Prevalence of Multidrug-Resistant Foodborne Pathogens and Indicator Bacteria from Edible Offal and Muscle Meats in Nashville, Tennessee
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.1.1. Detection of Salmonella Enterica serovar
2.1.2. Detection of Campylobacter spp.
2.1.3. Detection of Generic Escherichia coli and Escherichia coli O157:H7
2.1.4. Detection of Enterococcus spp.
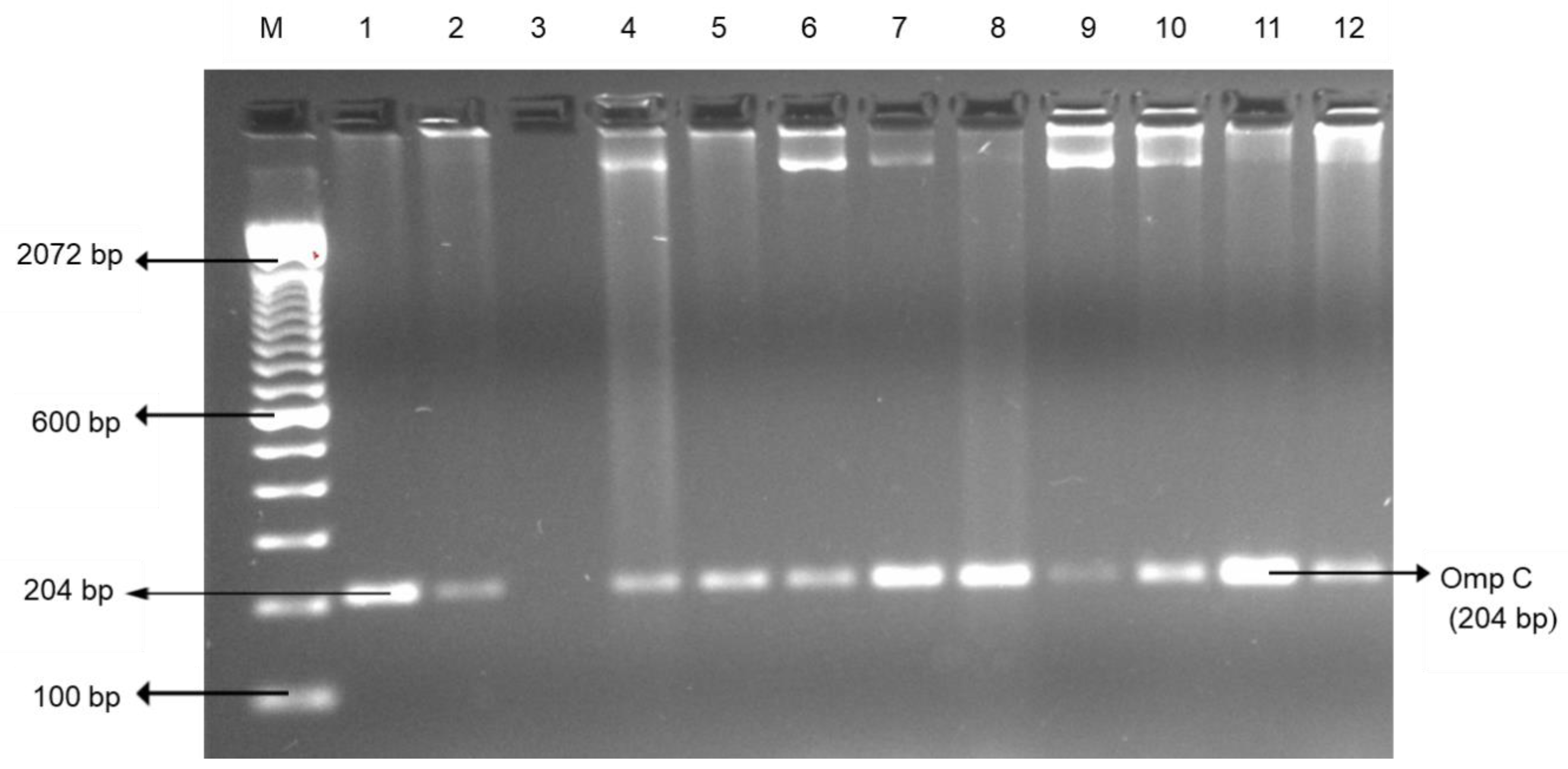
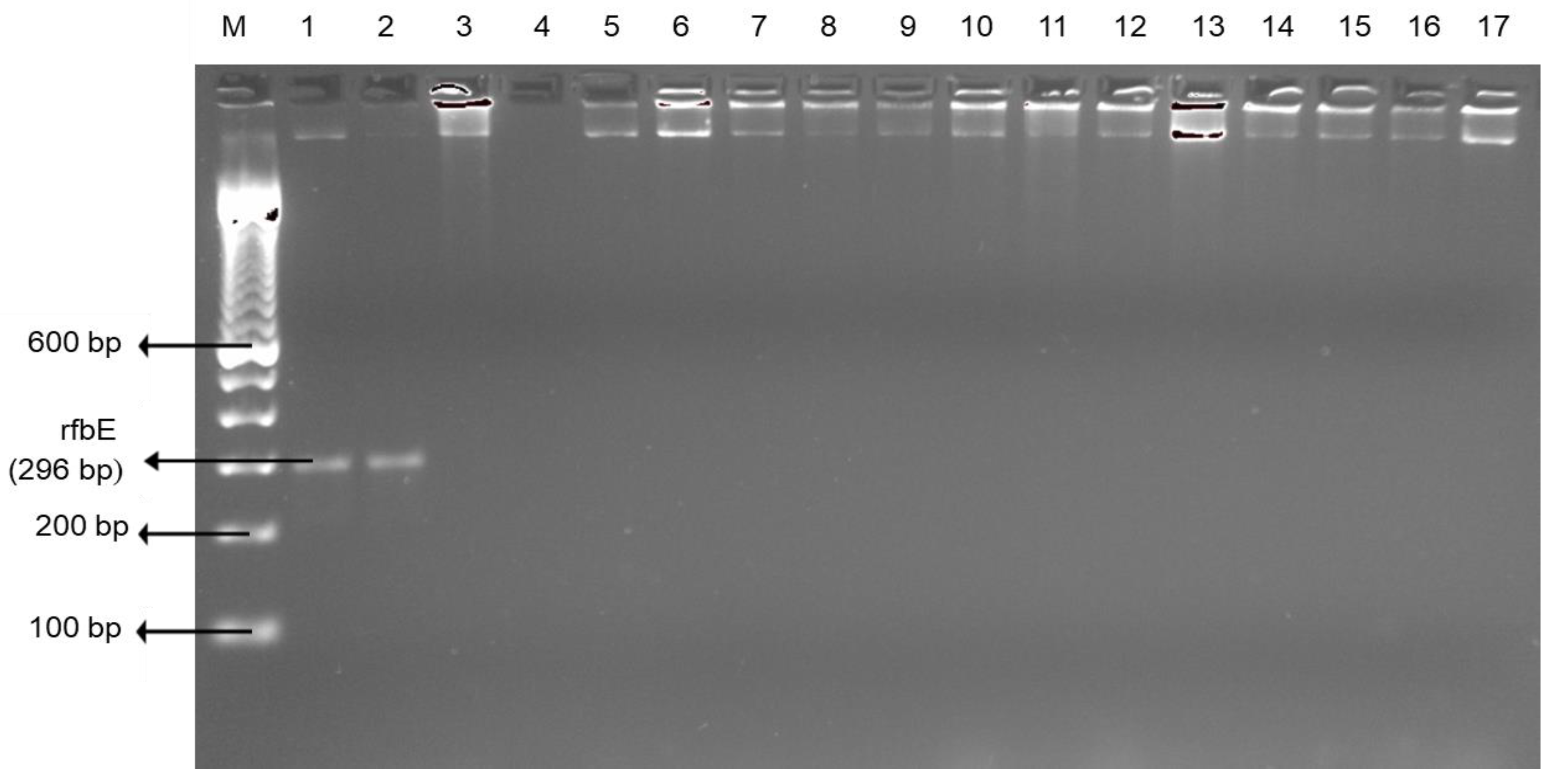
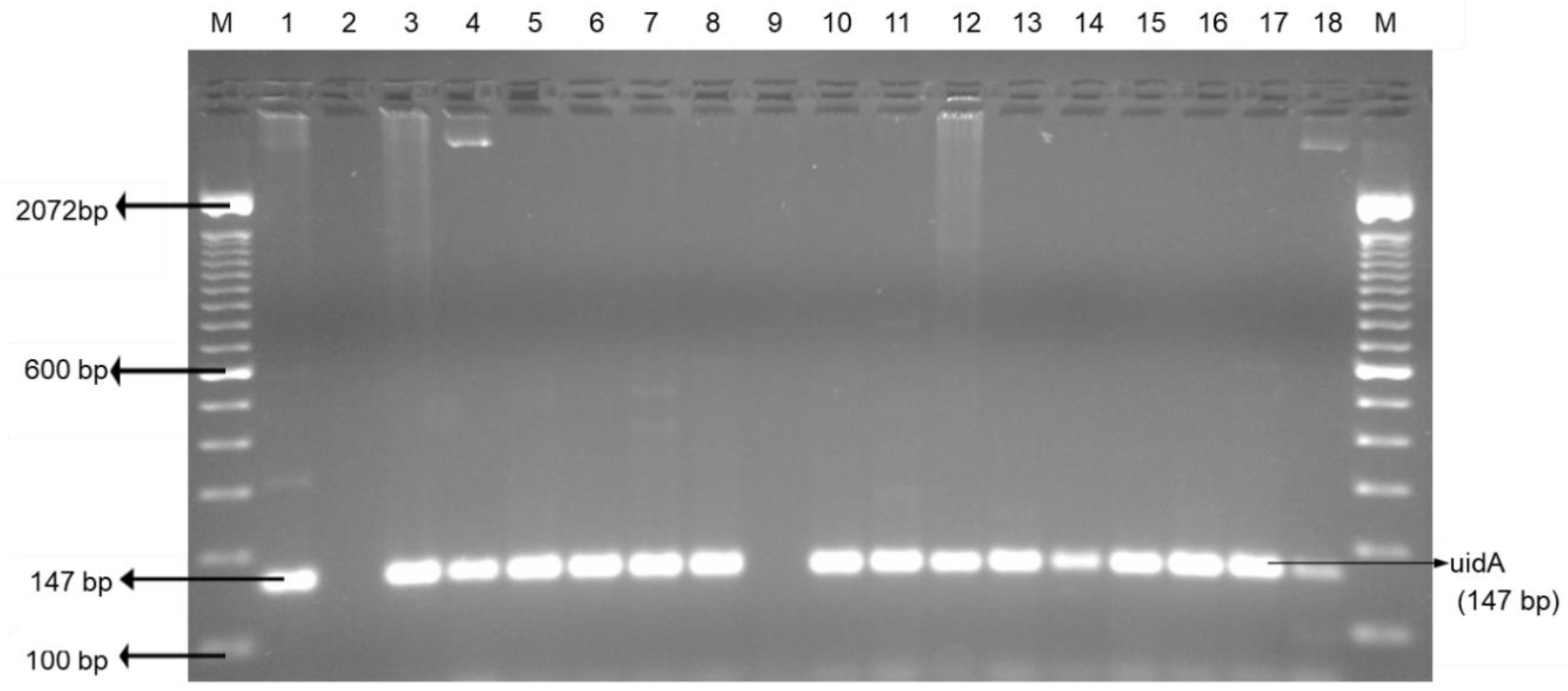
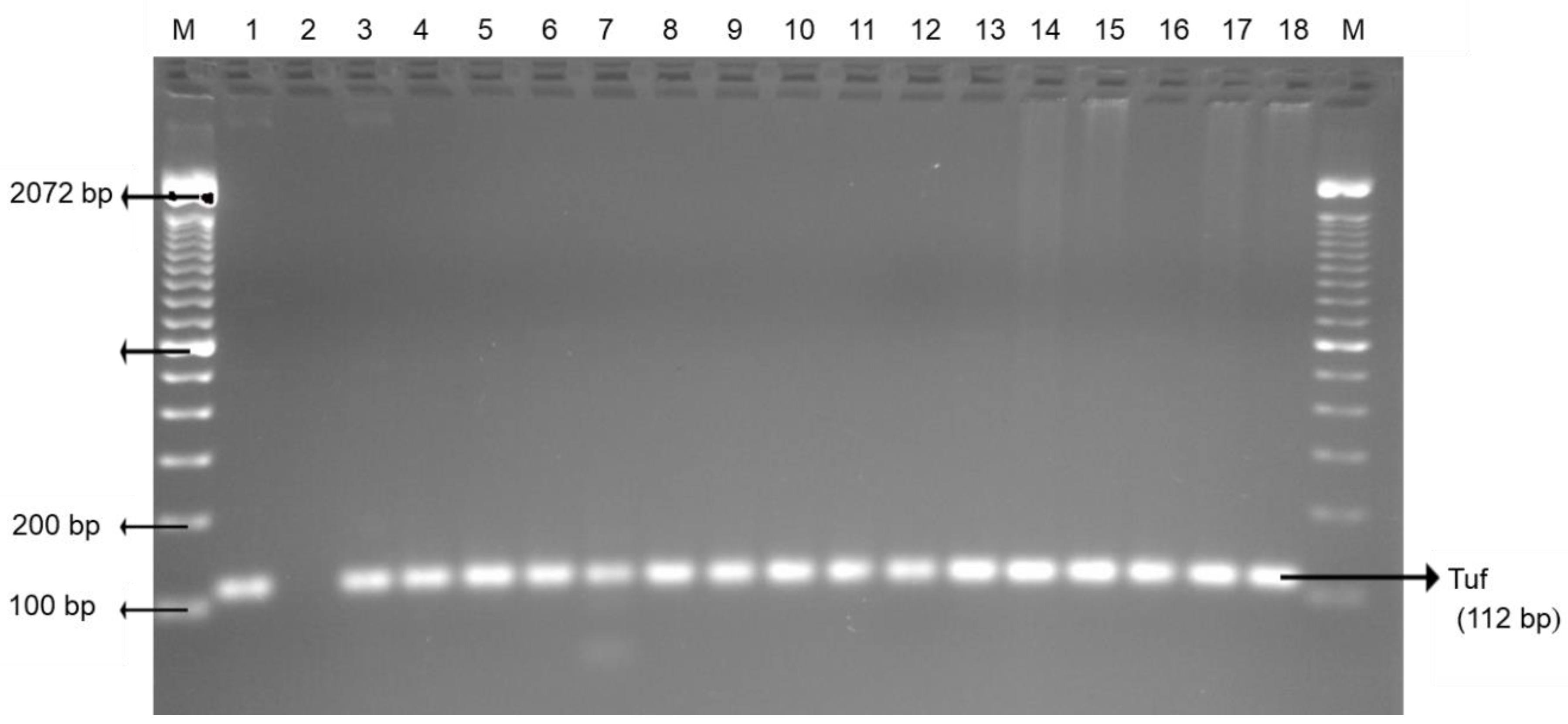
2.2. Bacterial DNA Preparation and Bacteria Confirmation
2.3. Antibiotic Resistant Profiles
2.4. Statistical Analysis
3. Results and Discussion
3.1. Pathogenic Bacteria: Salmonella enterica serovar and Campylobacter spp.
3.2. Indicator Bacteria: Escherichia coli and enterococci
3.3. Antimicrobial Drug Resistance in Pathogenic Bacteria
3.4. Antimicrobial Drug Resistance in Escherichia coli and Enterococci
3.5. Multidrug Resistant Patterns of Pathogenic and Indicator Bacteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The potential of animal by-products in food systems: Production, prospects, and challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Seong, P.N.; Kang, G.H.; Park, K.M.; Cho, S.H.; Kang, S.M.; Park, B.Y.; Moon, S.S.; Van Ba, H. Characterization of Hanwoo bovine by-products by means of yield, physicochemical, and nutritional compositions. Korean J. Food Sci. An. 2014, 34, 434. [Google Scholar] [CrossRef]
