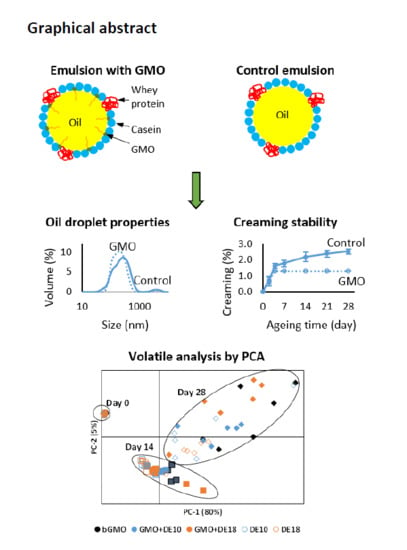
Application of a Novel Instantized Glycerol Monooleate Ingredient in a Protein-Stabilized Oil-In-Water Emulsion †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Instantized GMO Powders by Spray-Drying
- a = weight of water added to the powder in g;
- b = weight of powder used in g;
- c = moisture content (%) in the powder;
- d = dry matter (%) in the reconstituted emulsion after it has passed through the sieve.
2.3. Model Emulsions
2.4. Characterization of Model Emulsions
2.4.1. Emulsion Particle Size and Polydispersity
2.4.2. ζ-Potential
2.4.3. Viscosity
2.4.4. pH
2.4.5. Creaming Index
2.4.6. Emulsion Stability
2.4.7. Volatile Analysis Using Headspace Solid-Phase Microextraction (HS-SPME) and GC-MS
2.4.8. Statistical Analysis
3. Results and Discussion
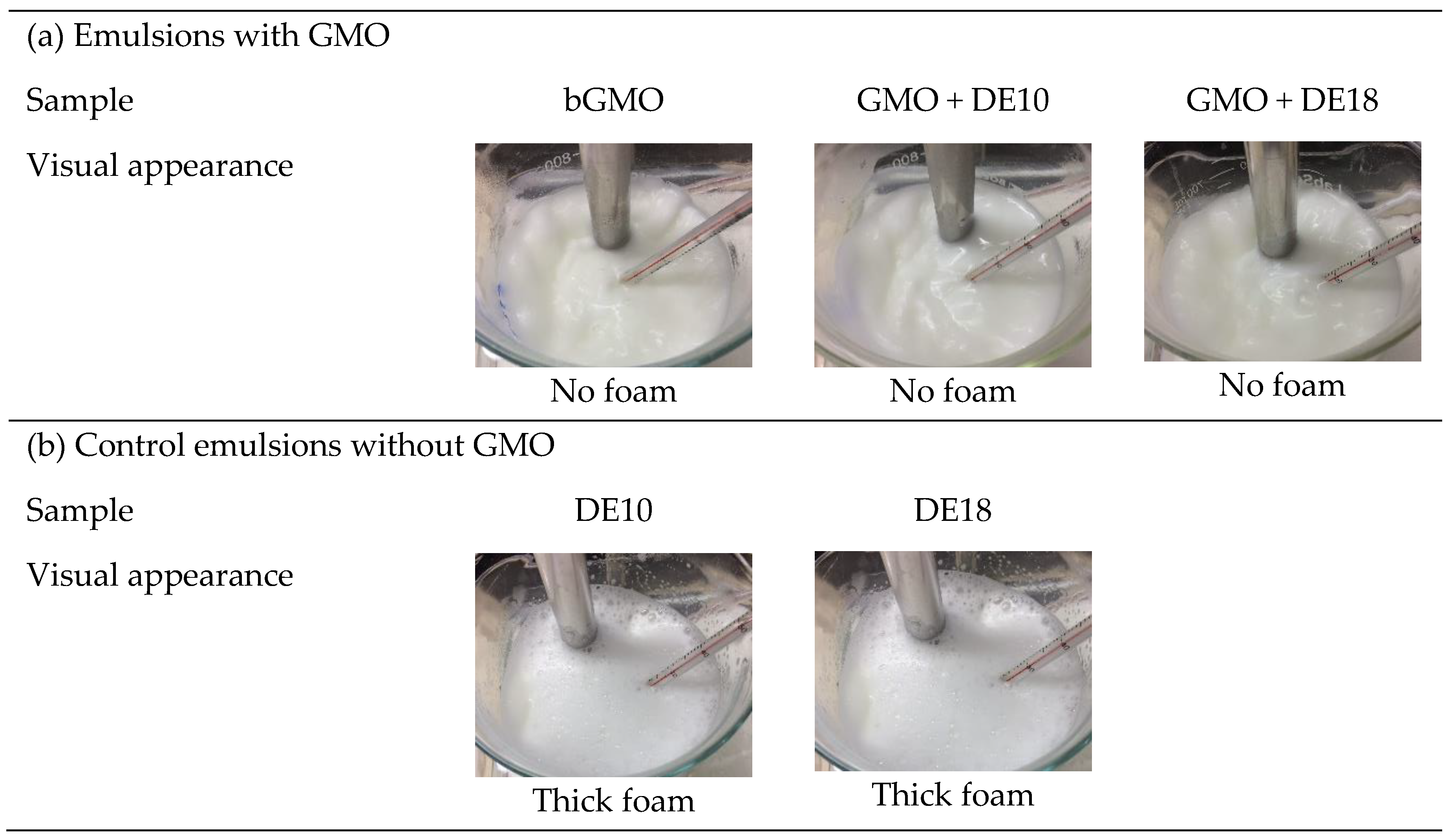
3.1. Observation of Foaming Behavior during Homogenization
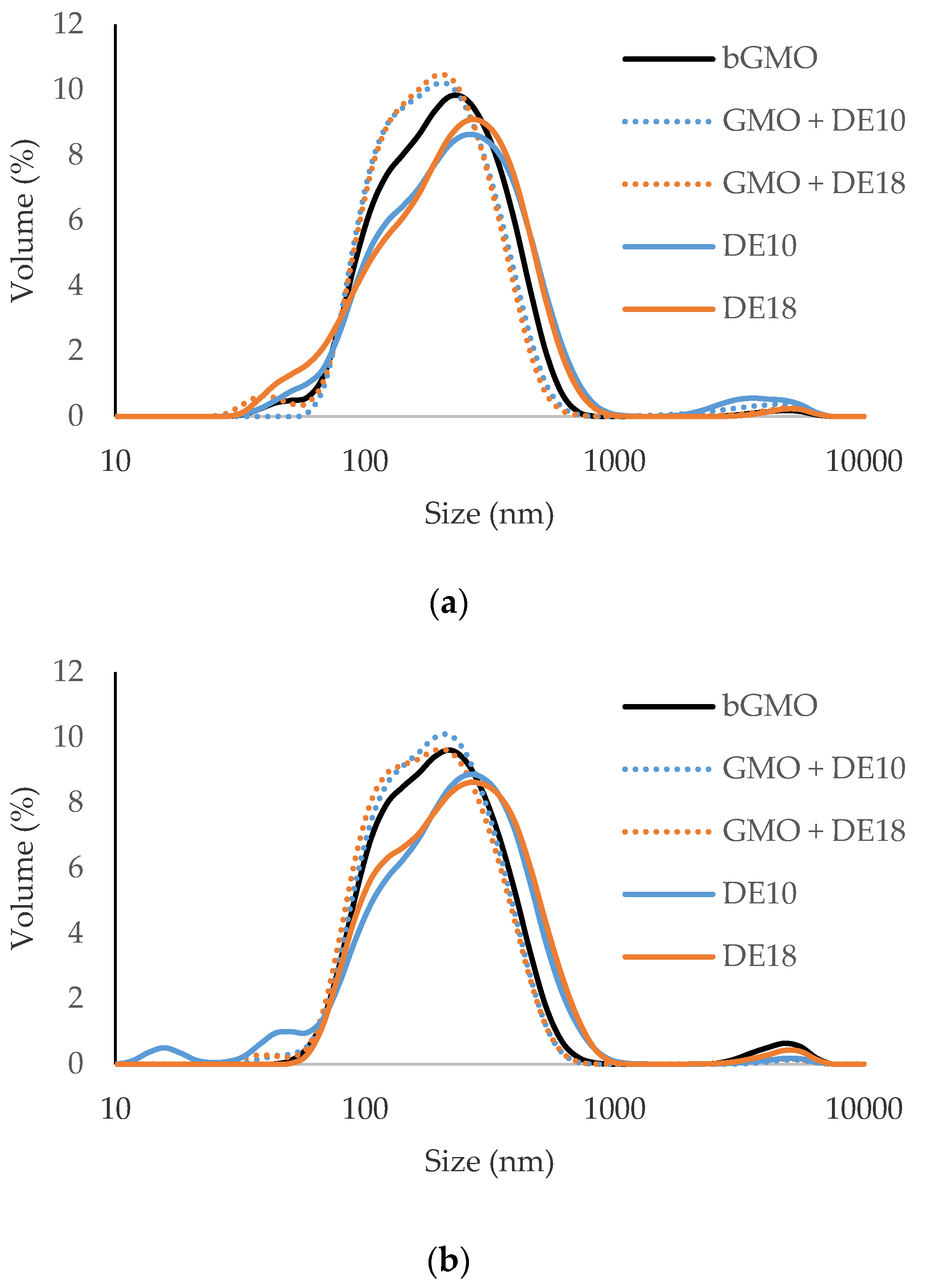
3.2. Droplet Size and Polydispersity Index
3.3. ζ-Potential
3.4. Viscosity
3.5. pH
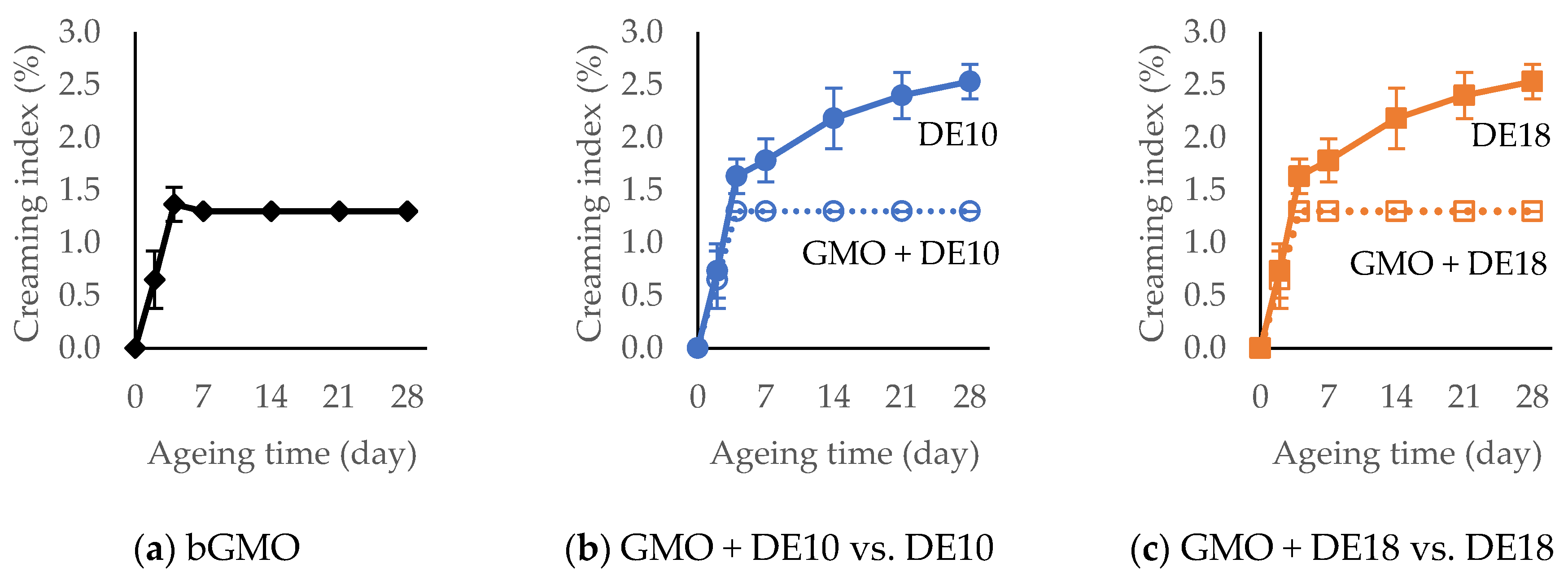
3.6. Creaming Index
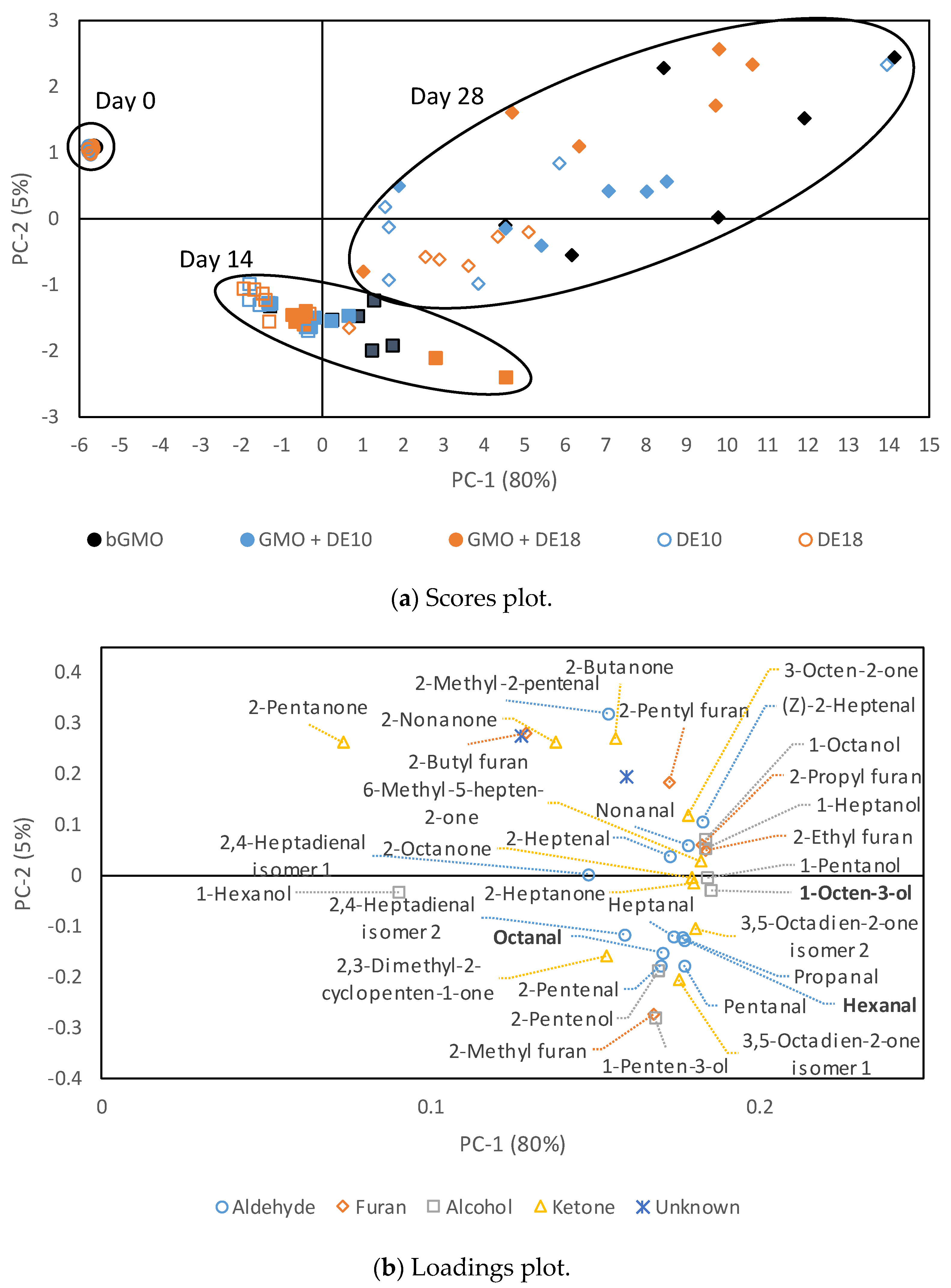
3.7. Oxidative Stability of Model Emulsions Measured Using Volatile Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hu, Y.-T.; Ting, Y.; Hu, J.-Y.; Hsieh, S.-C. Techniques and methods to study functional characteristics of emulsion systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Walstra, P. Physical Chemistry of Foods; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Bos, M.A.; van Vliet, T. Interfacial rheological properties of adsorbed protein layers and surfactants: A review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef]
