The Human Microbial Metabolism of Quercetin in Different Formulations: An In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Products
2.3. Growth Medium and Fecal Slurry Preparation
2.4. In Vitro Fecal Fermentation
2.5. Fecal Metabolite Extraction
2.6. uHPLC/MSn Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Products
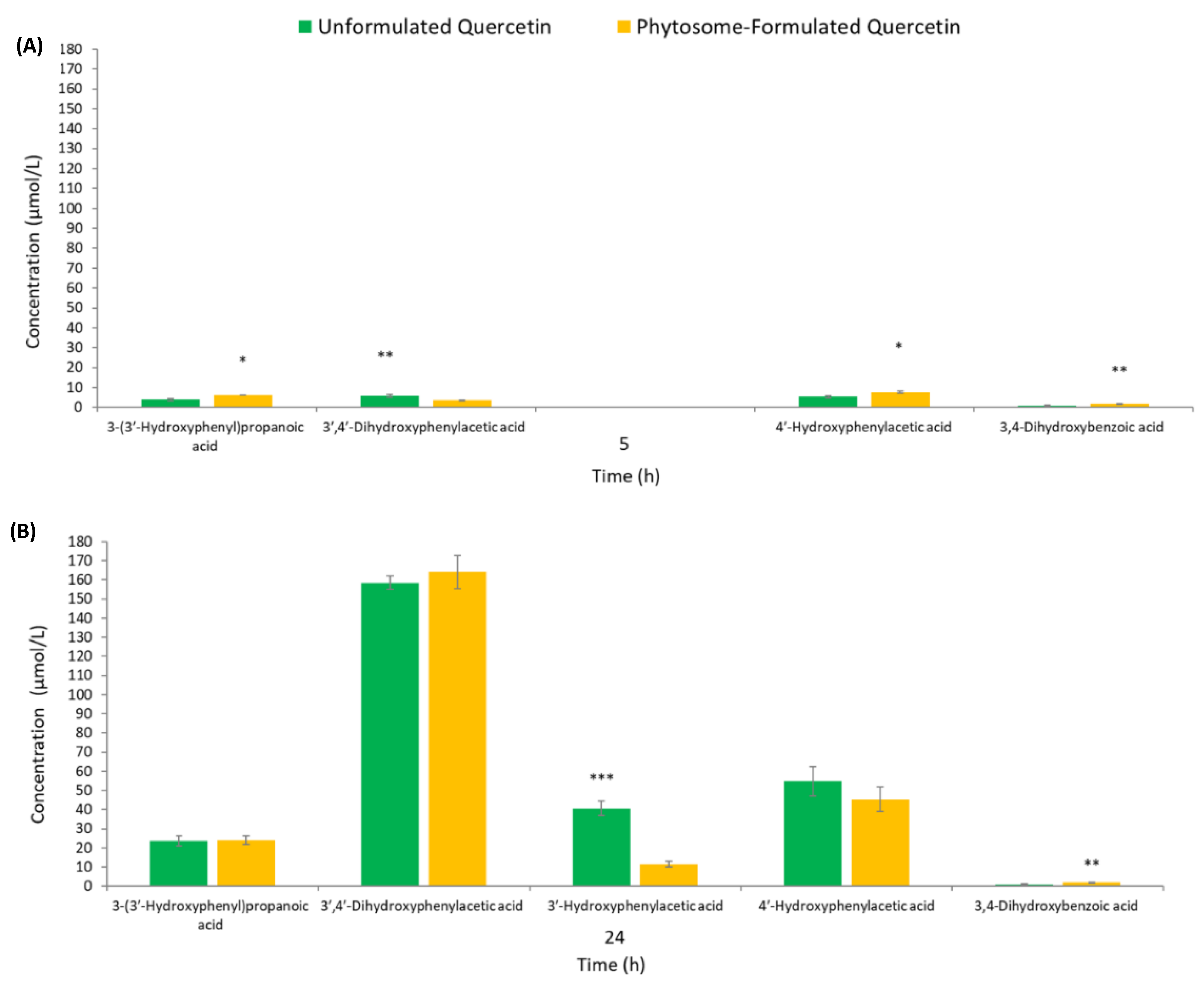
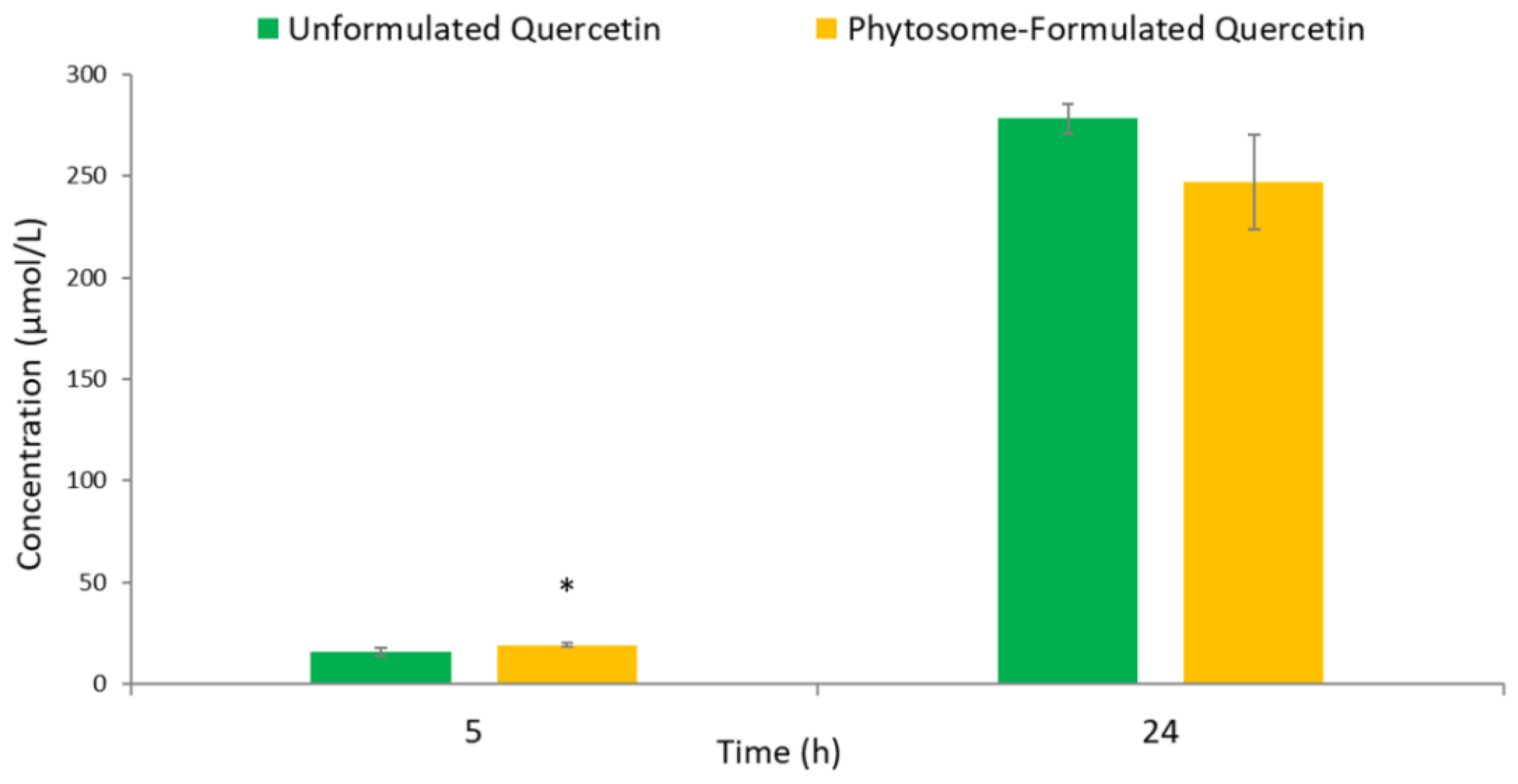
3.2. Human Colonic Metabolism of Quercetin
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid Content of U.S. Fruits, Vegetables, and Nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L.; Emésy, C.R.; Emésy, E.; Jiménez, J. Critical Reviews in Food Science and Nutrition Dietary Polyphenols and the Prevention of Diseases Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2014, 7, 452–490. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Riva, A.; Vitale, J.A.; Belcaro, G.; Hu, S.; Feragalli, B.; Vinciguerra, G.; Cacchio, M.; Bonanni, E.; Giacomelli, L.; Eggenhöffner, R.; et al. Quercetin phytosome® in triathlon athletes: A pilot registry study. Minerva Med. 2018, 109, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary prevention and management of asthma with quercetin phytosome: A pilot registry. Minerva Med. 2019, 110, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Nácher, A.; Díez-Sales, O.; Merino-Sanjuán, M.; Fadda, A.M.; Manconi, M. Chitosan-xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin. J. Microencapsul. 2014, 31, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Hu, T.G.; Li, L.; Zong, M.H.; Wu, H. A colon-specific delivery system for quercetin with enhanced cancer prevention based on co-axial electrospinning. Food Funct. 2018, 9, 5999–6009. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (poly)phenols and cardiovascular health. J. Agric. Food Chem. 2014, 62, 3842–3851. [Google Scholar] [CrossRef]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Serra, A.; MacIà, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Bresciani, L.; Favari, C.; Calani, L.; Francinelli, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Mena, P.; Del Rio, D. The Effect of Formulation of Curcuminoids on Their Metabolism by Human Colonic Microbiota. Molecules 2020, 25, 940. [Google Scholar] [CrossRef]
