Enzymatic Assessment of the State of Oil-Contaminated Soils in the South of Russia after Bioremediation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Buluktaev, A.A. Phytotoxicity and Enzymatic Activity of Kalmykian Soils under Oil Pollution. South Russ. Ecol. Dev. 2017, 12, 147–156. [Google Scholar] [CrossRef]
- Kuzina, A.A.; Kolesnikov, S.I.; Minnikova, T.V.; Gaivoronskii, V.G.; Nevedomaya, E.N.; Ter-Misakyants, T.A.; Kazeev, K.S. Ecologically safe concentrations of oil in the soils of the Black Sea coast of the Caucasus. Ecol. Ind. Russ. 2021, 25, 61–65. (In Russian) [Google Scholar] [CrossRef]
- Dindar, E.; Şağban, F.O.T.; Başkaya, H.S. Variations of soil enzyme activities in petroleum-hydrocarbon contaminated soil. Int. Biodeterior. Biodegrad. 2015, 105, 268–275. [Google Scholar] [CrossRef]
- Minnikova, T.V.; Kolesnikov, S.I.; Kazeev, K.S. Impact of ameliorants on the biological condition of oil-contaminated black soil. Soil Env. 2019, 38, 170–180. [Google Scholar] [CrossRef]
- Minnikova, T.; Kolesnikov, S.; Minkina, T.; Mandzhieva, S. Assessment of Ecological Condition of Haplic Chernozem Calcic Contaminated with Petroleum Hydrocarbons during Application of Bioremediation Agents of Various Natures. Land 2021, 10, 169. [Google Scholar] [CrossRef]
- Minnikova, T.; Kolesnikov, S.; Ruseva, A.; Kazeev, K.; Minkina, T.; Mandzhieva, S.; Sushkova, S. Influence of the Biochar on Petroleum Hydrocarbon Degradation Intensity and Ecological Condition of Haplic Chernozem. Eurasian J. Soil Sci. 2022, 11, 157–166. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N.; Trofimov, S.Y.; Shoba, S.A. Promising approaches to the purification of soils and groundwater from hydrocarbons (a review). Eurasian Soil Sci. 2016, 49, 705–713. [Google Scholar] [CrossRef]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of Petroleum-Contaminated Soils with Microbial and Microbial Combined Methods: Advances, Mechanisms, and Challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, M. Bioremediation of Petroleum-Contaminated Soil. Environ. Chem. Recent Pollut. Control. Approaches 2020, 34, 161–172. [Google Scholar]