- Damodaran, S. Protein stabilization of emulsions and foams. J. Food. Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Fredrick, E.; Heyman, B.; Moens, K.; Fischer, S.; Verwijlen, T.; Moldenaers, P.; Van der Meeren, P.; Dewettinck, K. Monoacylglycerols in dairy recombined cream: II. The effect on partial coalescence and whipping properties. Food Res. Int. 2013, 51, 936–945. [Google Scholar] [CrossRef]
- Krog, N. Emulsifiers. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 61–71. [Google Scholar] [CrossRef]
- Loi, C.C.; Eyres, G.T.; Birch, E.J. Effect of mono- and diglycerides on physical properties and stability of a protein-stabilised oil-in-water emulsion. J. Food Eng. 2019, 240, 56–64. [Google Scholar] [CrossRef]
- Augustin, M.A.; Oliver, C.M. Use of milk proteins for encapsulation of food ingredients. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 211–226. [Google Scholar] [CrossRef]
- Damerau, A.; Kamlang-ek, P.; Moisio, T.; Lampi, A.-M.; Piironen, V. Effect of SPME extraction conditions and humidity on the release of volatile lipid oxidation products from spray-dried emulsions. Food Chem. 2014, 157, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loi, C.C.; Eyres, G.T.; Silcock, P.; Birch, E.J. Preparation and characterisation of a novel emulsifier system based on glycerol monooleate by spray-drying. J. Food Eng. 2020, 285, 110100. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- González, A.; Martínez, M.L.; Paredes, A.J.; León, A.E.; Ribotta, P.D. Study of the preparation process and variation of wall components in chia (Salvia hispanica L.) oil microencapsulation. Powder Technol. 2016, 301, 868–875. [Google Scholar] [CrossRef]