- Podpečan, B.; Pengov, A.; Vadnjal, S. The source of contamination of ground meat for production of meat products with bacteria Staphylococcus aureus. Slov. Vet. Res. 2007, 44, 25–30. [Google Scholar]
- Jribi, H.; Sellami, H.; Mariam, S.; Smaoui, S.; Ghorbel, A.; Hachicha, S.; Benejat, L.; Messadi-Akrout, F.; Mégraud, F.; Gdoura, R. Isolation and Identification of Campylobacter spp. from Poultry and Poultry By-Products in Tunisia by Conventional Culture Method and Multiplex Real-Time PCR. J. Food Prot. 2017, 80, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Rajagunalan, S.; Tiwari, R.; Verma, A.; Singh, S. Food-borne pathogens of poultry having public health significance. Poult. World 2013, 16, 8–12. [Google Scholar]
- Chotinantakul, K.; Chansiw, N.; Okada, S. Antimicrobial resistance of Enterococcus spp. isolated from Thai fermented pork in Chiang Rai Province, Thailand. J. Glob. Antimicrob. Resist. 2018, 12, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Mellata, M. Human and avian extraintestinal pathogenic Escherichia coli: Infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef]
- Tyson, G.H.; Nyirabahizi, E.; Crarey, E.; Kabera, C.; Lam, C.; Rice-Trujillo, C.; McDermott, P.F.; Tate, H. Prevalence and antimicrobial resistance of Enterococci isolated from retail meats in the United States, 2002 to 2014. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Abdalrahman, L.S.; Wells, H.; Fakhr, M.K. Staphylococcus aureus is more prevalent in retail beef livers than in pork and other beef cuts. Pathogens 2015, 4, 182–198. [Google Scholar] [CrossRef]