- Cui, J.Q.; He, Q.S.; Liu, M.H.; Chen, H.; Sun, M.B.; Wen, J.P. Comparative Study on Different Remediation Strategies Applied in Petroleum-Contaminated Soils. Int. J. Environ. Res. Public Health 2020, 17, 1606. [Google Scholar] [CrossRef]
- Korneykova, M.; Myazin, V.; Fokina, N. Restoration of Oil-Contaminated Soils in Mountain Tundra (Murmansk Region, Russia). In Systems Thinking and Moral Imagination; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Zhao, N.; Guo, J.; Xu, W.; Ma, M.; Li, X. Remediation of Crude Oil-Polluted Soil by the Bacterial Rhizosphere Community of Suaeda Salsa Revealed by 16S rRNA Genes. Int. J. Environ. Res. Public Health 2020, 17, 1471. [Google Scholar] [CrossRef] [PubMed]
- Zahed, M.A.; Salehi, S.; Madadi, R.; Hejabi, F. Biochar as a sustainable product for remediation of petroleum contaminated soil. Curr. Res. Green Sust. Chem. 2021, 4, 100055. [Google Scholar] [CrossRef]
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Choppala, G.; Kirkham, M.B.; Bolan, N.S. Rhizoremediation as a green technology for the remediation of petroleum hydrocarbon-contaminated soils. J. Hazardous Mat. 2021, 401, 123282. [Google Scholar] [CrossRef]
- Rafikova, G.F.; Kuzina, E.V.; Korshunova, T.Y. Influence of bioremediation on the biological activity of leached chernozem contaminated with oil and lead. Eurasian Soil Sci. 2022, 3, 354–369. (In Russian) [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N.; Pandey, A. Bioremediation of oily sludge polluted soil employing a novel strain of Pseudomonas aeruginosa and phytotoxicity of petroleum hydrocarbons for seed germination. Sci. Total Environ. 2020, 737, 139766. [Google Scholar] [CrossRef]