- Sarkar, A.; Arfsten, J.; Golay, P.-A.; Acquistapace, S.; Heinrich, E. Microstructure and long-term stability of spray dried emulsions with ultra-high oil content. Food Hydrocoll. 2016, 52, 857–867. [Google Scholar] [CrossRef]
- Loi, C.C.; Eyres, G.T.; Birch, E.J. Effect of milk protein composition on physicochemical properties, creaming stability and volatile profile of a protein-stabilised oil-in-water emulsion. Food Res. Int. 2019, 120, 83–91. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Hanselmann, W.; Windhab, E. Flow characteristics and modelling of foam generation in a continuous rotor/stator mixer. J. Food Eng. 1998, 38, 393–405. [Google Scholar] [CrossRef]
- Berton, C.; Genot, C.; Ropers, M.-H. Quantification of unadsorbed protein and surfactant emulsifiers in oil-in-water emulsions. J. Colloid Interface Sci. 2011, 354, 739–748. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.B.; Andersen, M.L. Partial coalescence in emulsions: The impact of solid fat content and fatty acid composition. Eur. J. Lipid Sci. Technol. 2015, 117, 1627–1635. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Saurel, R.; Chambin, O.; Cases, E.; Voilley, A.; Cayot, P. Utilisation of pectin coating to enhance spray-dry stability of pea protein-stabilised oil-in-water emulsions. Food Chem. 2010, 122, 447–454. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Sidzhakova, D.; Campbell, B. Effect of thermal treatment, ionic strength, and pH on the short-term and long-term coalescence stability of β-lactoglobulin emulsions. Langmuir 2006, 22, 6042–6052. [Google Scholar] [CrossRef]
- Post, A.E.; Arnold, B.; Weiss, J.; Hinrichs, J. Effect of temperature and pH on the solubility of caseins: Environmental influences on the dissociation of αS- and β-casein. J. Dairy Sci. 2012, 95, 1603–1616. [Google Scholar] [CrossRef]