- New Zealand Food Safety (NZFS). Safe Cooking of Livers: Information for Chefs. 2017. Available online: https://www.mpi.govt.nz/food-safety/food-safety-codes-and-standards/good-operating-practice/documents/safe-cooking-of-livers (accessed on 1 July 2020).
- Lanier, W.A.; Hale, K.R.; Allen, L.; White, P.; Bachert, J.; Dewey-Mattia, D.; Geissler, A. Chicken Liver–Associated Illness Outbreaks, United States, 2000–2015—Identifying Opportunities for Prevention; The Council of State and Territorial Epidemiologists: Atlanta, Georgia, 2017. [Google Scholar]
- Topp, E. Agriculture and Agri-Food Canada’s research program on antimicrobial resistance. Can. Commun. Dis. Rep. 2017, 43, 224–227. [Google Scholar] [CrossRef]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals’, Clinical infectious diseases: An official publication of the Infectious Diseases. Soc. Am. 2017, 34, S93–S106. [Google Scholar]
- Bosilevac, J.M.; Gassem, M.A.; Al Sheddy, I.A.; Almaiman, S.A.; Al-Mohizea, I.S.; Alowaimer, A.; Koohmaraie, M. Prevalence of Escherichia coli O157: H7 and Salmonella in camels, cattle, goats, and sheep harvested for meat in Riyadh. J. Food Prot. 2015, 78, 89–96. [Google Scholar] [CrossRef]
- Toldrá, F.; Aristoy, M.C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef]