- Varjani, S.; Pandey, A.; Upasani, V.N. Petroleum sludge polluted soil remediation: Integrated approach involving novel bacterial consortium and nutrient application. Sci. Total Environ. 2021, 763, 142934. [Google Scholar] [CrossRef]
- Turov, Y.P.; Guznyaeva, M.Y.; Lazarev, D.A.; Petrova, Y.Y.; Zhdanova, G.O.; Stom, D.I. Study of the processes of sorption and removal of oil hydrocarbons from soil samples. Eurasian Soil Sci. 2022, 6, 747–758. (In Russian) [Google Scholar]
- Gałązka, A.; Grządziel, J.; Gałązka, R.; Ukalska-Jaruga, A.; Strzelecka, J.; Smreczak, B. Genetic and Functional Diversity of Bacterial Microbiome in Soils with Long Term Impacts of Petroleum Hydrocarbons. Front. Microbiol. 2018, 9, 1923. [Google Scholar] [CrossRef]
- Jain, P.K.; Gupta, V.K.; Gaur, R.K.; Lowry, M.; Jaroli, D.P.; Chauhan, U.K. Bioremediation of Petroleum Oil Contaminated Soil and Water. Res. J. Environ. Toxicol. 2011, 5, 1–26. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; p. 234. [Google Scholar]
- Terekhova, V.A.; Fedoseeva, E.V.; Panova, M.I.; Chukov, S.N. Biotesting of humic products as potential ameliorants (review). Eurasian Soil Sci. 2022, 7, 795–807. (In Russian) [Google Scholar]
- PND F 16.1: 2.2.22-98. 1998. Quantitative Chemical Analysis of Soils. Methods for Measuring the Massfraction of Petroleum Products in Mineral, Organogenic, Organomineral Soils and Bottom Sediments by IR Spectrometry. Available online: https://www.russiangost.com/p-162437-pnd-f-1612222-98.aspx (accessed on 25 January 2022).
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Khaziev, F.K. Methods of Soil Enzymology; Nauka: Moscow, Russia, 2005; p. 252. [Google Scholar]
- Kolesnikov, S.I.; Kazeev, K.S.; Akimenko, Y.V. Development of regional standards for pollutants in the soil using biological parameters. Environ. Monit. Assess. 2019, 191, 544. [Google Scholar] [CrossRef] [PubMed]
- Minnikova, T.V.; Kolesnikov, S.I.; Denisova, T.V. Influence of nitrogen and humic fertilizers on the biochemical state of oil-contaminated chernozem. South Russ. Ecol. Dev. 2019, 14, 189–201. (In Russian) [Google Scholar] [CrossRef]
