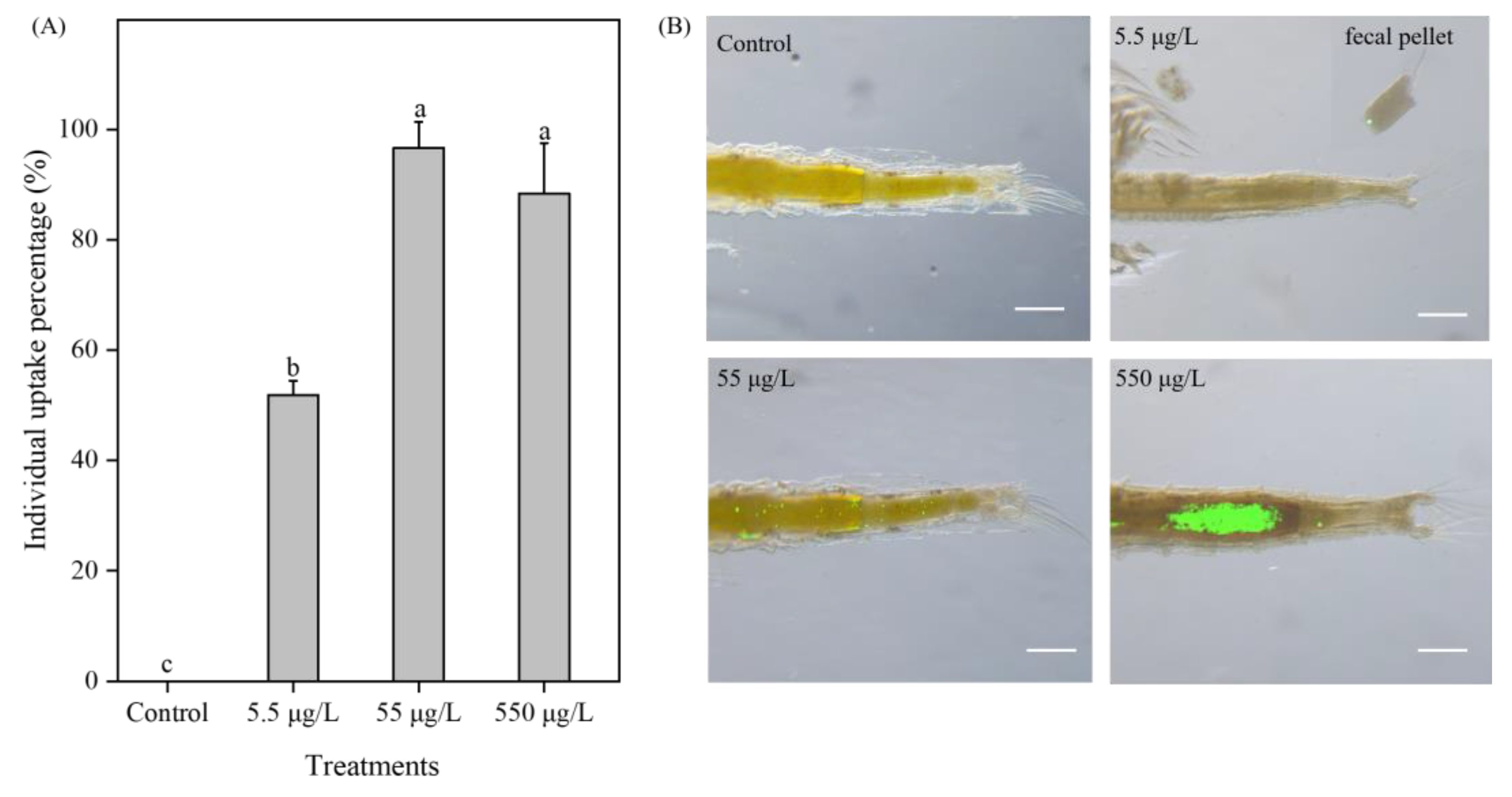
3.6. Microplastic Uptake by Brine Shrimp upon Exposures to 1 μm PS-NH2 When Providing High Food Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthews, S.; Mai, L.; Jeong, C.-B.; Lee, J.-S.; Zeng, E.Y.; Xu, E.G. Key mechanisms of micro- and nanoplastic (MNP) toxicity across taxonomic groups. Comp. Biochem. Phys. C 2021, 247, 109056. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Beaumont, N.; Cole, M.; Hopkins, F.E.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability of microplastics to marine zooplankton: Effect of shape and infochemicals. Environ. Sci. Technol. 2020, 54, 12024–12033. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Shim, W.J.; Kwon, O.Y.; Kang, J.-H. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Soto, N.; Hatfield, J.; Katsumiti, A.; Duroudier, N.; Lacave, J.M.; Bilbao, E.; Orbea, A.; Navarro, E.; Cajaraville, M.P. Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis. Sci. Total Environ. 2019, 684, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, B.; Jong, M.C.; Ma, C.; Zuo, C.; Chen, Q.; Shi, H. Process-oriented impacts of microplastic fibers on behavior and histology of fish. J. Hazard Mater. 2023, 448, 130856. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, H.; Liu, Y.; Zhan, J.; Li, W.; Yang, K.; Yi, X. Acute and chronic combined effect of polystyrene microplastics and dibutyl phthalate on the marine copepod Tigriopus japonicus. Chemosphere 2020, 261, 127711. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Sun, X.; Liang, J.; Zhu, M.; Zhao, Y.; Zhang, B. Microplastics in seawater and zooplankton from the Yellow Sea. Environ. Pollut. 2018, 242, 585–595. [Google Scholar] [CrossRef]
- Yu, J.; Tian, J.Y.; Xu, R.; Zhang, Z.Y.; Yang, G.P.; Wang, X.D.; Lai, J.G.; Chen, R. Effects of microplastics exposure on ingestion, fecundity, development, and dimethylsulfide production in Tigriopus japonicus (Harpacticoida, copepod). Environ. Pollut. 2020, 267, 115429. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Zhang, M.; Ding, G.; Sun, J.; Du, M.; Cong, Y.; Jin, F.; Zhang, W.; Wang, J. The uptake and elimination of polystyrene microplastics by the brine shrimp, Artemia parthenogenetica, and its impact on its feeding behavior and intestinal histology. Chemosphere 2019, 234, 123–131. [Google Scholar] [CrossRef]
- Zhang, C.; Jeong, C.B.; Lee, J.S.; Wang, D.; Wang, M. Transgenerational proteome plasticity in resilience of a marine copepod in response to environmentally relevant concentrations of microplastics. Environ. Sci. Technol. 2019, 53, 8426–8436. [Google Scholar] [CrossRef]