- Alvarez, J.; Sota, M.; Vivanco, A.B.; Perales, I.; Cisterna, R.; Rementeria, A.; Garaizar, J. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J. Clin. Microbiol. 2004, 42, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, E.; Ameri, M. Antimicrobial resistance patterns of Campylobacter spp. isolated from raw chicken, turkey, quail, partridge, and ostrich meat in Iran. Food Control 2011, 22, 1165–1170. [Google Scholar] [CrossRef]
- Bai, J.; Shi, X.; Nagaraja, T. A multiplex PCR procedure for the detection of six major virulence genes in Escherichia coli O157: H7. J. Microbiol. Methods 2010, 82, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Tantawiwat, S.; Tansuphasiri, U.; Wongwit, W.; Wongchotigul, V.; Kitayaporn, D. Development of multiplex PCR for the detection of total coliform bacteria for Escherichia coli and Clostridium perfringens in drinking water. Southeast Asian J. Trop. Med. Public Health 2005, 36, 162–169. [Google Scholar] [PubMed]
- Iweriebor, B.C.; Obi, L.C.; Okoh, A.I. Virulence and antimicrobial resistance factors of Enterococcus spp. isolated from fecal samples from piggery farms in Eastern Cape, South Africa. BMC Microbiol. 2015, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; CLSI Supplement M100S; CLSI: Wayne, PA, USA, 2016. [Google Scholar]
- FDA. NARMS Integrated Report: 2014-The National Antimicrobial Resistance Monitoring System: Enteric Bacteria; FDA: White Oak, MD, USA, 2016.