- Gospodarek, J.; Rusin, M.; Barczyk, G.; Nadgórska-Socha, A. The Effect of Petroleum-Derived Substances and Their Bioremediation on Soil Enzymatic Activity and Soil Invertebrates. Agronomy 2021, 11, 80. [Google Scholar] [CrossRef]
- Eremin, D.M.; Eremina, D.V. Influence of Granulometric Composition Structure of Anthropogenic—Reformed Soil on Ecology of Infrastructure. Proc. Eng. 2016, 165, 788–793. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil—A field study. Int. Biodeterior. Biodegrad. 2018, 126, 57–68. [Google Scholar] [CrossRef]
- Shigaeva, T.D.; Polyak, Y.M.; Kudryavtseva, V.A. Oxidation-reduction potential as an indicator of the state of environmental objects. Biosphere 2020, 12, 111–124. (In Russian) [Google Scholar] [CrossRef]
- Phogat, V.K.; Tomar, V.S.; Dahiya, R. Soil Physical Properties. Soil Science: An Introduction Edition; Indian Society of Soil Science: Delhi, India, 2015; pp. 135–172. [Google Scholar]
- Buluktaev, A.A. Changes in the salt composition of the soils of the Black Lands under oil pollution. South Russ. Ecol. Dev. 2018, 2, 184–195. [Google Scholar] [CrossRef]
- Korneykova, M.V.; Myazin, V.A.; Fokina, N.V.; Chaporgina, A.A. Bioremediation of Soil of The Kola Peninsula (Murmansk Region) Contaminated with Diesel Fuel. Geo. Env. Sust. 2021, 14, 171–176. [Google Scholar] [CrossRef]
- Kashirskaya, N.N.; Plekhanova, L.N.; Chernysheva, E.V.; Eltsov, M.V.; Udaltsov, S.N.; Borisov, A.V. Spatial and temporal features of phosphatase activity in natural and anthropogenically transformed soils. Eurasian Soil Sci. 2020, 1, 89–101. (In Russian) [Google Scholar]
- Cheverdin, Y.I.; Titova, T.V.; Bespalov, V.A.; Saprykin, S.V.; Garmashova, L.V.; Cheverdin, A.Y. Relationship between microbiological parameters and physical properties of segregation chernozems. Systems 2017, 21, 2. (In Russian) [Google Scholar]
