Green/Roasted Coffee May Reduce Cardiovascular Risk in Hypercholesterolemic Subjects by Decreasing Body Weight, Abdominal Adiposity and Blood Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Dietary Control and Compliance
2.4. Anthropometric Measurements and Physical Activity
2.5. Lipid Profile. Diabetes and Appetite Biomarker Analysis
2.6. Statistical Analysis
3. Results
3.1. Volunteer Characteristics and Dietary Intake
3.2. Anthropometric Measurements and Physical Activity
3.3. Lipid Profile. Appetite and Diabetes Biomarkers
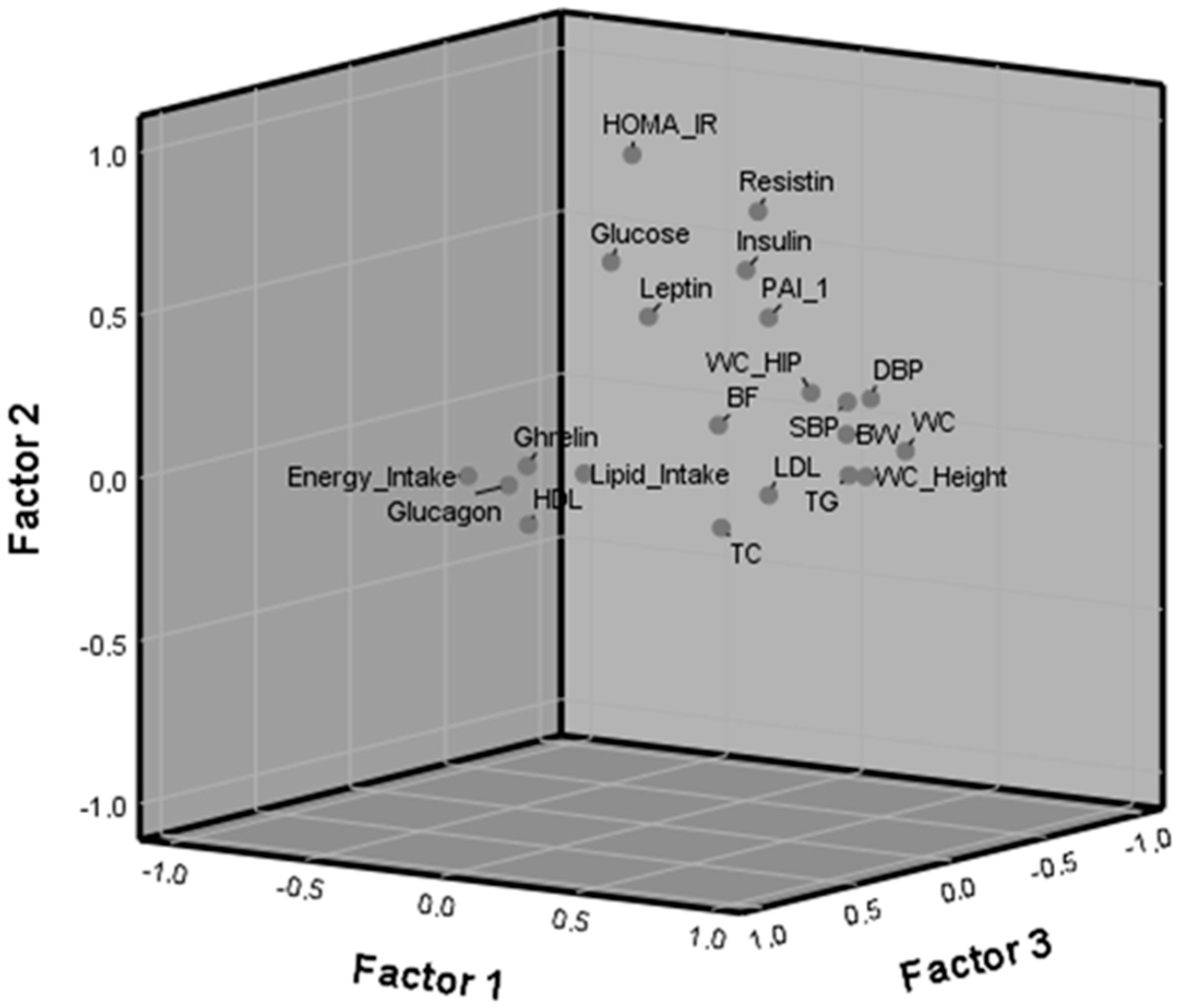
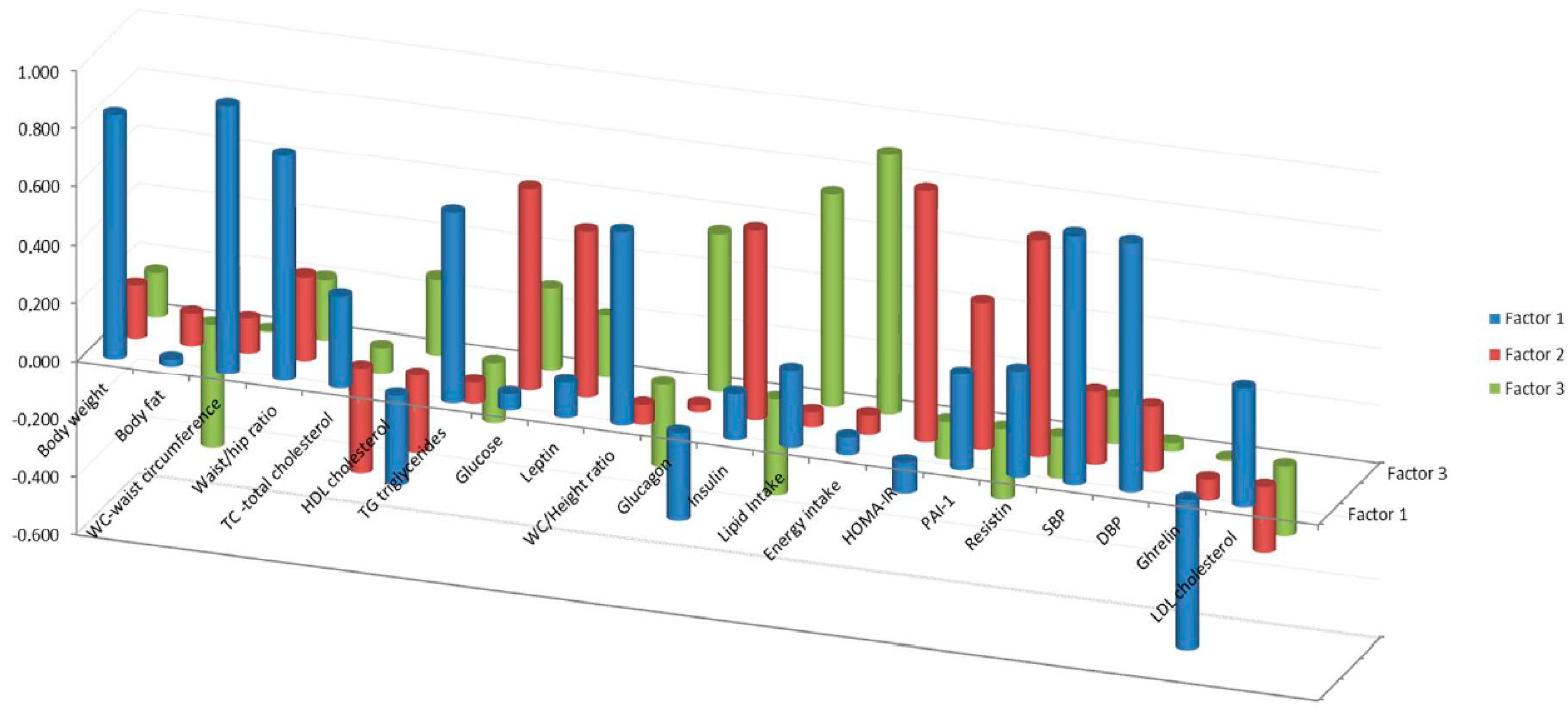
3.4. Factor Component Analysis
4. Discussion
4.1. Effects of the Coffee Blend on Anthropometric Measurements in Normocholesterolemic and Hypercholesterolemic Subjects
4.2. Factor Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New dietary supplements for obesity: What we currently know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Tunnicliffe, J.M.; Shearer, J. Coffee, glucose homeostasis, and insulin resistance: Physiological mechanisms and mediators. Appl. Physiol. Nutr. Metab. 2008, 33, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Watanabe, T.; Kobayashi, S.; Yamaguchi, T.; Hibi, M.; Fukuhara, I.; Osaki, N. Coffee abundant in chlorogenic acids reduces abdominal fat in overweight adults: A randomized, double-blind, controlled trial. Nutrients 2019, 11, 1617. [Google Scholar] [CrossRef]
- Pan, M.H.; Tung, Y.C.; Yang, G.; Li, S.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct. 2016, 7, 4481–4491. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Boozer, C.N.; Geliebter, A. Coffee, diabetes, and weight control. Am. J. Clin. Nutr. 2006, 84, 682–693. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Gökcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef]
- Martini, D.; Rosi, A.; Tassotti, M.; Antonini, M.; Dall’Asta, M.; Bresciani, L.; Fantuzzi, F.; Spigoni, V.; Domínguez-Perles, R.; Angelino, D.; et al. Effect of coffee and cocoa-based confectionery containing coffee on markers of cardiometabolic health: Results from the pocket-4-life project. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Sarriá, B.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Long-term consumption of a green/roasted coffee blend positively affects glucose metabolism and insulin resistance in humans. Food Res. Intern. 