- Mezdour, S.; Korolczuk, J. Zeta potential of sodium caseinate in water-ethanol solutions. Milchwissenschaft 2010, 65, 392–395. [Google Scholar]
- Ross, S.; Morrison, I.D. Colloidal Systems and Interfaces; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Liang, Y.; Wong, S.S.; Pham, S.Q.; Tan, J.J. Effects of globular protein type and concentration on the physical properties and flow behaviors of oil-in-water emulsions stabilized by micellar casein-globular protein mixtures. Food Hydrocoll. 2016, 54, 89–98. [Google Scholar] [CrossRef]
- Matsumiya, K.; Takahashi, W.; Inoue, T.; Matsumura, Y. Effects of bacteriostatic emulsifiers on stability of milk-based emulsions. J. Food Eng. 2010, 96, 185–191. [Google Scholar] [CrossRef]
- Huang, X.; Kakuda, Y.; Cui, W. Hydrocolloids in emulsions: Particle size distribution and interfacial activity. Food Hydrocoll. 2001, 15, 533–542. [Google Scholar] [CrossRef]
- Berton, C.; Ropers, M.-H.; Bertrand, D.; Viau, M.; Genot, C. Oxidative stability of oil-in-water emulsions stabilised with protein or surfactant emulsifiers in various oxidation conditions. Food Chem. 2012, 131, 1360–1369. [Google Scholar] [CrossRef]
- Vandamme, J.; Nikiforov, A.; De Roose, M.; Leys, C.; De Cooman, L.; Van Durme, J. Controlled accelerated oxidation of oleic acid using a DBD plasma: Determination of volatile oxidation compounds. Food Res. Int. 2016, 79, 54–63. [Google Scholar] [CrossRef]
- Genot, C.; Meynier, A.; Riaublanc, A. Lipid oxidation in emulsions. In Lipid Oxidation Pathways; Kamal-Eldin, A., Ed.; AOCS Publishing: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Osborn, H.T.; Akoh, C.C. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid-based oil-in-water emulsions. Food Chem. 2004, 84, 451–456. [Google Scholar] [CrossRef]