- Kramarev, S.M.; Pashova, V.T.; Gmytsyk, A.A.; Khoroshun, K.A.; Kramarev, A.S. Phosphate state of ordinary chernozems and the financial mechanism for its improvement. Soil Sci. Agrochem. 2017, 1, 53–70. (In Russian) [Google Scholar]
- Kutovaya, O.V.; Grebennikov, A.M.; Tkhakakhova, A.K.; Isaev, V.A.; Garmashov, V.M.; Bespalov, V.A.; Cheverdin, Y.I.; Belobrov, V.P. Changes in soil biological processes and the structure of the microbial community of agrochernozems under different methods of tillage. Bull. Soil Inst. V.V. Dokuchaev. 2018, 92, 35–61. (In Russian) [Google Scholar] [CrossRef]
- Nosov, V.V.; Biryukova, O.A.; Bozhkov, D.V. The content of mobile forms of phosphorus in ordinary chernozems of the Rostov region and the efficiency of using phosphorus from fertilizers by corn plants. Mosc. Econ. J. 2016, 3, 44. (In Russian) [Google Scholar]
- Kravtsova, N.E.; Mokrikov, G.V.; Kazeev, K.S.; Minnikova, T.V.; Kolesnikov, S.I. Influence of soil treatment methods on the dynamics of the content of nutrients in ordinary chernozems of the Rostov region. Agrochem. Bull. 2019, 1, 33–36. (In Russian) [Google Scholar]
- Medvedeva, A.M.; Biryukova, O.A.; Minkina, T.M.; Kuchmenko, E.V.; Mandzhieva, S.S. Mobility and distribution of phosphorus in ordinary chernozem under various agricultural technologies. Izv. High. Educ. Institutions. North Cauc. Region. Nat. Sci. 2021, 4, 95–102. (In Russian) [Google Scholar]
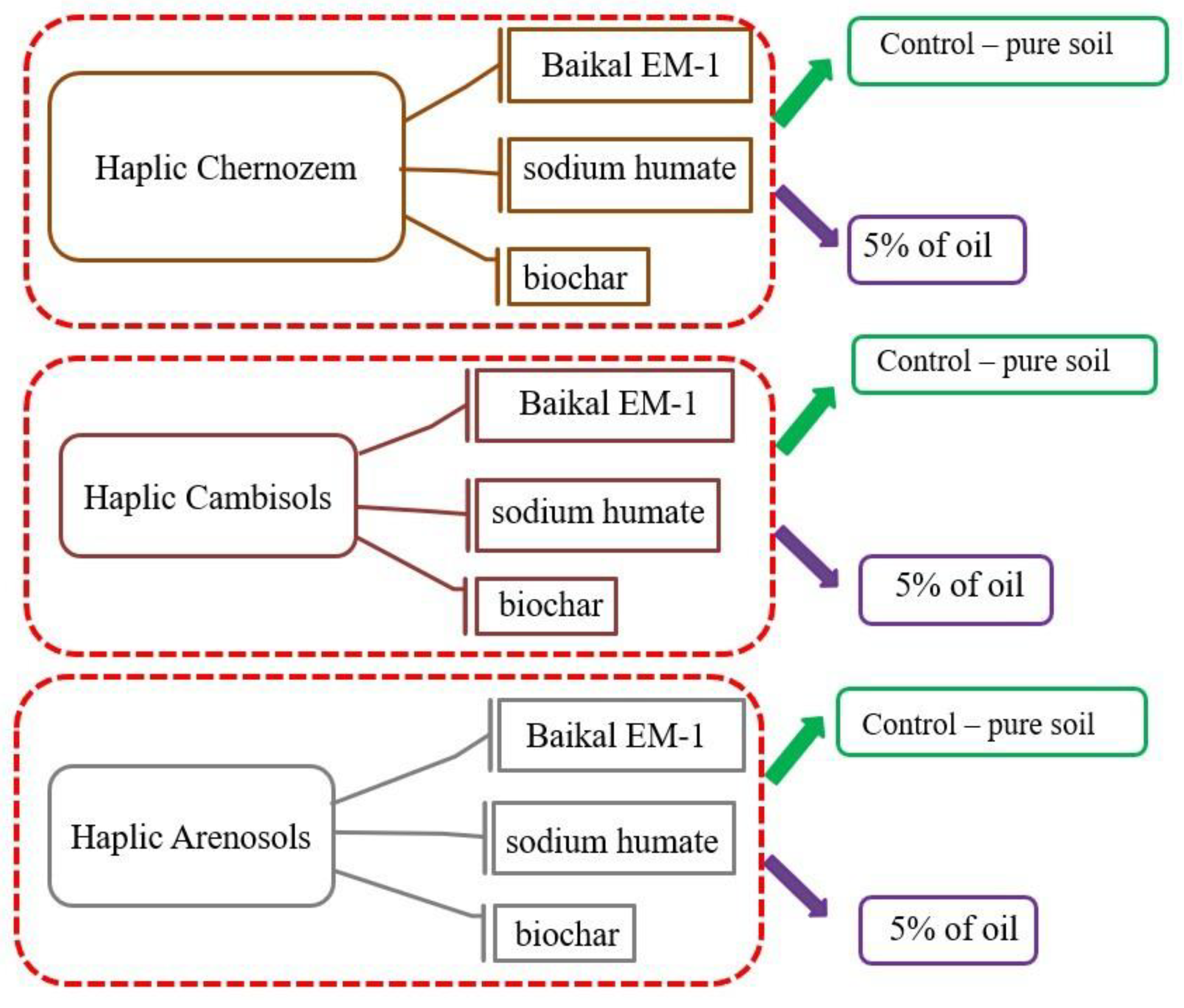
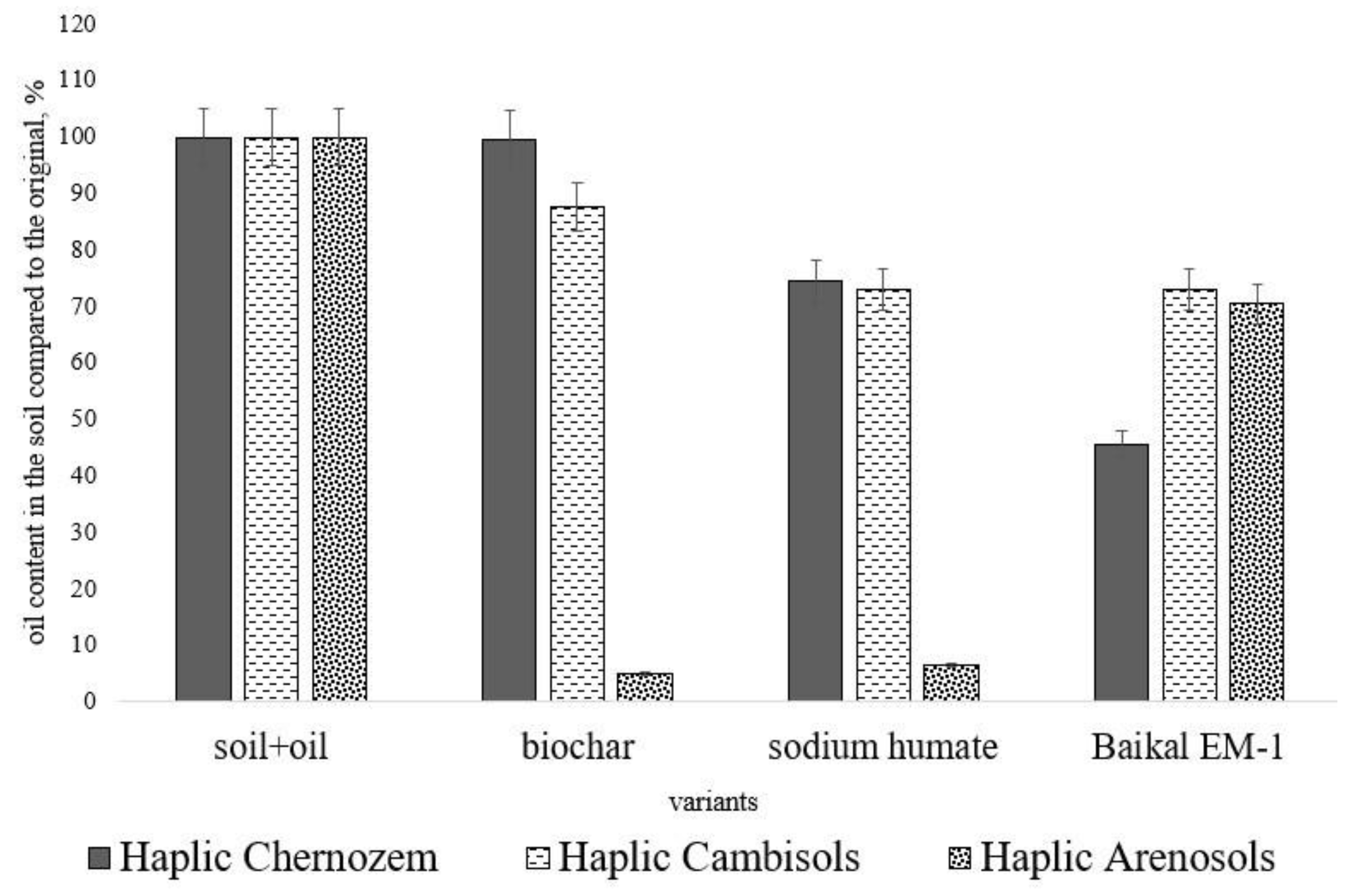
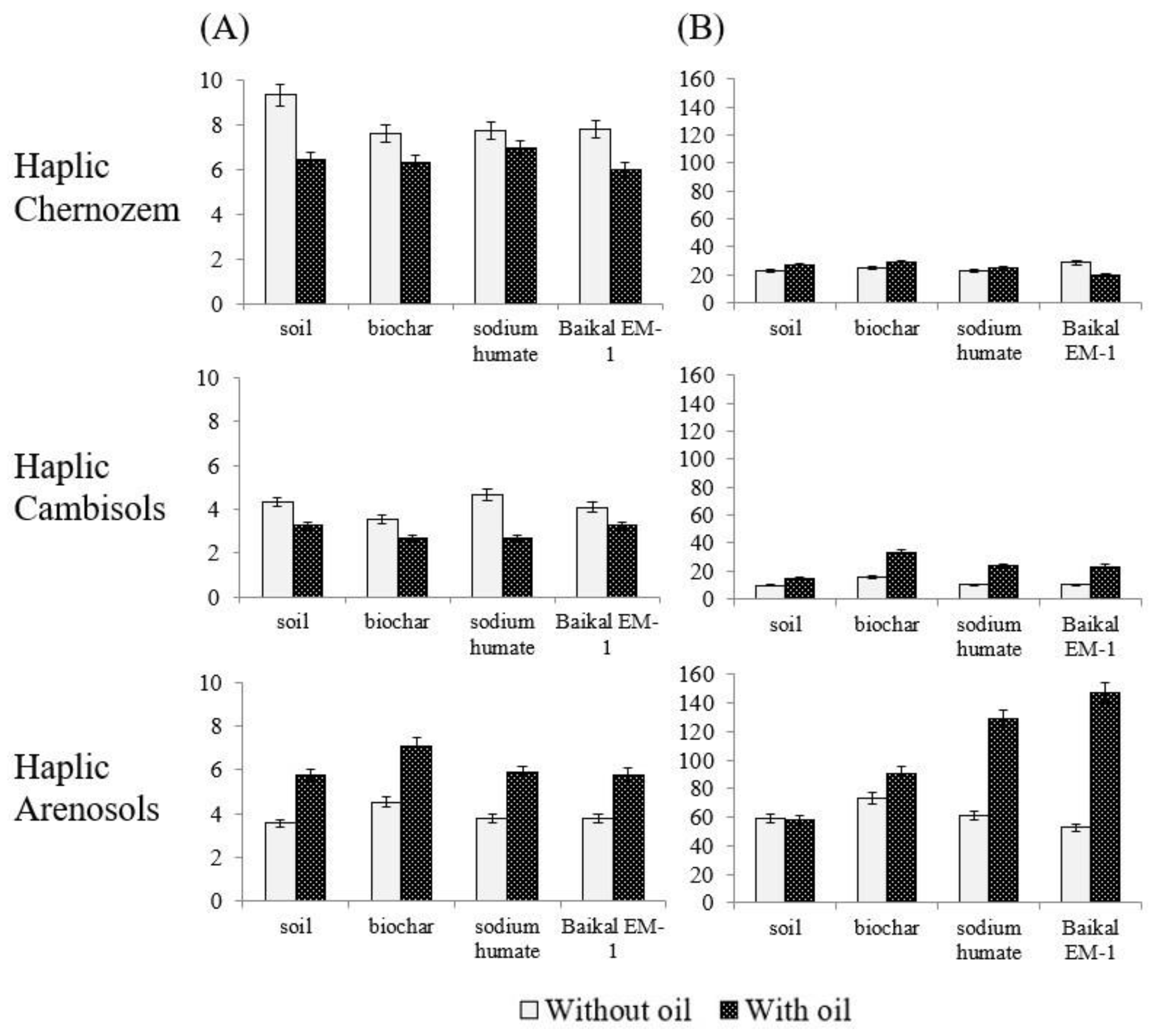


| Soil Type | Sampling Location | Coordinates | Land Use Type | Soil Texture | Soil Organic Carbon, % | pH |
|---|---|---|---|---|---|---|
| Haplic Chernozem | Botanical Garden of SFU (Rostov-on-Don) | 47°14′17.54 N 39°38′33.22 E | arable land | heavy loamy | 3.7 ± 0.2 | 7.1 ± 0.5 |
| Haplic Arenosols | Ust’-Donetsk region, (Rostov region) | 47°46.015 N 40°51.700 E | arable land | light loamy | 1.8 ± 0.1 | 5.1 ± 0.3 |