2016, 89, 1023–1028. [Google Scholar] [CrossRef]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo-Clemente, L. Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome. Eur. J. Nutr. 2018, 57, 269–278. [Google Scholar] [CrossRef]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, S.; Hatzold, T.; El Yamani, N.; Stavro, P.M.; Lorenzo, Y.; Dusinska, M.; Reus, A.; Pasman, W.; Collins, A. Coffee and oxidative stress: A human intervention study. Eur. J. Nutr. 2018, 57, 533–544. [Google Scholar] [CrossRef]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. In Techniques for Measuring Body Composition; Brozek, J., Henschel, A., Eds.; National Academy of Sciences—National Research Council: Washington, DC, USA, 1961; pp. 223–244. [Google Scholar]
- Durnin, J.V.; Womersley, J.V.G.A. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO/UNO. Expert Consultation Report. Human Energy Requirements; United Nations University World Health Organization Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Garaulet, M.; Perez-Llamas, F.; Canteras, M.; Tebar, F.J.; Zamora, S. Endocrine, metabolic and nutritional factors in obesity and their relative significance as studied by factor analysis. Int. J. Obes. 2001, 25, 243–250. [Google Scholar] [CrossRef][Green Version]
- FAO/WHO/UNO. Expert Consultation Report Energy and Protein Requirements; Technical Report Series No 724; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- Martini, D.; Chiavaroli, L.; González-Sarrías, A.; Bresciani, L.; Palma-Duran, S.A.; Dall’Asta, M.; Deligiannidou, G.E.; Massaro, M.; Scoditti, E.; Combet, E.; et al. Impact of Foods and Dietary Supplements Containing Hydroxycinnamic Acids on Cardiometabolic Biomarkers: A Systematic Review to Explore Inter-Individual Variability. Nutrients 2019, 11, 1805. [Google Scholar] [CrossRef]
- Lee, A.; Lim, W.; Kim, S.; Khil, H.; Cheon, E.; An, S.; Hong, S.; Lee, D.H.; Kang, S.S.; Oh, H.; et al. Coffee Intake and Obesity: A Meta-Analysis. Nutrients 2019, 11, 1274. [Google Scholar] [CrossRef]