- Dimakou, C.P.; Kiokias, S.N.; Tsaprouni, I.V.; Oreopoulou, V. Effect of processing and storage parameters on the oxidative deterioration of oil-in-water emulsions. Food Biophys. 2007, 2, 38. [Google Scholar] [CrossRef]
| Sample | GMO + DE10 | GMO + DE18 |
|---|---|---|
| (a) Formulation of Instantized GMO Powders a | ||
| Core Materials | 48.6% | 48.6% |
| (i) Glycerol monooleate | 33.6% | 33.6% |
| (ii) Canola oil | 15.0% | 15.0% |
| Wall Materials | 51.4% | 51.4% |
| (iii) Sodium caseinate | 3.0% | 3.0% |
| (iv) Sodium stearate | 1.4% | 1.4% |
| (v) Maltodextrin DE 10 | 47.0% | |
| (vi) Maltodextrin DE 18 | 47.0% | |
| (b) Properties of Instantized GMO Powders | ||
| Encapsulation efficiency (%) b | 93.3 ± 1.0 | 93.9 ± 1.2 |
| Surface oil (%) c | 3.0 ± 0.4 | 3.1 ± 0.6 |
| Moisture (%) | 2.1 ± 0.6 | 2.1 ± 0.5 |
| Dispersibility (%) d | 74.0 ± 0.8 | 87.3 ± 2.0 |
| Initial droplet diameter (nm) e | 153.1 ± 2.7 | 153.9 ± 2.8 |
| Reconstituted droplet diameter (nm) e | 267.1 ± 8.4 | 295.0 ± 11.8 |
| Sample | bGMO | GMO + DE10 | GMO + DE18 | DE10 | DE18 |
|---|---|---|---|---|---|
| (a) Oil Phase | |||||
| Canola oil | 4.0% | 4.0% | 4.0% | 4.0% | 4.0% |
| GMO | 0.2% | ||||
| (b) Water Phase | |||||
| Caster sugar | 6.0% | 6.0% | 6.0% | 6.0% | 6.0% |
| Sodium caseinate | 0.80% | 0.80% | 0.80% | 0.80% | 0.80% |
| WPC | 0.20% | 0.20% | 0.20% | 0.20% | 0.20% |
| Sodium azide | 0.02% | 0.02% | 0.02% | 0.02% | 0.02% |
| Maltodextrin DE 10 | 0.28% | ||||
| Maltodextrin DE 18 | 0.28% | ||||
| GMO + DE10 powder | 0.60% | ||||
| GMO + DE18 powder | 0.60% | ||||
| Deionized water | 88.8% | 88.4% | 88.4% | 88.7% | 88.7% |
| Sample | Day 0 | Day 7 | Day 14 | Day 28 |
