Behaviour of Non-O157 STEC and Atypical EPEC during the Manufacturing and Ripening of Raw Milk Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Collection of Raw Cow’s Milk and Experimental Design
2.3. Cheesemaking Process
2.4. Microbiological Analysis
2.5. Physicochemical Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters during Cheesemaking and Ripening
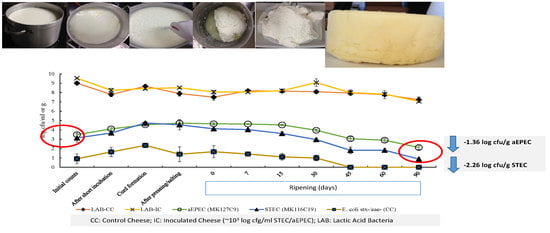
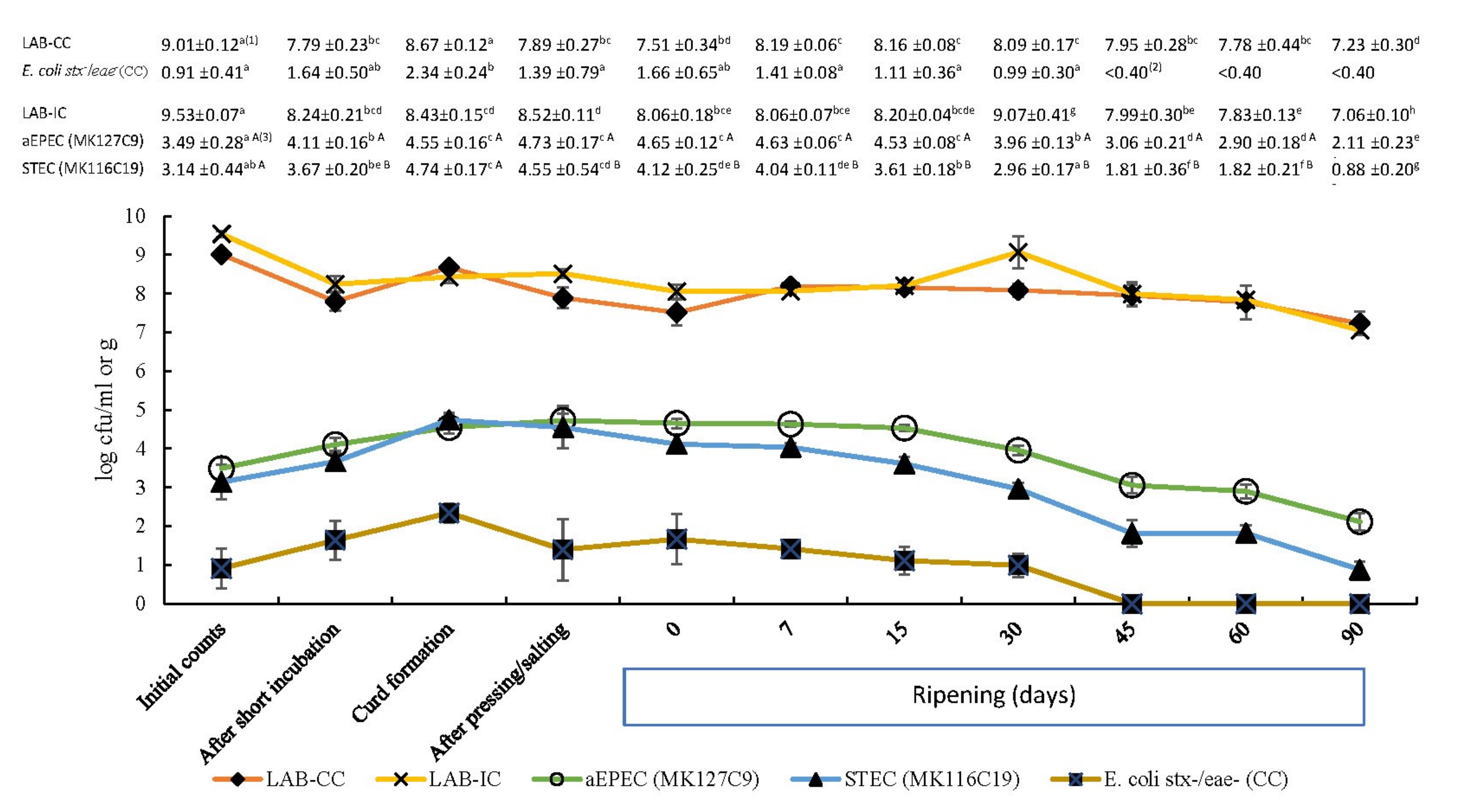
3.2. Analysis of E. coli Populations in Cheese Samples
3.3. Fate of aEPEC and STEC Strains in Raw Milk Cheese
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gyles, C.L. Shiga toxin-producing Escherichia coli: An overview. J. Anim. Sci. 2007, 85, E45–E62. [Google Scholar] [CrossRef]
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A.; et al. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 2013, 162, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, C.W.; Doyle, M.E. White Paper on Non-O157 Shiga-toxin Producing E. coli from Meat and Non-Meat Sources. 2009. Available online: https://meatpoultryfoundation.org/namif/wp-content/uploads/08-402.pdf (accessed on 2 August 2020).
- European Centre for Disease Prevention and Control. Shiga toxin/verocytotoxin-producing Escherichia coli (STEC/VTEC) infection. In CDC. Annual Epidemiological Report for 2018; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2020. [Google Scholar]
- European Centre for Desease Prevention and Control; European Centre for Desease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Centers for Desease Control and Prevention. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet 2015 Surveillance Report (Final Data); U.S.Department of Health and Human Service: Atlanta, GA, USA, 2017.
- Pearson, J.S.; Giogha, C.; Wong Fok Lung, T.; Hartland, E.L. The genetics of enteropathogenic Escherichia coli virulence. Annu. Rev. Genet. 2016, 50, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.A.; Santos, J.; García-Meniño, I.; Flament-Simon, S.C.; Blanco, J.; García-López, M.-L.; Otero, A.; Rodríguez-Calleja, J.M. Characterisation, antimicrobial resistance and diversity of atypical EPEC and STEC isolated from cow’s milk, cheese and dairy cattle farm environments. LWT 2019, 108, 319–325. [Google Scholar] [CrossRef]