- Gavrieli, A.; Fragopoulou, E.; Mantzoros, C.S.; Yannakoulia, M. Gender and body mass index modify the effect of increasing amounts of caffeinated coffee on postprandial glucose and insulin concentrations; a randomized, controlled, clinical trial. Metabolism 2013, 62, 1099–1106. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzman, O.J.; Munoz-Durango, K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Lima, D.R. Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur. J. Nutr. 2016, 55, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-J.; Liu, C.-Y.; Chiu, J.-P.; Hsu, C.-H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Bravo, L.; Mateos, R.; Sarriá, B. Preventive effect of coffee against cardiovascular diseases. In Coffee: Chemistry, Quality and Health Implications; Farah, A., Ed.; Cambridge, Royal Society of Chemistry: London, UK, 2019; pp. 105–146. [Google Scholar]
- Lee, C.M.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- Momin, M.; Fan, F.; Li, J.; Jia, J.; Zhang, L.; Zhang, Y.; Huo, Y. Joint Effects of Body Mass Index and Waist Circumference on the Incidence of Hypertension in a Community-Based Chinese Population. Obes. Facts 2019. [Google Scholar] [CrossRef]
- Song, Y.H. The association of blood pressure with body mass index and waist circumference in normal weight and overweight adolescents. Korean J. Pediatr. 2014, 57, 79–84. [Google Scholar] [CrossRef][Green Version]
- Berchtold, P.; Berger, M.; Jörgens, V.; Daweke, C.; Chantelau, E. Cardiovascular risk factors and HDL-cholesterol levels in obesity. Int. J. Obes. 1981, 5, 1–10. [Google Scholar]
- Terry, R.B.; Wood, P.D.; Haskell, W.L.; Stefanick, M.L.; Krauss, R.M. Regional adiposity patterns in relation to lipids, lipoprotein cholesterol, and lipoprotein subfraction mass in men. J. Clin. Endocrinol. Metab. 1989, 68, 191–199. [Google Scholar] [CrossRef]
- Albrink, M.J.; Krauss, R.M.; Lindgrem, F.T.; von der Groeben, J.; Pan, S.; Wood, P.D. Intercorrelations among plasma high density lipoprotein, obesity and triglycerides in a normal population. Lipids 1980, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sanchez, K.; Leyva, M.U.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschop, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, R5–R9. [Google Scholar] [CrossRef]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef] [PubMed]
- Denroche, H.C.; Huynh, F.K.; Kieffe, T.J. The role of leptin in glucose homeostasis. J. Diabetes Investig. 2012, 3, 115–129. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bordin Barbieri, N.; Weinmann, T.; Ziegelmann, P.K.; Duncan, B.B.; Schmidt, M.I. Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci. Rep. 2016, 6, 17714. [Google Scholar] [CrossRef]