|---|---|---|---|---|
| (a) Droplet Size (nm) | ||||
| bGMO | 186.0 ± 5.6 ab | 183.2 ± 5.2 a | 182.3 ± 4.0 a | 184.1 ± 3.1 a |
| GMO + DE10 | 178.1 ± 1.5 a | 175.7 ± 2.3 a | 175.1 ± 3.2 a | 177.9 ± 2.3 a |
| GMO + DE18 | 175.8 ± 0.8 a | 174.1 ± 0.6 a | 172.3 ± 1.0 a | 174.0 ± 0.9 a |
| DE10 | 198.4 ± 8.5 c | 199.8 ± 10.5 b | 196.4 ± 9.2 b | 199.5 ± 9.6 b |
| DE18 | 196.9 ± 6.1 bc | 196.1 ± 5.0 b | 194.7 ± 4.4 b | 198.4 ± 3.5 b |
| F-value | 15.2 | 16.3 | 18.7 | 22.7 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| (b) Polydispersity index | ||||
| bGMO | 0.162 ± 0.022 ab | 0.170 ± 0.012 a | 0.177 ± 0.009 a | 0.177 ± 0.019 a |
| GMO + DE10 | 0.163 ± 0.019 ab | 0.162 ± 0.015 a | 0.162 ± 0.008 a | 0.165 ± 0.010 a |
| GMO + DE18 | 0.158 ± 0.014 a | 0.158 ± 0.006 a | 0.161 ± 0.009 a | 0.160 ± 0.006 a |
| DE10 | 0.201 ± 0.022 b | 0.204 ± 0.022 b | 0.202 ± 0.021 b | 0.202 ± 0.018 b |
| DE18 | 0.194 ± 0.015 ab | 0.209 ± 0.009 b | 0.198 ± 0.011 b | 0.200 ± 0.018 b |
| F-value | 4.6 | 11.5 | 10.0 | 6.6 |
| p-value | <0.05 | <0.001 | <0.001 | <0.01 |
| Sample | Day 0 | Day 7 | Day 14 | Day 28 |
|---|---|---|---|---|
| bGMO | −51.6 ± 1.4 a | −47.8 ± 0.5 c | −47.7 ± 0.4 a | −46.9 ± 1.1 b |
| GMO + DE10 | −51.9 ± 0.2 a | −49.1 ± 0.3 b | −49.0 ± 0.5 b | −47.6 ± 0.2 ab |
| GMO + DE18 | −52.1 ± 0.4 a | −49.0 ± 0.3 b | −49.2 ± 0.5 b | −47.7 ± 0.1 ab |
| DE10 | −53.0 ± 0.3 a | −49.8 ± 0.5 ab | −49.9 ± 0.7 b | −48.1 ± 0.9 ab |
| DE18 | −52.9 ± 0.8 a | −50.3 ± 0.0 a | −50.0 ± 0.8 b | −49.3 ± 1.1 a |
| F-value | 2.7 | 16.9 | 10.0 | 4.8 |
| p-value | 0.072 | <0.001 | <0.001 | <0.05 |
| Retention Index | Volatile Compound | Day | Concentration (µg/L) a | Source of Lipid Oxidation [31] | ||||