- Hernandes, R.T.; Elias, W.P.; Vieira, M.A.M.; Gomes, T.A.T. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 2009, 297, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Suárez, M.E.; Otero, A.; García-López, M.L.; Dahbi, G.; Blanco, M.; Mora, A.; Blanco, J.; Santos, J.A. Genetic characterization of Shiga toxin-producing Escherichia coli (STEC) and atypical enteropathogenic Escherichia coli (EPEC) isolates from goat’s milk and goat farm environment. Int. J. Food Microbiol. 2016, 236, 148–154. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Barletta, F.; Contreras, C.; Mercado, E. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 852–856. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, C.A.F.; Corassin, C.H.; Lee, S.H.I.; Gonçalves, B.L.; Barancelli, G.V. Pathogenic bacteria in cheese, Their implications for human health and prevention strategies. In Nutrients in Dairy and their Implications for Health and Disease; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 61–75. ISBN 9780128097625. [Google Scholar]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Front. Cell. Infect. Microbiol. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Smith, J.L.; Fratamico, P.M.; Launchi, N.R. Update on non-O157 Shiga toxin-producing E. coli as a foodborne pathogen: Analysis and control. In Advances in Microbial Food Safety; Sofos, J., Ed.; Elsevier: Cambridge, UK, 2015; pp. 3–32. ISBN 9781782421153. [Google Scholar]
- Torres, A.G. Escherichia coli in the Americas; Torres, A.G., Ed.; Springer: Gewerbestrasse, Germany, 2016; ISBN 978-3-319-45091-9. [Google Scholar]
- Little, C.L.; Rhoades, J.R.; Sagoo, S.K.; Harris, J.; Greenwood, M.; Mithani, V.; Grant, K.; McLauchlin, J. Microbiological quality of retail cheeses made from raw, thermized or pasteurized milk in the UK. Food Microbiol. 2008, 25, 304–312. [Google Scholar] [CrossRef]
- Madic, J.; Vingadassalon, N.; de Garam, C.P.; Marault, M.; Scheutz, F.; Brugere, H.; Jamet, E.; Auvray, F. Detection of Shiga toxin-producing Escherichia coli serotypes O26:H11, O103:H2, O111:H8, O145:H28, and O157:H7 in raw-milk cheeses by using multiplex real-time PCR. Appl. Environ. Microbiol. 2011, 77, 2035–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marozzi, S.; De Santis, P.; Lovari, S.; Condoleo, R.; Bilei, S.; Marcianò, R.; Mezher, Z. Prevalence and molecular characterisation of Shiga-toxin-producing Escherichia coli in raw milk cheeses from Lazio region, Italy. Ital. J. Food Saf. 2016, 5, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Giezendanner, N.; Corti, S.; Krause, G.; Beutin, L.; Danuser, J.; Stephan, R. Characteristics of Shiga toxin-producing Escherichia coli isolated from Swiss raw milk cheese within a 3-year monitoring program. J. Food Prot. 2010, 73, 88–91. [Google Scholar] [CrossRef]