- Papillo, V.A.; Arlorio, M.; Locatelli, M.; Fuso, L.; Pellegrini, N.; Fogliano, V. In vitro evaluation of gastro-intestinal digestion and colonic biotransformation of curcuminoids considering different formulations and food matrices. J. Funct. Foods 2019, 59, 156–163. [Google Scholar] [CrossRef]
- Mena, P.; Sánchez-Salcedo, E.M.; Tassotti, M.; Martínez, J.J.; Hernández, F.; Del Rio, D. Phytochemical evaluation of eight white (Morus alba L.) and black (Morus nigra L.) mulberry clones grown in Spain based on UHPLC-ESI-MSn metabolomic profiles. Food Res. Int. 2016, 89, 1116–1122. [Google Scholar] [CrossRef]
- Bresciani, L.; Dall’asta, M.; Favari, C.; Calani, L.; Del Rio, D.; Brighenti, F. An: In vitro exploratory study of dietary strategies based on polyphenol-rich beverages, fruit juices and oils to control trimethylamine production in the colon. Food Funct. 2018, 9, 6470–6483. [Google Scholar] [CrossRef]
- Dall’Asta, M.; Calani, L.; Tedeschi, M.; Jechiu, L.; Brighenti, F.; Del Rio, D. Identification of microbial metabolites derived from invitro fecal fermentation of different polyphenolic food sources. Nutrition 2012, 28, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Kalpravidh, R.W.; Suksamrarn, S.; Chaivisuthangkura, A.; Chitchumroonchokchai, C.; Failla, M.L. Bioaccessibility, biotransformation, and transport of α-mangostin from Garcinia mangostana (Mangosteen) using simulated digestion and Caco-2 human intestinal cells. Mol. Nutr. Food Res. 2009, 53. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Calani, L.; Bresciani, L.; Dall’asta, M.; Faccini, A.; Augustin, M.A.; Gras, S.L.; Del Rio, D. The degradation of curcuminoids in a human faecal fermentation model. Int. J. Food Sci. Nutr. 2015, 66, 790–796. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Li, H. Bin; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Aura, A.M.; Mattila, I.; Seppänen-Laakso, T.; Miettinen, J.; Oksman-Caldentey, K.M.; Orešič, M. Microbial metabolism of catechin stereoisomers by human faecal microbiota: Comparison of targeted analysis and a non-targeted metabolomics method. Phytochem. Lett. 2008, 1, 18–22. [Google Scholar] [CrossRef]