|---|---|---|---|---|---|---|---|---|
| bGMO | GMO + DE10 | GMO + DE18 | DE10 | DE18 | ||||
| 1082 | Hexanal | 0 | 25.0 ± 8.4 a | 17.7 ± 7.1 a | 21.9 ± 6.1 a | 16.2 ± 8.8 a | 18.7 ± 6.0 a | n-6 linoleic acid |
| 14 | 567.8 ± 118.8 bc | 490.7 ± 102.7 abc | 670.1 ± 294.3 c | 294.8 ± 121.1 a | 361.9 ± 20.8 ab | |||
| 28 | 945.0 ± 364.6 a | 819.7 ± 309.6 a | 902.4 ± 266.7 a | 739.5 ± 262.5 a | 726.8 ± 143.8 a | |||
| 1292 | Octanal | 0 | n.d. | n.d. | n.d. | n.d. | n.d. | n-9 oleic acid |
| 14 | 180.8 ± 52.4 a | 141.0 ± 18.6 a | 226.0 ± 145.4 a | 145.2 ± 24.7 a | 166.2 ± 82.4 a | |||
| 28 | 380.3 ± 111.3 a | 292.0 ± 68.5 a | 297.7 ± 66.5 a | 232.1 ± 77.6 a | 273.4 ± 68.6 a | |||
| 1441 | 1-Octen-3-ol | 0 | 2.1 ± 0.1 | n.d. | n.d. | n.d. | n.d. | n-6 linoleic acid |
| 14 | 28.7 ± 4.2 a | 26.1 ± 2.9 a | 29.5 ± 8.1 a | 19.4 ± 1.5 a | 20.0 ± 1.9 a | |||
| 28 | 60.3 ± 15.8 a | 49.8 ± 8.7 a | 54.7 ± 14.9 a | 43.4 ± 18.4 a | 38.9 ± 5.6 a | |||
| Retention Index | Compound | TIC Peak Area (Million au) a | ||||
|---|---|---|---|---|---|---|
| bGMO | GMO + DE10 | GMO + DE18 | DE10 | DE18 | ||
| 794 | Propanal | 33.11 ± 6.62 c | 28.12 ± 6.23 bc | 30.96 ± 7.61 c | 18.28 ± 4.98 ab | 16.22 ± 2.54 a |
| 956 | 2-Ethyl furan | 171.70 ± 41.12 a | 129.93 ± 34.55 a | 137.76 ± 41.87 a | 132.89 ± 79.60 a | 101.67 ± 22.18 a |
| 982 | Pentanal | 56.61 ± 13.02 a | 48.06 ± 13.51 a | 43.86 ± 11.09 a | 46.29 ± 20.04 a | 42.55 ± 7.87 a |
| 1232 | 2-Pentyl furan | 85.45 ± 31.15 a | 55.89 ± 17.00 a | 60.65 ± 16.57 a | 60.20 ± 58.76 a | 38.99 ± 7.59 a |
| 1278 | (Z)-2-Heptenal | 14.51 ± 4.85 a | 11.11 ± 2.71 a | 12.76 ± 4.90 a | 9.55 ± 4.06 a | 8.99 ± 1.85 a |
| 1397 | Nonanal | 38.51 ± 12.78 a | 30.27 ± 7.12 a | 31.51 ± 9.50 a | 25.94 ± 17.65 a | 21.06 ± 7.23 a |