- Ombarak, R.A.; Hinenoya, A.; Awasthi, S.P.; Iguchi, A.; Shima, A.; Elbagory, A.-R.M.; Yamasaki, S. Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. Int. J. Food Microbiol. 2016, 221, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, S.; Agaoglu, S. Behavior of Escherichia coli O157:H7 during the ripening of herby cheese manufactured from raw milk. J. Food Heal. Sci. 2016, 2, 49–56. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, D.J.; Druart, M.J.; Donnelly, C.W. Behavior of Escherichia coli O157:H7 during the manufacture and aging of Gouda and stirred-curd Cheddar cheeses manufactured from raw milk. J. Food Prot. 2010, 73, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Miszczycha, S.D.; Perrin, F.; Ganet, S.; Jamet, E.; Tenenhaus-Aziza, F.; Montel, M.C.; Thevenot-Sergentet, D. Behavior of different Shiga toxin-producing Escherichia coli serotypes in various experimentally contaminated raw-milk cheeses. Appl. Environ. Microbiol. 2013, 79. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Hoffmann, W.; Bockelmann, W.; Hummerjohann, J.; Stephan, R.; Hammer, P. Fate of Shiga toxin-producing and generic Escherichia coli during production and ripening of semihard raw milk cheese. J. Dairy Sci. 2013, 96, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Spano, G.; Goffredo, E.; Beneduce, L.; Tarantino, D.; Dupuy, A.; Massa, S. Fate of Escherichia coli O157:H7 during the manufacture of Mozzarella cheese. Lett. Appl. Microbiol. 2003, 36, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Stephan, R.; Schumacher, S.; Corti, S.; Krause, G.; Danuser, J.; Beutin, L. Prevalence and Characteristics of Shiga Toxin-Producing Escherichia coli in Swiss Raw Milk Cheeses Collected at Producer Level. J. Dairy Sci. 2008, 91, 2561–2565. [Google Scholar] [CrossRef] [Green Version]
- Baylis, C.L. Raw milk and raw milk cheeses as vehicles for infection by Verocytotoxin-producing Escherichia coli. Int. J. Dairy Technol. 2009, 62, 293–307. [Google Scholar] [CrossRef]
- Pollard, D.R.; Johnson, W.M.; Lior, H.; Tyler, S.D.; Rozee, K.R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J. Clin. Microbiol. 1990, 28, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, J.; Aabo, S.; Hill, W.; Notermans, S.; Wernars, K.; Granum, P.; Popovic, P.; Rasmussen, H.; Olsvik, Ø. Probes and polymerase chain reaction for detection of food-borne bacterial pathogens. Int. J. Food Microbiol. 1995, 28, 1–78. [Google Scholar] [CrossRef]