- Al-Massadi, O.; Fernø, J.; Diéguez, C.; Nogueiras, R.; Quiñones, M. Glucagon control on food intake and energy balance. Int. J. Mol. Sci. 2019, 20, 3905. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Men and women | Suffering chronic pathology other than hypercholesterolemia |
| 18–55 y old | Smoking |
| BMI 20–25 kg/m2 | Vegetarianism |
| Pregnancy in women | |
| Attending to T-C levels in blood two groups: Normocholesterolemics (T-C < 200 mg/dL) Hypercholesterolemics (T-C 200–240 mg/dL) | Having taken dietary supplements, laxatives, or antibiotics six months before the beginning of the study |
| Normocholesterolemics (n = 25) | Hypercholesterolemics (n = 27) | p Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Control | Coffee | Baseline | Control | Coffee | Coffee | Group | |
| Body weight (kg) | 63.0 ± 2.2 | 62.8 ± 2.8 | 62.5 ± 2.3 | 63.0 ± 2.8 | 62.9 ± 2.8 | 62.0 ± 2.8 | 0.017 | N.S. |
| Body fat (%) | 22.8 ± 1.4 | 23.7 ± 1.4 | 21.5 ± 1.2 | 25.4 ± 1.3 | 24.4 ± 1.2 | 23.6 ± 1.3 | 0.001 | N.S. |
| BMI (kg/m2) | 21.5 ± 0.5 a | 21.5 ± 0.5 a | 21.3 ± 0.5 b | 23.8 ± 0.5 a | 23.6 ± 0.6 a,b | 23.4 ± 0.6 b | 0.012 | N.S. |
| Waist circumference (cm) | 70.4 ± 1.3 | 70.6 ± 1.4 | 70.9 ± 1.4 | 76.8 ± 2.5 | 76.6 ± 2.5 | 75.6 ±2.5 | N.S. | 0.007 |
| Hip circumference (cm) | 97.2 ± 1.1 | 96.7 ± 1.2 | 96.4 ± 1.3 | 96.4 ± 1.3 | 96.6 ± 1.4 | 96.3 ± 1.2 | N.S. | N.S. |
| Thigh circumference (cm) | 52.0 ± 0.6 | 52.3 ± 0.6 | 51.8 ± 0.7 | 51.3 ± 1.0 | 50.9 ± 0.8 | 50.5 ± 0.9 | N.S. | N.S. |
| Arm circumference (cm) | 27.2 ± 0.5 a | 27.4 ± 0.5 a,b | 27.7 ± 0.5 b | 27.2 ± 0.8 | 27.3 ± 0.8 | 27.2 ± 0.8 | N.S. | N.S. |
| Waist/Hip ratio | 0.72 ± 0.0 | 0.73 ± 0.0 | 0.73 ± 0.0 | 0.80 ± 0.0 | 0.80 ± 0.0 | 0.79 ± 0.0 | N.S. | 0.001 |
| Waist/height ratio | 0.43 ± 0.0 | 0.43 ± 0.0 | 0.43 ± 0.0 | 0.46 ± 0.0 | 0.46 ± 0.0 | 0.45 ± 0.0 | N.S | 0.002 |
| Tricipital fold (mm) | 17.1 ± 1.2 | 16.7 ± 1.2 | 15.9 ± 1.2 | 16.1 ± 1.1 | 16.3 ± 1.2 | 15.9 ± 1.3 | N.S. | N.S. |
| Subscapular fold (mm) | 10.8 ± 0.7 | 10.5 ± 0.6 | 10.2 ± 0.6 | 13.6 ± 1.3 | 13.1 ± 1.2 | 12.9 ± 1.2 | N.S. | N.S. |
| Loadings of Variables by Factors | |||
|---|---|---|---|
| F1 | F2 | F3 | |
| Waist circumference | 0.919 | 0.119 | 0.005 |
| Diastolic blood pressure | 0.862 | 0.225 | 0.029 |