- Gandhi, A.; Dutta, A.; Pal, A.; Bakshi, P. Recent Trends of Phytosomes for Delivering Herbal Extract with Improved Bioavailability. J. Pharmacogn. Phytochem. 2012, 1, 6–14. [Google Scholar]
- Jenner, A.M.; Rafter, J.; Halliwell, B. Human fecal water content of phenolics: The extent of colonic exposure to aromatic compounds. Free Radic. Biol. Med. 2005, 38, 763–772. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Russell, W.R.; Duncan, S.H.; Scobbie, L.; Duncan, G.; Cantlay, L.; Calder, A.G.; Anderson, S.E.; Flint, H.J. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol. Nutr. Food Res. 2013, 57, 523–535. [Google Scholar] [CrossRef]
- Aura, A.M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.M.; Oksman-Caldentey, K.M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Gu¨tschow, M.; Gu¨tschow, G.; Engst, W.; Blaut, M.; Mikrobiologie, A.G. Degradation of Quercetin and Luteolin by Eubacterium ramulus Downloaded from. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Paz de Peña, M.; Concepción, C.; Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. BioFactors 2013, 39, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Catabolism of raw and cooked green pepper (Capsicum annuum) (poly)phenolic compounds after simulated gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2016, 27, 201–213. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Victoria Moreno-Arribas, M.; Bartolomé, B. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Victoria Moreno-Arribas, M.; Bartolomé, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targetted analysis of precursor compounds, intermediate metabolites and end-products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]

| Compound | RT (min) | [M − H]− (m/z) | MS2 Ions (m/z) | MS3 Ions (m/z) | Unformulated Quercetin | Phytosome-Formulated Quercetin |
|---|---|---|---|---|---|---|
| Quercetin | 6.17 | 301 | 179 *, 151, 273, 257 | 959.2 ± 95.0 | 382.1 ± 25.1 | |
| Kaempferol | 7.02 | 285 | 285, 151 | n.q. | n.q. | |
| Isorhamnetin | 7.2 | 315 | 300 | n.q. | n.q. | |
| Rutin | 4.27 | 609 | 301, 343, 179 | 301:179, 151, 273, 257 | n.q. | n.q. |
| Compound | RT (min) | [M − H]− (m/z) | MS2 Ions (m/z) | LOD (µmol/L) | LOQ (µmol/L) | Quantification |