| 1414 | 3-Octen-2-one | 52.50 ± 21.47 b | 38.44 ± 8.94 ab | 43.51 ± 17.97 ab | 23.13 ± 9.97 a | 21.48 ± 5.39 a |
| 1448 | 1-Heptanol | 55.12 ± 17.99 a | 38.75 ± 7.59 a | 44.75 ± 15.39 a | 34.80 ± 21.37 a | 30.01 ± 8.11 a |
| 1505 | 2,4-Heptadienal isomer 2 | 14.27 ± 5.21 b | 9.44 ± 1.57 ab | 10.36 ± 3.00 ab | 6.73 ± 3.59 a | 5.25 ± 0.80 a |
| 1527 | 3,5-Octadien-2-one isomer 1 | 94.63 ± 22.08 b | 77.37 ± 10.67 ab | 85.60 ± 19.21 ab | 66.08 ± 14.70 a | 59.94 ± 5.82 a |
| 1551 | 1-Octanol | 29.96 ± 10.32 a | 21.77 ± 4.74 a | 25.05 ± 8.87 a | 19.16 ± 12.92 a | 16.78 ± 4.37 a |
| 1581 | 3,5-Octadien-2-one isomer 2 | 338.93 ± 54.61 a | 294.74 ± 32.67 a | 315.95 ± 59.86 a | 280.03 ± 41.43 a | 264.48 ± 25.33 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loi, C.C.; Eyres, G.T.; Silcock, P.; Birch, E.J. Application of a Novel Instantized Glycerol Monooleate Ingredient in a Protein-Stabilized Oil-In-Water Emulsion. Foods 2020, 9, 1237. https://doi.org/10.3390/foods9091237
Loi CC, Eyres GT, Silcock P, Birch EJ. Application of a Novel Instantized Glycerol Monooleate Ingredient in a Protein-Stabilized Oil-In-Water Emulsion. Foods. 2020; 9(9):1237. https://doi.org/10.3390/foods9091237
Chicago/Turabian StyleLoi, Chia Chun, Graham T. Eyres, Patrick Silcock, and E. John Birch. 2020. "Application of a Novel Instantized Glycerol Monooleate Ingredient in a Protein-Stabilized Oil-In-Water Emulsion" Foods 9, no. 9: 1237. https://doi.org/10.3390/foods9091237
APA StyleLoi, C. C., Eyres, G. T., Silcock, P., & Birch, E. J. (2020). Application of a Novel Instantized Glycerol Monooleate Ingredient in a Protein-Stabilized Oil-In-Water Emulsion. Foods, 9(9), 1237. https://doi.org/10.3390/foods9091237


_Loi.jpg)