- Erickson, A.K.; Murray, D.L.; Ruesch, L.A.; Thomas, M.; Lau, Z.; Scaria, J. Genotypic and Phenotypic Characterization of Salmonella Isolated from Fresh Ground Meats Obtained from Retail Grocery Stores in the Brookings, South Dakota, Area. J. Food Prot. 2018, 81, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.; Khai, L.T.L.; Ogasawara, N.; Tam, N.T.; Okatani, A.T.; Akiba, M.; Hayashidani, H. Contamination of Salmonella in retail meats and shrimps in the Mekong Delta, Vietnam. J. Food Prot. 2005, 68, 1077–1080. [Google Scholar] [CrossRef]
- Wideman, N.; Bailey, M.; Bilgili, S.F.; Thippareddi, H.; Wang, L.; Bratcher, C.; Sanchez-Plata, M.; Singh, M. Evaluating best practices for Campylobacter and Salmonella reduction in poultry processing plants. Poult. Sci. 2016, 95, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Samadpour, M.; Barbour, M.W.; Nguyen, T.; Cao, T.M.; Buck, F.; Depavia, G.A.; Mazengia, E.; Yang, P.; Alfi, D.; Lopes, M.; et al. Incidence of enterohemorrhagic Escherichia coli, Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in retail fresh ground beef, sprouts, and mushrooms. J. Food Prot. 2006, 69, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Noda, T.; Onozuka, D.; Sera, N. Salmonella in liquid eggs and other foods in Fukuoka Prefecture, Japan. Int. J. Microbiol. 2013. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly-prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong, China. Front Microbiol. 2018, 9, 2104. [Google Scholar] [CrossRef]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; McCarthy, M.; Resconi, V.C.; Troy, D. Meat consumption: Trends and quality matters. Meat Sci. 2014, 98, 561–568. [Google Scholar] [CrossRef]
- Trokhymchuk, A.; Waldner, C.; Chaban, B.; Gow, S.; Hill, J.E. Prevalence and diversity of Campylobacter species in Saskatchewan retail ground beef. J. Food Prot. 2014, 77, 2106–2110. [Google Scholar] [CrossRef]
- Enokimoto, M.; Kubo, M.; Bozono, Y.; Mieno, Y.; Misawa, N. Enumeration and identification of Campylobacter species in the liver and bile of slaughtered cattle. Int. J. Food Microbiol. 2007, 118, 259–263. [Google Scholar] [CrossRef]
- Noormohamed, A.; Fakhr, M. A higher prevalence rate of Campylobacter in retail beef livers compared to other beef and pork meat cuts. Int. J. Environ. Res. Public Health 2013, 10, 2058–2068. [Google Scholar] [CrossRef]
- Strachan, N.; MacRae, M.; Thomson, A.; Rotariu, O.; Ogden, I.; Forbes, K. Source attribution, prevalence, and enumeration of Campylobacter spp. from retail liver. Int. J. Food Microbiol. 2012, 153, 234–236. [Google Scholar] [CrossRef]
- Ghafir, Y.; China, B.; Dierick, K.; De Zutter, L.; Daube, G. A seven-year survey of Campylobacter contamination in meat at different production stages in Belgium. Int. J. Food Microbiol. 2007, 116, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Vipham, J.L.; Brashears, M.M.; Loneragan, G.H.; Echeverry, A.; Brooks, J.C.; Chaney, W.E.; Miller, M.F. Salmonella and Campylobacter baseline in retail ground beef and whole-muscle cuts purchased during 2010 in the United States. J. Food Prot. 2012, 75, 2110–2115. [Google Scholar] [CrossRef] [PubMed]
- Zbrun, M.V.; Olivero, C.; Romero-Scharpen, A.; Rossler, E.; Soto, L.P.; Astesana, D.M.; Blajman, J.E.; Berisvil, A.; Signorini, M.L.; Frizzo, L.S. Antimicrobial resistance in thermotolerant Campylobacter isolated from different stages of the poultry meat supply chain in Argentina. Food Control 2015, 57, 136–141. [Google Scholar] [CrossRef]
- Bolton, D.J. Campylobacter virulence and survival factors. Food Microbiol. 2015, 48, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.; Rigby, D.; Burton, M.; Millman, C.; Williams, N.J.; Jones, T.R.; Wigley, P.; O’Brien, S.J.; Cross, P.; ENIGMA Consortium. Restaurant cooking trends and increased risk for Campylobacter infection. Emerg. Infect. Dis. 2016, 22, 1208. [Google Scholar] [CrossRef]
- Scott, M.K.; Geissler, A.; Poissant, T.; DeBess, E.; Melius, B.; Eckmann, K.; Salehi, E.; Cieslak, P.R. Notes from the field: Campylobacteriosis outbreak associated with consuming undercooked chicken liver pâté-Ohio and Oregon, December 2013–January 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 399. [Google Scholar]
- Lahti, E.; Löfdahl, M.; Ågren, J.; Hansson, I.; Olsson Engvall, E. Confirmation of a campylobacteriosis outbreak associated with chicken liver pâté using PFGE and WGS. Zoonoses Public Health 2017, 64, 14–20. [Google Scholar] [CrossRef]
- Food Authority. Microbiological Quality of Beef, Lamb and Pork Meat Cuts and Offal. Available online: http://www.foodauthority.nsw.gov.au/_Documents/scienceandtechnical/campylobacter_in_meat_and_offal.pdf (accessed on 10 June 2020).
- Faten, S.H.; Amani, M.S.; Mervat, S.H.; Gaafar, M.H. Enterobacteriaceae in edible offal. J. Ben Vet Med. 2013, 25, 77–87. [Google Scholar]
- Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992–June 2001, issued August 2001. Am. J. Infect. Control 2001, 29, 404–421. [Google Scholar] [CrossRef]
- Shar, A.H.; Kazi, Y.F.; Kanhar, N.A.; Soomro, I.H.; Zia, S.M.; Ghumro, P.B. Drinking water quality in Rohri city, Sindh, Pakistan. Afr. J. Biotechnol. 2010, 9, 7102–7107. [Google Scholar]
- World Health Organization. Critically Important Antimicrobials for Human Medicine-5th Revision; WHO Press: Geneva, Switzerland, 2016; ISBN 978-92-4-151222-0. [Google Scholar]
- Thung, T.Y.; Radu, S.; Mahyudin, N.A.; Rukayadi, Y.; Zakaria, Z.; Mazlan, N.; Tan, B.H.; Lee, E.; Yeoh, S.L.; Chin, Y.Z.; et al. Prevalence, virulence genes, and antimicrobial resistance profiles of Salmonella serovars from retail beef in Selangor, Malaysia. Front Microbiol. 2018, 8, 2697. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Foods that Sickened People in Outbreaks with a Single Known Source, 2009–2016. Available online: https://www.cdc.gov/foodsafety/cdc-and-food-safety.html (accessed on 25 June 2020).
- Klochko, A. Salmonella Infection (Salmonellosis) Treatment & Management. Available online: https://emedicine.medscape.com/article/228174-treatment (accessed on 2 May 2020).