|---|---|---|---|---|---|---|
| Native compound | ||||||
| Quercetin | 6.17 | 301 | 179 *, 151, 273, 257 | 0.005 | 0.01 | R.S. |
| Breakdown metabolites | ||||||
| 3-(3′,4′-Dihydroxyphenyl)propanoic acid | 2.35 | 181 | 137, 119, 109 | 0.05 | 1.00 | <LOQ |
| 3-(4′-Hydroxyphenyl)propanoic acid | 3.18 | 165 | 121, 93 | 2.00 | 25.00 | R.S. |
| 3-(3′-Hydroxyphenyl)propanoic acid | 3.59 | 165 | 121, 119 | 0.05 | 1.00 | R.S. |
| 3-Phenylpropanoic acid | 5.54 | 149 | 105 | 5.00 | 50.00 | <LOD |
| 3′,4′-Dihydroxyphenylacetic acid | 1.41 | 167 | 123 | 0.25 | 1.00 | R.S. |
| 4′-Hydroxyphenylacetic acid | 2.26 | 151 | 107 | 1.00 | 10.00 | R.S. |
| 3′-Hydroxyphenylacetic acid | 2.60 | 151 | 107 | 0.25 | 1.00 | R.S. |
| Phenylacetic acid | 4.18 | 135 | 91 | 2.00 | 10.00 | R.S. |
| 3,4-Dihydroxybenzoic acid | 1.20 | 153 | 109 | 0.05 | 1.00 | R.S. |
| 4-Hydroxybenzoic acid | 2.07 | 137 | 93 | 0.25 | 1.00 | <LOQ |
| 3-Hydroxybenzoic acid | 2.58 | 137 | 93 | 0.25 | 1.00 | <LOD |
| Benzene-1,3,5-triol | 0.65 | 125 | 5.00 | 5.00 | <LOD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pede, G.; Bresciani, L.; Calani, L.; Petrangolini, G.; Riva, A.; Allegrini, P.; Del Rio, D.; Mena, P. The Human Microbial Metabolism of Quercetin in Different Formulations: An In Vitro Evaluation. Foods 2020, 9, 1121. https://doi.org/10.3390/foods9081121
Di Pede G, Bresciani L, Calani L, Petrangolini G, Riva A, Allegrini P, Del Rio D, Mena P. The Human Microbial Metabolism of Quercetin in Different Formulations: An In Vitro Evaluation. Foods. 2020; 9(8):1121. https://doi.org/10.3390/foods9081121
Chicago/Turabian StyleDi Pede, Giuseppe, Letizia Bresciani, Luca Calani, Giovanna Petrangolini, Antonella Riva, Pietro Allegrini, Daniele Del Rio, and Pedro Mena. 2020. "The Human Microbial Metabolism of Quercetin in Different Formulations: An In Vitro Evaluation" Foods 9, no. 8: 1121. https://doi.org/10.3390/foods9081121
APA StyleDi Pede, G., Bresciani, L., Calani, L., Petrangolini, G., Riva, A., Allegrini, P., Del Rio, D., & Mena, P. (2020). The Human Microbial Metabolism of Quercetin in Different Formulations: An In Vitro Evaluation. Foods, 9(8), 1121. https://doi.org/10.3390/foods9081121