| Haplic Cambisols | Nickel village, (Rep. Adygea) | 44°10.649 N 40°9.469 E | hornbeam-beech forest | heavy loamy | 2.3 ± 0.1 | 8.2 ± 0.3 |
| № | Indicator | Method of Measurement | Units of Measurement |
|---|---|---|---|
| Physical, physico-chemica,l and chemical indicators | |||
| 1. | Residual oil content | Extraction from the soil with carbon tetrachloride followed by determination of the quantitative content by IR spectroscopy | mg/kg |
| 2. | pH | Potentiometric method | - |
| 3. | The content of easily soluble salts (Cl−, SO−24, HCO3− Ca2+, Mg2+) | Conductometric method | mg/kg |
| 4. | Redox potential | Voltammetric method | mV |
| Enzyme activity | |||
| 5. | Catalase activity | Gasometric method for the decomposition of hydrogen peroxide | mL O2/1 g /1 min |
| 6. | Dehydrogenases activity | Reduction in tetrazolium chloride salts to triphenylformazans (TPF) | mg TPF/10 g of soil/24 h |
| 7. | Invertase activity | Colorimetric method to change the content of reducing sugars | mg glucose/1 g of soil/24 h |
| 8. | Urease activity | Colorimetric method according to the amount of released ammonia | мг NH3/10 g of soil/24 h |
| 9. | Phosphatase activity | Colorimetric method according to the amount of released ammonia | µg p-nitrophenol/1 g of soil/1 h |
| № | pH | SS | RP |
|---|---|---|---|
| Haplic Chernozem | |||
| Without ameliorants | 7.1 ± 0.05 7.9 ± 0.35 | 0.27 ± 0.01 0.16 ± 0.01 | 273 ± 3.00 271 ± 9.00 |
| +biochar | 7.4 ± 0.20 7.2 ± 0.04 | 0.20 ± 0.01 0.32 ± 0.01 | 288 ± 2.50 325 ± 8.00 |
| +sodium humate | 6.4 ± 0.36 6.8 ± 0.05 | 0.11 ± 0.01 0.16 ± 0.04 | 371 ± 1.00 362 ± 2.00 |
| +Baikal EM-1 | 7.0 ± 0.22 7.2 ± 0.15 | 0.15 ± 0.02 0.11 ± 0.03 | 344 ± 4.50 269 ± 2.50 |
| Haplic Cambisols | |||
| Without ameliorants | 5.1 ± 0.08 5.3 ± 0.07 | 0.15 ± 0.02 0.18 ± 0.04 | 288 ± 3.90 289 ± 2.50 |
| +biochar | 5.9 ± 0.01 5.5 ± 0.30 | 0.13 ± 0.03 0.28 ± 0.08 | 343 ± 2.00 246 ± 4.50 |