- Bergami, E.; Bocci, E.; Vannuccini, M.L.; Monopoli, M.; Salvati, A.; Dawson, K.A.; Corsi, I. Nano-sized polystyrene affects feeding, behavior and physiology of brine shrimp Artemia franciscana larvae. Ecotoxicol. Environ. Saf. 2016, 123, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Botterell, Z.L.R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.H.; Sun, Z.X.; Feng, L.S.; Jin, T.; Xing, J.C.; Wen, X.L. Algal density affects the influences of polyethylene microplastics on the freshwater rotifer Brachionus calyciflorus. Chemosphere 2021, 270, 128613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Zhang, M.; Mu, J.; Ding, G.; Mao, Z.; Cao, Y.; Jin, F.; Cong, Y.; Wang, L.; et al. Effects of ingested polystyrene microplastics on brine shrimp, Artemia parthenogenetica. Environ. Pollut. 2019, 244, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Bergami, E.; Pugnalini, S.; Vannuccini, M.L.; Manfra, L.; Faleri, C.; Savorelli, F.; Dawson, K.A.; Corsi, I. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana. Aquat. Toxicol. 2017, 189, 159–169. [Google Scholar] [CrossRef]
- Materić, D.; Holzinger, R.; Niemann, H. Nanoplastics and ultrafine microplastic in the Dutch Wadden Sea—The hidden plastics debris? Sci. Total Environ. 2022, 846, 157371. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Wang, D.; Ru, S.; Zhang, W.; Zhang, Z.; Li, Y.; Zhao, L.; Li, L.; Wang, J. Impacts of nanoplastics on life-history traits of marine rotifer (Brachionus plicatilis) are recovered after being transferred to clean seawater. Environ. Sci. Pollut. Res. Int. 2022, 29, 42780–42791. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.D.; Weinstein, J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Nielsen, T.G. Microplastic exposure studies should be environmentally realistic. Proc. Natl. Acad. Sci. USA 2016, 113, E4121–E4122. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Ding, G.; Shi, H.; Cong, Y.; Li, Z.; Wang, J. Polystyrene microplastics alleviate adverse effects of benzo[a]pyrene on tissues and cells of the marine mussel, Mytilus galloprovincialis. Aquat. Toxicol. 2023, 256, 106430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Tang, Y.; Li, A.; Liu, C.; Xie, C.; Xiao, L.; Lu, S. Phytoplankton community and HAB species in the South China Sea detected by morphological and metabarcoding approaches. Harmful Algae 2022, 118, 102297. [Google Scholar] [CrossRef] [PubMed]
- Varg, J.E.; Kunce, W.; Outomuro, D.; Svanback, R.; Johansson, F. Single and combined effects of microplastics, pyrethroid and food resources on the life-history traits and microbiome of Chironomus riparius. Environ. Pollut. 2021, 289, 117848. [Google Scholar] [CrossRef]