| Systolic blood pressure | 0.860 | 0.251 | 0.161 |
| Body weight | 0.837 | 0.183 | 0.153 |
| Waist/hip ratio | 0.776 | 0.289 | 0.209 |
| Waist/height ratio | 0.665 | −0.067 | −0.285 |
| Triglycerides | 0.665 | −0.072 | −0.211 |
| HOMA-IR | −0.106 | 0.864 | −0.131 |
| Resistin | 0.368 | 0.749 | −0.144 |
| Glucose | 0.057 | 0.691 | 0.283 |
| Insulin | 0.160 | 0.652 | −0.330 |
| Leptin | 0.123 | 0.571 | 0.212 |
| PAI-1 | 0.331 | 0.508 | −0.241 |
| Energy intake | 0.061 | 0.067 | 0.894 |
| Lipid intake | 0.268 | 0.052 | 0.731 |
| Glucagon | −0.303 | 0.025 | 0.545 |
| HDL-cholesterol | −0.309 | −0.267 | 0.262 |
| Ghrelin | −0.513 | −0.072 | −0.008 |
| Total-cholesterol | 0.318 | −0.364 | −0.089 |
| LDL-cholesterol | 0.410 | −0.227 | −0.239 |
| Body fat percentage | 0.024 | 0.112 | −0.423 |
| F1 | F2 | F3 | |
|---|---|---|---|
| Baseline | 29.08 (12.24) | 5.03 (4.51) | 5.89 (2.97) |
| After the coffee intervention | 18.89 (14.86) | 3.16 (4.43) | 4.00 (3.50) |
| p < 0.001 | N.S. | N.S. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarriá, B.; Sierra-Cinos, J.L.; García-Diz, L.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Green/Roasted Coffee May Reduce Cardiovascular Risk in Hypercholesterolemic Subjects by Decreasing Body Weight, Abdominal Adiposity and Blood Pressure. Foods 2020, 9, 1191. https://doi.org/10.3390/foods9091191
Sarriá B, Sierra-Cinos JL, García-Diz L, Martínez-López S, Mateos R, Bravo-Clemente L. Green/Roasted Coffee May Reduce Cardiovascular Risk in Hypercholesterolemic Subjects by Decreasing Body Weight, Abdominal Adiposity and Blood Pressure. Foods. 2020; 9(9):1191. https://doi.org/10.3390/foods9091191
Chicago/Turabian StyleSarriá, Beatriz, José Luis Sierra-Cinos, Luis García-Diz, Sara Martínez-López, Raquel Mateos, and Laura Bravo-Clemente. 2020. "Green/Roasted Coffee May Reduce Cardiovascular Risk in Hypercholesterolemic Subjects by Decreasing Body Weight, Abdominal Adiposity and Blood Pressure" Foods 9, no. 9: 1191. https://doi.org/10.3390/foods9091191
APA StyleSarriá, B., Sierra-Cinos, J. L., García-Diz, L., Martínez-López, S., Mateos, R., & Bravo-Clemente, L. (2020). Green/Roasted Coffee May Reduce Cardiovascular Risk in Hypercholesterolemic Subjects by Decreasing Body Weight, Abdominal Adiposity and Blood Pressure. Foods, 9(9), 1191. https://doi.org/10.3390/foods9091191