| +sodium humate | 5.2 ± 0.05 5.8 ± 0.09 | 0.14 ± 0.01 0.12 ± 0.01 | 344 ± 6.50 273 ± 5.00 |
| +Baikal EM-1 | 5.7 ± 0.37 5.6 ± 0.15 | 0.17 ± 0.02 0.24 ± 0.01 | 355 ± 7.00 238 ± 2.50 |
| Haplic Arenosols | |||
| Without ameliorants | 8.2 ± 0.13 8.0 ± 0.26 | 8.35 ± 0.25 0.05 ± 0.01 | 223 ± 7.50 247 ± 6.50 |
| +biochar | 8.3 ± 0.02 8.1 ± 0.02 | 0.15 ± 0.01 0.15 ± 0.08 | 217 ± 2.00 205 ± 2.00 |
| +sodium humate | 8.2 ± 0.10 7.6 ± 0.60 | 5.69 ± 0.31 0.05 ± 0.01 | 242 ± 2.00 238 ± 6.00 |
| +Baikal EM-1 | 7.7 ± 0.60 7.6 ± 0.69 | 6.34 ± 0.01 4.00 ± 0.10 | 268 ± 2.50 267 ± 3.00 |
| Ameliorant | Acat | Adeh | Ainv | Aur | Aphos |
|---|---|---|---|---|---|
| biochar | −0.16 | 0.71 * | −0.89 ** | 0.95 * | 0.98 * |
| sodium humate | −0.68 * | 0.52 | −0.58 * | −0.87 ** | 1.00 * |
| Baikal EM-1 | −0.87 ** | −0.81 ** | −1.00 ** | 0.95 * | −0.96 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnikova, T.; Kolesnikov, S.; Revina, S.; Ruseva, A.; Gaivoronsky, V. Enzymatic Assessment of the State of Oil-Contaminated Soils in the South of Russia after Bioremediation. Toxics 2023, 11, 355. https://doi.org/10.3390/toxics11040355
Minnikova T, Kolesnikov S, Revina S, Ruseva A, Gaivoronsky V. Enzymatic Assessment of the State of Oil-Contaminated Soils in the South of Russia after Bioremediation. Toxics. 2023; 11(4):355. https://doi.org/10.3390/toxics11040355
Chicago/Turabian StyleMinnikova, Tatyana, Sergey Kolesnikov, Sofia Revina, Anna Ruseva, and Vladimir Gaivoronsky. 2023. "Enzymatic Assessment of the State of Oil-Contaminated Soils in the South of Russia after Bioremediation" Toxics 11, no. 4: 355. https://doi.org/10.3390/toxics11040355
APA StyleMinnikova, T., Kolesnikov, S., Revina, S., Ruseva, A., & Gaivoronsky, V. (2023). Enzymatic Assessment of the State of Oil-Contaminated Soils in the South of Russia after Bioremediation. Toxics, 11(4), 355. https://doi.org/10.3390/toxics11040355