- Paton, A.W.; Paton, J.C. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 1998, 36, 598–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosikowski, F. Analysis. In Cheese and Fermented Milk Foods; Kosikowski, F., Ed.; Brooktondale: New York, NY, USA, 1978; pp. 560–597. [Google Scholar]
- Ballesteros, C.; Poveda, J.M.; González-Viñas, M.A.; Cabezas, L. Microbiological, biochemical and sensory characteristics of artisanal and industrial Manchego cheeses. Food Control 2006, 17, 249–255. [Google Scholar] [CrossRef]
- Cabezas, L.; Sánchez, I.; Poveda, J.M.; Seseña, S.; Palop, M.L. Comparison of microflora, chemical and sensory characteristics of artisanal Manchego cheeses from two dairies. Food Control 2007, 18, 11–17. [Google Scholar] [CrossRef]
- Rowe, M.T.; Kirk, R. An investigation into the phenomenon of cross-protection in Escherichia coli O157:H7. Food Microbiol. 1999, 16, 157–164. [Google Scholar] [CrossRef]
- European Commission Regulation (EC). No 2073/2005 of the Commission of 15 November 2005 laying down microbiological criteria on food products. Off. J. Eur. Union 2005, L338. Available online: http://data.europa.eu/eli/reg/2005/2073/oj (accessed on 2 August 2020).
- Vidovic, S.; Mangalappalli-Illathu, A.K.; Korber, D.R. Prolonged cold stress response of Escherichia coli O157 and the role of rpoS. Int. J. Food Microbiol. 2011, 146, 163–169. [Google Scholar] [CrossRef]
- Oh, J.-H.; Vinay-Lara, E.; McMinn, R.; Glass, K.A.; Johnson, M.E.; Steele, J.L. Evaluation of NaCl, pH, and lactic acid on the growth of Shiga toxin-producing Escherichia coli in a liquid Cheddar cheese extract. J. Dairy Sci. 2014, 97, 6671–6679. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Tasara, T.; Hummer Johann, J.; Stephan, R. An overview of molecular stress response mechanisms in Escherichia coli contributing to survival of Shiga toxin–producing Escherichia coli during raw milk cheese production. J. Food Prot. 2011, 74, 849–864. [Google Scholar] [CrossRef]
- Fernandes, C.F.; Shahani, K.M. Modulation of antibiosis by lactobacilli and other lactic cultures and fermented foods. Microbiol. Aliment. Nutr. 1989, 7, 337–352. [Google Scholar]
- Dineen, S.S.; Takeuchi, K.; Soudah, J.E.; Boor, K.J. Persistence of Escherichia coli O157:H7 in dairy fermentation systems. J. Food Prot. 1998, 61, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- European Parliament; Council Regulation (EC). No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L139. Available online: http://data.europa.eu/eli/reg/2004/853/oj (accessed on 2 August 2020).