- Hoss, S.; Rauchschwalbe, M.T.; Fueser, H.; Traunspurger, W. Food availability is crucial for effects of 1-μm polystyrene beads on the nematode Caenorhabditis elegans in freshwater sediments. Chemosphere 2022, 298, 134101. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, N.; Wang, M. Effects of microplastics on marine copepods. Ecotoxicol. Environ. Saf. 2021, 217, 112243. [Google Scholar] [CrossRef]
- Pikuda, O.; Xu, E.G.; Berk, D.; Tufenkji, N. Toxicity assessments of micro- and nanoplastics can be confounded by preservatives in commercial formulations. Environ. Sci. Technol. Lett. 2019, 6, 21–25. [Google Scholar] [CrossRef]
| Factors | MSE | F | p |
|---|---|---|---|
| Survival rate | |||
| C | 0.233 | F(3,32) = 20.000 | <0.001 * |
| F | 1.841 | F(1,32) = 157.786 | <0.001 * |
| S | 0.021 | F(1,32) = 1.786 | 0.191 |
| C *F | 0.132 | F(3,32) = 11.310 | <0.001 * |
| C *S | 0.005 | F(3,32) = 0.452 | 0.717 |
| F *S | 0.013 | F(1,32) = 1.143 | 0.293 |
| C *F *S | 0.007 | F(3,32) = 0.571 | 0.638 |
| Body length | |||
| C | 0.173 | F(3,32) = 2.657 | 0.065 |
| F | 49.066 | F(1,32) = 753.944 | <0.001 * |
| S | 0.239 | F(1,32) = 3.675 | 0.064 |
| C *F | 0.917 | F(3,32) = 14.095 | <0.001 * |
| C *S | 0.087 | F(3,32) = 1.341 | 0.279 |
| F *S | 0.305 | F(1,32) = 4.686 | 0.038 |
| C *F *S | 0.206 | F(3,32) = 3.170 | 0.038 |
| Instar | |||
| C | 2.264 | F(3,32) = 6.272 | 0.002 * |
| F | 107.786 | F(1,32) = 298.604 | <0.001 * |
| S | 6.017 | F(1,32) = 16.670 | <0.001 * |
| C *F | 1.115 | F(3,32) = 3.088 | 0.041 * |
| C *S | 0.793 | F(3,32) = 2.198 | 0.107 |
| F *S | 0.800 | F(1,32) = 2.215 | 0.146 |
| C *F *S | 1.085 | F(3,32) = 3.006 | 0.045 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zhang, M.; Li, Z.; Cao, S.; Lou, Y.; Cong, Y.; Jin, F.; Wang, Y. Long-Term Toxicity of 50-nm and 1-μm Surface-Charged Polystyrene Microbeads in the Brine Shrimp Artemia parthenogenetica and Role of Food Availability. Toxics 2023, 11, 356. https://doi.org/10.3390/toxics11040356
Shen Y, Zhang M, Li Z, Cao S, Lou Y, Cong Y, Jin F, Wang Y. Long-Term Toxicity of 50-nm and 1-μm Surface-Charged Polystyrene Microbeads in the Brine Shrimp Artemia parthenogenetica and Role of Food Availability. Toxics. 2023; 11(4):356. https://doi.org/10.3390/toxics11040356
Chicago/Turabian StyleShen, Yu, Mingxing Zhang, Zhaochuan Li, Shuo Cao, Yadi Lou, Yi Cong, Fei Jin, and Ying Wang. 2023. "Long-Term Toxicity of 50-nm and 1-μm Surface-Charged Polystyrene Microbeads in the Brine Shrimp Artemia parthenogenetica and Role of Food Availability" Toxics 11, no. 4: 356. https://doi.org/10.3390/toxics11040356
APA StyleShen, Y., Zhang, M., Li, Z., Cao, S., Lou, Y., Cong, Y., Jin, F., & Wang, Y. (2023). Long-Term Toxicity of 50-nm and 1-μm Surface-Charged Polystyrene Microbeads in the Brine Shrimp Artemia parthenogenetica and Role of Food Availability. Toxics, 11(4), 356. https://doi.org/10.3390/toxics11040356