- Ge, B.; White, D.G.; McDermott, P.F.; Girard, W.; Zhao, S.; Hubert, S.; Meng, J. Prevalence and Antimicrobial. Appl. Environ. Microbiol. 2003, 69, 3005–3007. [Google Scholar] [CrossRef] [PubMed]
- Carev, M.; Kovačić, A.; Novak, A.; Tonkić, M.; Jerončić, A. Campylobacter jejuni strains co-resistant to tetracycline and ciprofloxacin in patients with gastroenteritis in Croatia. Infect. Dis. 2017, 49, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Osek, J. Antimicrobial resistance mechanisms among Campylobacter. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef]
- Andersen, S.R.; Saadbye, P.; Shukri, N.M.; Rosenquist, H.; Nielsen, N.L.; Boel, J. Antimicrobial resistance among Campylobacter jejuni isolated from raw poultry meat at retail level in Denmark. Int. J. Food Microbiol. 2006, 107, 250–255. [Google Scholar] [CrossRef]
- Maćkiw, E.; Korsak, D.; Rzewuska, K.; Tomczuk, K.; Rożynek, E. Antibiotic resistance in Campylobacter jejuni and Campylobacter coli isolated from food in Poland. Food Control 2012, 23, 297–301. [Google Scholar] [CrossRef]
- Nisar, M.; Mushtaq, M.H.; Shehzad, W.; Hussain, A.; Muhammad, J.; Nagaraja, K.V.; Goyal, S.M. Prevalence and antimicrobial resistance patterns of Campylobacter spp. isolated from retail meat in Lahore, Pakistan. Food Control 2017, 80, 327–332. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamamoto, S. Campylobacter contamination in retail poultry meats and by-products in the world: A literature survey. J. Vet. Med. Sci. 2009, 71, 255–261. [Google Scholar] [CrossRef]
- Karczmarczyk, M.; Martins, M.; Quinn, T.; Leonard, N.; Fanning, S. Mechanisms of fluoroquinolone resistance in Escherichia coli isolates from food-producing animals. Appl. Environ. Microbiol. 2011, 77, 7113–7120. [Google Scholar] [CrossRef]
- Little, C.; Richardson, J.; Owen, R.; De Pinna, E.; Threlfall, E. Campylobacter and Salmonella in raw red meats in the United Kingdom: Prevalence, characterization, and antimicrobial resistance pattern, 2003–2005. Food Microbiol. 2008, 25, 538–543. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Tanaka, M.; Ohtsuka, T.; Takatori, K.; Hara-Kudo, Y. Comparison of detection methods for Escherichia coli O157 in beef livers and carcasses. Foodborne Pathog. Dis. 2010, 7, 1563–1567. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Aslam, M.; Diarra, M.S.; Checkley, S.; Bohaychuk, V.; Masson, L. Characterization of antimicrobial resistance and virulence genes in Enterococcus spp. isolated from retail meats in Alberta, Canada. Int. J. Food Microbiol. 2012, 156, 222–230. [Google Scholar] [CrossRef]
- Klare, I.; Konstabel, C.; Badstübner, D.; Werner, G.; Witte, W. Occurrence and spread of antibiotic resistances in Enterococcus faecium. Int. J. Food Microbiol. 2003, 88, 269–290. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B.; Hiott, L.M.; Woodley, T.A. Prevalence of streptogramin resistance in enterococci from animals: Identification of vatD from animal sources in the USA. Int. J. Antimicrob. 2007, 30, 60–66. [Google Scholar] [CrossRef]
- Carvalho, I.; del Campo, R.; Sousa, M.; Silva, N.; Carrola, J.; Marinho, C.; Santos, T.; Carvalho, S.; Nóvoa, M.; Quaresma, M.; et al. Antimicrobial-resistant Escherichia coli and Enterococcus spp. isolated from Miranda donkey (Equus asinus): An old problem from a new source with a different approach. J. Med. Microbiol. 2017, 66, 191–202. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266. [Google Scholar] [CrossRef]
- Nguyen, D.T.A.; Kanki, M.; Do Nguyen, P.; Le, H.T.; Ngo, P.T.; Tran, D.N.M.; Le, N.H.; Van Dang, C.; Kawai, T.; Kawahara, R.; et al. Prevalence, antibiotic resistance, and extended-spectrum and AmpC β-lactamase productivity of Salmonella isolates from raw meat and seafood samples in Ho Chi Minh City, Vietnam. Int. J. Food Microbiol. 2016, 236, 115–122. [Google Scholar] [CrossRef]