- Reitsma, C.J.; Henning, D.R. Survival of enterohemorrhagic Escherichia coli O157:H7 during the manufacture and curing of cheddar cheese. J. Food Prot. 1996, 59, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.M.; Thormann, K.; Frunzke, J. Impact of spontaneous prophage induction on the fitness of bacterial populations and host-microbe interactions. J. Bacteriol. 2015, 197, 410–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Mercer, R.G.; McMullen, L.M.; Gänzle, M.G. Induction of Shiga toxin en coding prophage by abiotic environmental stress in food. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
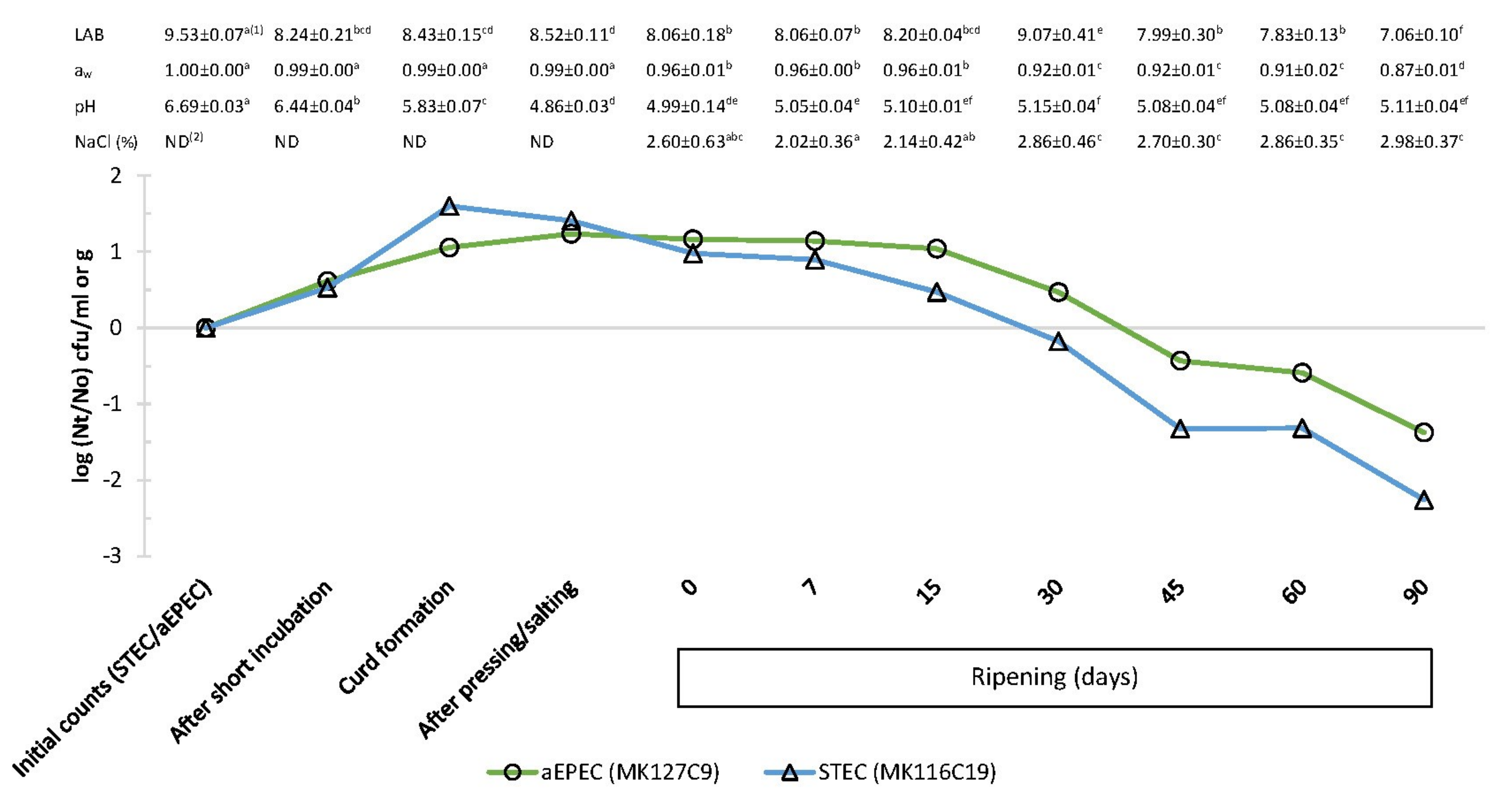
| Steps of Manufacture | Control Cheese 1 | Inoculated Cheese 2 | ||||
|---|---|---|---|---|---|---|
| pH | aw | NaCl | pH | aw | NaCl | |
| Milk | 6.76 ± 0.01a 3 | 1.00 ± 0.00 a | ND 4 | 6.70 ± 0.03 a | 1.00 ± 0.00 a | ND |
| After short incubation | 6.44 ± 0.03 b | 1.00 ± 0.00 a | ND | 6.44 ± 0.04 b | 0.99 ± 0.00 a | ND |
| Freshly cut curd | 5.67 ± 0.06 c | 0.99 ± 0.00 ab | ND | 5.83 ± 0.07 c | 0.99 ± 0.00 a | ND |
| Curd after moulding | 5.00 ± 0.09 d | 0.99 ± 0.00 ab | ND | 4.86 ± 0.03 f | 0.99 ± 0.00 a | ND |
| After pressing/salting (0 d) | 5.19 ± 0.02 g | 0.97 ± 0.01 bc | 1.90 ± 0.42 a | 4.99 ± 0.14 e | 0.96 ± 0.01 b | 2.60 ± 0.63 abc |
| Cheese ripening: | ||||||
| 7 d | 5.08 ± 0.02 def | 0.96 ± 0.01 c | 2.45 ± 0.66 ab | 5.05 ± 0.04 de | 0.96 ± 0.00 b | 2.02 ± 0.36 a |
| 15 d | 5.13 ± 0.04 fg | 0.95 ± 0.01 cd | 2.66 ± 0.60 abc | 5.10 ± 0.01 d | 0.96 ± 0.01 b | 2.14 ± 0.42 ab |
| 30 d | 5.04 ± 0.04 def | 0.93 ± 0.02 de | 2.96 ± 0.56 bc | 5.15 ± 0.04 d | 0.92 ± 0.01 c | 2.86 ± 0.46 c |
| 45 d | 5.02 ± 0.05 de | 0.92 ± 0.01 e | 3.26 ± 0.19 c | 5.08 ± 0.03 de | 0.92 ± 0.01 cd | 2.70 ± 0.30b c |
| 60 d | 5.12 ± 0.07 efg | 0.91 ± 0.02 e | 3.02 ± 0.70 bc | 5.08 ± 0.04 de | 0.91 ± 0.02 d | 2.86 ± 0.35 c |
| 90 d | 5.10 ± 0.09 defg | 0.88 ± 0.01 f | 3.25 ± 0.17 c | 5.11 ± 0.04 d | 0.87 ± 0.01 e | 2.98 ± 0.37c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, E.A.; Ramos-Pereira, J.; Santos, J.A.; López-Díaz, T.M.; Otero, A.; Rodríguez-Calleja, J.M. Behaviour of Non-O157 STEC and Atypical EPEC during the Manufacturing and Ripening of Raw Milk Cheese. Foods 2020, 9, 1215. https://doi.org/10.3390/foods9091215
Rios EA, Ramos-Pereira J, Santos JA, López-Díaz TM, Otero A, Rodríguez-Calleja JM. Behaviour of Non-O157 STEC and Atypical EPEC during the Manufacturing and Ripening of Raw Milk Cheese. Foods. 2020; 9(9):1215. https://doi.org/10.3390/foods9091215
Chicago/Turabian StyleRios, Edson A., Juliana Ramos-Pereira, Jesús A. Santos, Teresa M. López-Díaz, Andrés Otero, and Jose M. Rodríguez-Calleja. 2020. "Behaviour of Non-O157 STEC and Atypical EPEC during the Manufacturing and Ripening of Raw Milk Cheese" Foods 9, no. 9: 1215. https://doi.org/10.3390/foods9091215
APA StyleRios, E. A., Ramos-Pereira, J., Santos, J. A., López-Díaz, T. M., Otero, A., & Rodríguez-Calleja, J. M. (2020). Behaviour of Non-O157 STEC and Atypical EPEC during the Manufacturing and Ripening of Raw Milk Cheese. Foods, 9(9), 1215. https://doi.org/10.3390/foods9091215