- Chatre, P.; Haenni, M.; Meunier, D.; Botrel, M.-A.; Calavas, D.; Madec, J.-Y. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from cattle between 2002 and 2006 in France. J. Food Prot. 2010, 73, 825–831. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34–35. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Dis. Contrl. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Whichard, J.M.; Medalla, F.; Hoekstra, R.M.; Mcdermott, P.F.; Joyce, K.; Chiller, T.; Barrett, T.J.; White, D.G. Evaluation of antimicrobial resistance phenotypes for predicting multidrug-resistant Salmonella recovered from retail meats and humans in the United States. J. Food Prot. 2010, 73, 445–451. [Google Scholar] [CrossRef]
- Eyý, A.; Arslan, S. Prevalence of Escherichia coli in retail poultry meat, ground beef, and beef. Med. Weter 2012, 68, 238. [Google Scholar]
- World Health Organization (WHO). Critically Important Antimicrobials for Human Medicine, 5th ed.; WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR); WHO Press: Geneva, Switzerland, 2016; ISBN 978-92-4-151222. [Google Scholar]

| Bacterial Species A | No. (%) of Edible Offal B | No. (%) of Muscle Meat B | ||||||
|---|---|---|---|---|---|---|---|---|
| CL (n = 72) | BL (n = 44) | BT (n = 44) | Total (n = 160) | CW (n = 72) | GC (n = 72) | GB (n = 44) | Total (n = 188) | |
| Salmonella enterica serovar | 7 (9.7) ay | 0 (0.0) cy | 0 (0.0) cy | 7 (4.4) aby | 3 (4.2) aby | 5 (4.2) aby | 0 (0.0) cy | 8 (4.3) aby |
| Campylobacter | 0 (0.0) by | 3 (6.9) ay | 0 (0.0) by | 3 (1.9) aby | 0 (0.0) by | 1 (1.4) aby | 1 (2.3) aby | 2 (1.1) aby |
| Escherichia coli | 67 (93.1) ax | 28 (63.6) bx | 32 (72.7) abx | 127 (79.4) abx | 67 (93.1) ax | 68 (94.4) ax | 33 (75.0) abx | 168 (89.4) ax |
| enterococci | 70 (97.2) ax | 36 (81.8) bx | 35 (79.5) bx | 141 (88.1) bx | 72 (100.0) ax | 72 (100.0) ax | 36 (81.8) bx | 180 (95.7) ax |
| Bacterial Species A | Isolates (n = 116) | No. (%) of Bacteria Isolates in the Edible Offal Resistant to Antimicrobial Agents B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | CTX | CAZ | CRO | CHL | CIP | ERY | IPM | TET | VAN | ||
| Offal meats | |||||||||||
| Salmonella enterica serovar | 7 | 0 (0.0) c | 3 (42.9) ab | 0 (0.0) c | 4 (57.1) ab | 5 (71.4) a | 0 (0.0) c | 7 (100) a | 0 (0.0)c | 6 (85.7) a | 7 (100) a |
| Campylobacter | 3 | 0 (0) a | 3 (100) a | 0 (0) a | 3 (100) a | 0 (0) a | 3 (100) a | 3 (100) a | 0 (0) a | 1 (33.3) a | 3 (100) a |
| Escherichia coli | 53 | 53 (100) a | 39 (73.6) ab | 9 (17) c | 39 (73.6) ab | 36 (67.9) ab | 18 (34) bc | 53 (100) a | 0 (0) c | 53 (100) a | 53 (100) a |
| Enterococci | 53 | 0 (0) c | 51 (96.2) a | 51 (96) a | 52 (98) a | 9 (17) bc | 32 (60) ab | 51 (96.2) a | 0 (0) c | 49 (92.5) a | 40 (75.5) ab |
| Total | 116 | 53 (46) bc | 96 (83) ab | 60 (52) bc | 98 (84.5) ab | 50 (43.1) bc | 53 (46) bc | 114 (98.3) a | 0 (0) c | 109 (94) a | 103 (88.8) ab |
| Muscle meats | |||||||||||
| Salmonella enterica serovar | 8 | 2 (25) bc | 5 (62.5) ab | 2 (25) bc | 5 (62.5) ab | 5 (62.5) ab | 0 (0.0) c | 8 (100) a | 0 (0) c | 8 (100) a | 8 (100) a |
| Campylobacter | 2 | 0 (0)a | 2 (100) a | 0 (0) a | 2 (100) a | 2 (100) a | 1 (50) a | 2 (100) a | 0 (0) a | 0 (0) a | 2 (100) a |
| Escherichia coli | 51 | 47 (92.2) a | 26 (51) ab | 9 (17.7) c | 28 (55) ab | 28 (55) ab | 14 (26) bc | 51 (100) a | 1 (2) c | 51 (100) a | 51 (100) a |
| Enterococci | 54 | 1 (1.9) c | 50 (92.6) a | 49 (90.7) a | 51 (94.4) a | 27 (50) ab | 49 (90.7) a | 53 (98.1) a | 0 (0) c | 54 (100) a | 45 (83.3) a |
| Total | 115 | 50 (43.5) bc | 83 (71.2) b | 60 (52.2) bc | 86 (74.8) b | 62 (53.9) bc | 64 (55.7) bc | 114 (99.1) a | 1 (0.9) c | 113 (98.3) a | 106 (92.2) a |
| Bacterial Species A | Antibiotic Resistance Profiles B | No. (%) of Edible Offal Isolates C (n = 116) | No. (%) of Muscle Meat Isolates C (n = 115) |
|---|---|---|---|
| Salmonella enterica serovar (n = 15) | AMC, CTX, CRO, CHL, ERY, TET, VAN | 0 (0.0) c | 1 (0.9) c |
| AMC, CHL, ERY, TET, VAN | 0 (0.0) c | 1 (0.9) c | |
| CTX, CAZ, CRO, CHL, ERY, TET, VAN | 0 (0.0) c | 1 (0.9) c | |
| CTX, CAZ, ERY, TET, VAN | 0 (0.0) c | 1 (0.9) c | |
| CTX, CRO, CHL, ERY, TET, VAN | 2 (1.7) bc | 2 (1.7) bc | |
| CTX, CHL, ERY, TET, VAN | 1 (0.9) c | 0 (0.0) c | |
| CRO, CHL, ERY, TET, VAN | 1 (0.9) c | 0 (0.0) c | |
| ERY, TET, VAN | 2 (1.7) bc | 2 (1.7) bc | |
| ERY, VAN | 1 (0.9) c | 0 (0.0) c | |
| Campylobacter (n = 5) | CTX, CRO, CHL, ERY, VAN | 0 (0.0) c | 1 (0.9) c |
| CRO, CHL, ERY, VAN | 0 (0.0) c | 1 (0.9) c | |
| CRO, ERY, VAN | 1 (0.9) c | 0 (0.0) c | |
| CIP, ERY, VAN | 1 (0.9) c | 0 (0.0) c | |
| ERY, VAN | 1 (0.9) c | 0 (0.0) c | |
| E. coli (n = 104) | AMC, CTX, CRO, CHL, ERY, TET, VAN | 1 (0.9) c | 0 (0.0) c |
| AMC, CTX, ERY, TET, VAN | 0 (0.0) c | 1 (0.9) c | |
| AMC, CAZ, CRO, CHL, ERY, TET, VAN | 2 (1,7) bc | 2 (1.7) bc | |
| AMC, CAZ, CHL, ERY, TET, VAN | 9 (7.8) bx | 2 (1.7) bcy | |
| AMC, CRO, CHL, ERY, TET, VAN | 5 (4.3) bc | 1 (0.9) c | |
| AMC, CHL, CIP, ERY, TET, VAN | 1 (0.9) c | 0 (0.0) c | |
| AMC, CHL, ERY, TET, VAN | 23 (19.8) ax | 15 (13.0) aby | |
| AMC, ERY, IPM, TET, VAN | 0 (0.0) c | 1 (0.9) c | |
| AMC, ERY, TET, VAN | 12 (10.3) aby | 25 (21.7) ax | |
| ERY, TET, VAN | 0 (0.0) c | 4 (3.5) bc | |
| Enterococci (n = 107) | AMC, CTX, CRO, ERY, TET, VAN | 0 (0.0) c | 1 (0.9) c |
| CTX, CAZ, CRO, CIP, ERY, TET, VAN | 1 (0.9) c | 5 (4.3) bc | |
| CTX, CAZ, CRO, CHL, CIP, TET | 0 (0.0) c | 2 (1.7) bc | |
| CTX, CAZ, CRO, CIP, ERY, TET | 2 (1.7) bc | 0 (0.0) c | |
| CTX, CAZ, CRO, CIP, TET, VAN | 0 (0.0) bc | 2 (1.7) bc | |
| CTX, CAZ, CRO, ERY, TET, VAN | 6 (5.2) b | 8 (7.0) b | |
| CTX, CAZ, CHL, CIP, ERY, TET | 0 (0.0) c | 2 (1.7) bc | |
| CTX, CAZ, CRO, CIP, TET | 1 (0.9) c | 0 (0.0) c | |
| CTX, CAZ, CRO, ERY, TET | 10 (8.6) bx | 9 (7.8) by | |
| CTX, CAZ, CRO, TET, VAN | 2 (1.7) bc | 7 (6.1) b | |
| CTX, CAZ, CRO, ERY | 2 (1.7) bc | 0 (0.0) c | |
| CTX, CAZ, CRO, TET | 15 (12.9) abx | 6 (5.2) by | |
| CTX, CAZ, CRO, VAN | 3 (2.6) bc | 0 (0.0) c | |
| CTX, CAZ, ERY, TET | 2 (1.7) bc | 0 (0.0) c | |
| CTX, CAZ, CRO | 0 (0.0) c | 3 (2.6) bc | |
| CTX, CAZ, CIP | 2 (1.7) bc | 0 (0.0) c | |
| CAZ, CRO, ERY, TET, VAN | 0 (0.0) c | 1 (0.9) c | |
| CAZ, CRO, ERY, TET | 2 (1.7) bc | 0 (0.0) c | |
| CAZ, ERY, TET, VAN | 1 (0.9) c | 0 (0.0) c | |
| CAZ, CRO, TET | 0 (0.0) c | 1 (0.9) c | |
| CAZ, CRO | 0 (0.0) c | 3 (2.6) bc | |
| CAZ, TET | 2 (1.7) bc | 0 (0.0) c | |
| CHL, TET, VAN | 1 (0.9) c | 0 (0.0) c | |
| ERY, TET, VAN | 1 (0.9) c | 2 (1.7) bc | |
| ERY, VAN | 0 (0.0) c | 2 (1.7) bc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Kilonzo-Nthenge, A.; Nahashon, S.N.; Pokharel, B.; Mafiz, A.I.; Nzomo, M. Prevalence of Multidrug-Resistant Foodborne Pathogens and Indicator Bacteria from Edible Offal and Muscle Meats in Nashville, Tennessee. Foods 2020, 9, 1190. https://doi.org/10.3390/foods9091190
Liu S, Kilonzo-Nthenge A, Nahashon SN, Pokharel B, Mafiz AI, Nzomo M. Prevalence of Multidrug-Resistant Foodborne Pathogens and Indicator Bacteria from Edible Offal and Muscle Meats in Nashville, Tennessee. Foods. 2020; 9(9):1190. https://doi.org/10.3390/foods9091190
Chicago/Turabian StyleLiu, Siqin, Agnes Kilonzo-Nthenge, Samuel N. Nahashon, Bharat Pokharel, Abdullah Ibn Mafiz, and Maureen Nzomo. 2020. "Prevalence of Multidrug-Resistant Foodborne Pathogens and Indicator Bacteria from Edible Offal and Muscle Meats in Nashville, Tennessee" Foods 9, no. 9: 1190. https://doi.org/10.3390/foods9091190
APA StyleLiu, S., Kilonzo-Nthenge, A., Nahashon, S. N., Pokharel, B., Mafiz, A. I., & Nzomo, M. (2020). Prevalence of Multidrug-Resistant Foodborne Pathogens and Indicator Bacteria from Edible Offal and Muscle Meats in Nashville, Tennessee. Foods, 9(9), 1190. https://doi.org/10.3390/foods9091190






