Living the Sweet Life: How Liquorilactobacillus hordei TMW 1.1822 Changes Its Behavior in the Presence of Sucrose in Comparison to Glucose
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media and Growth Conditions
2.2. Proteomic Analysis
2.2.1. Experimental Setup
2.2.2. Peptide Preparation, Separation and Mass Spectrometry
2.2.3. Data Availability
2.2.4. Protein Identification and Quantification
2.2.5. GO-Enrichment Analysis
2.3. Analysis of Culture Supernatants of Glucose and Sucrose-Treated Cells
2.3.1. Quantification of Sugar Consumption and Acid Formation
2.3.2. SDS-PAGE, Staining and Zymogram
2.4. Determination of Growth Parameters in Different Sugars
2.5. Screening for Dextransucrases in Other L. hordei Strains by PCR
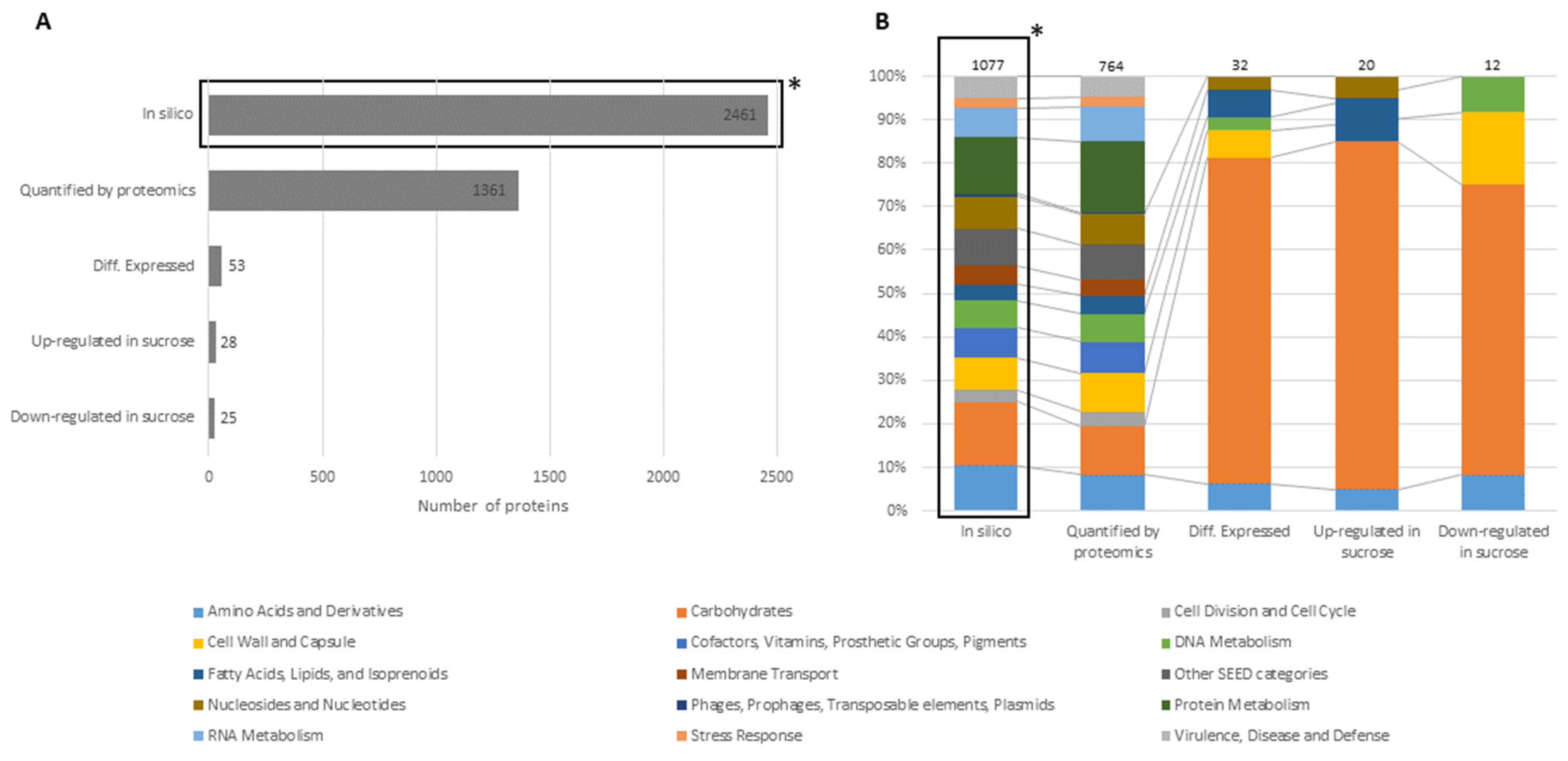
3. Results
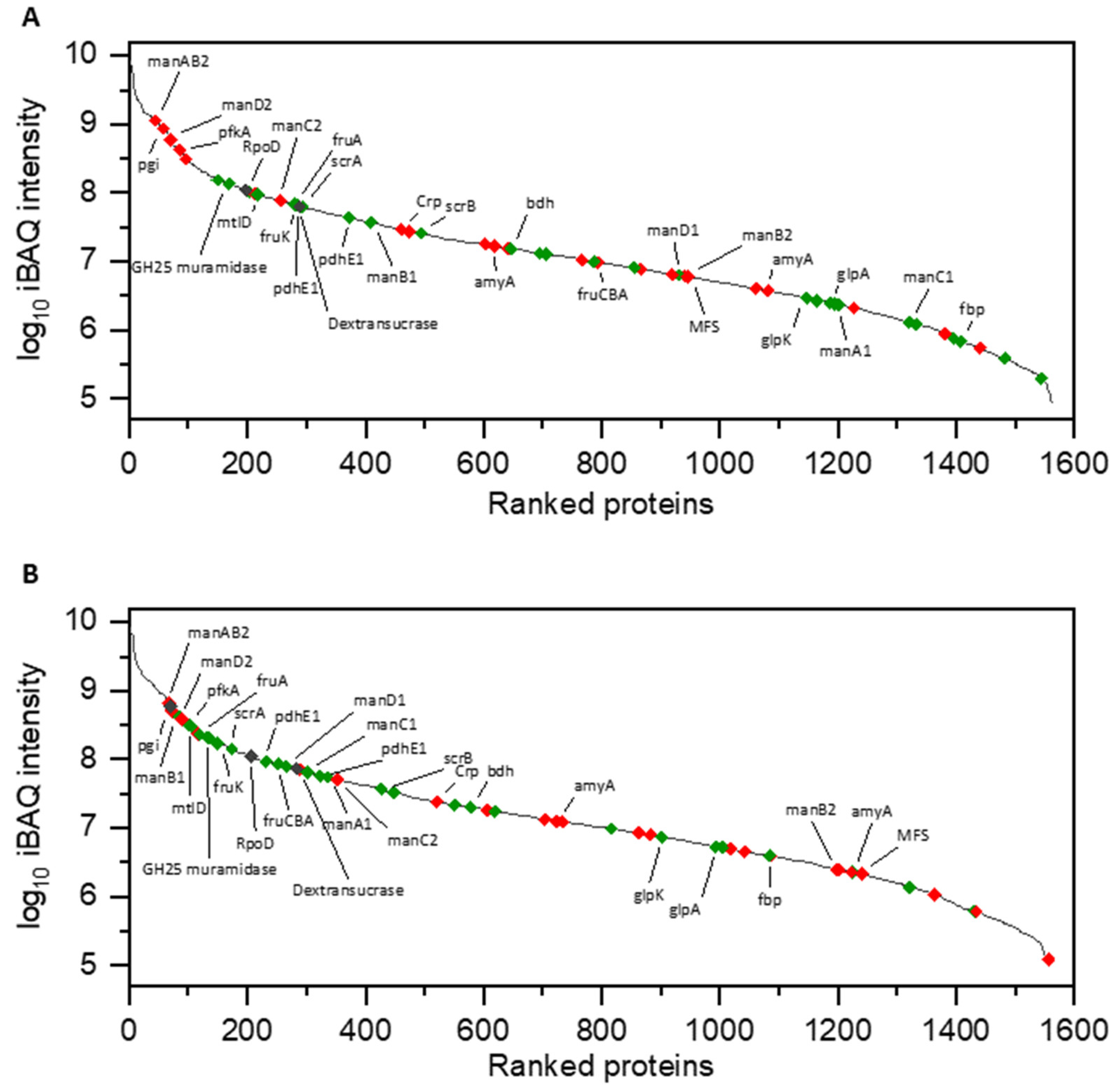
3.1. Proteomic Analysis of Cell Lysates and Exoproteomes
3.1.1. Differential Proteomics of Cell Lysates
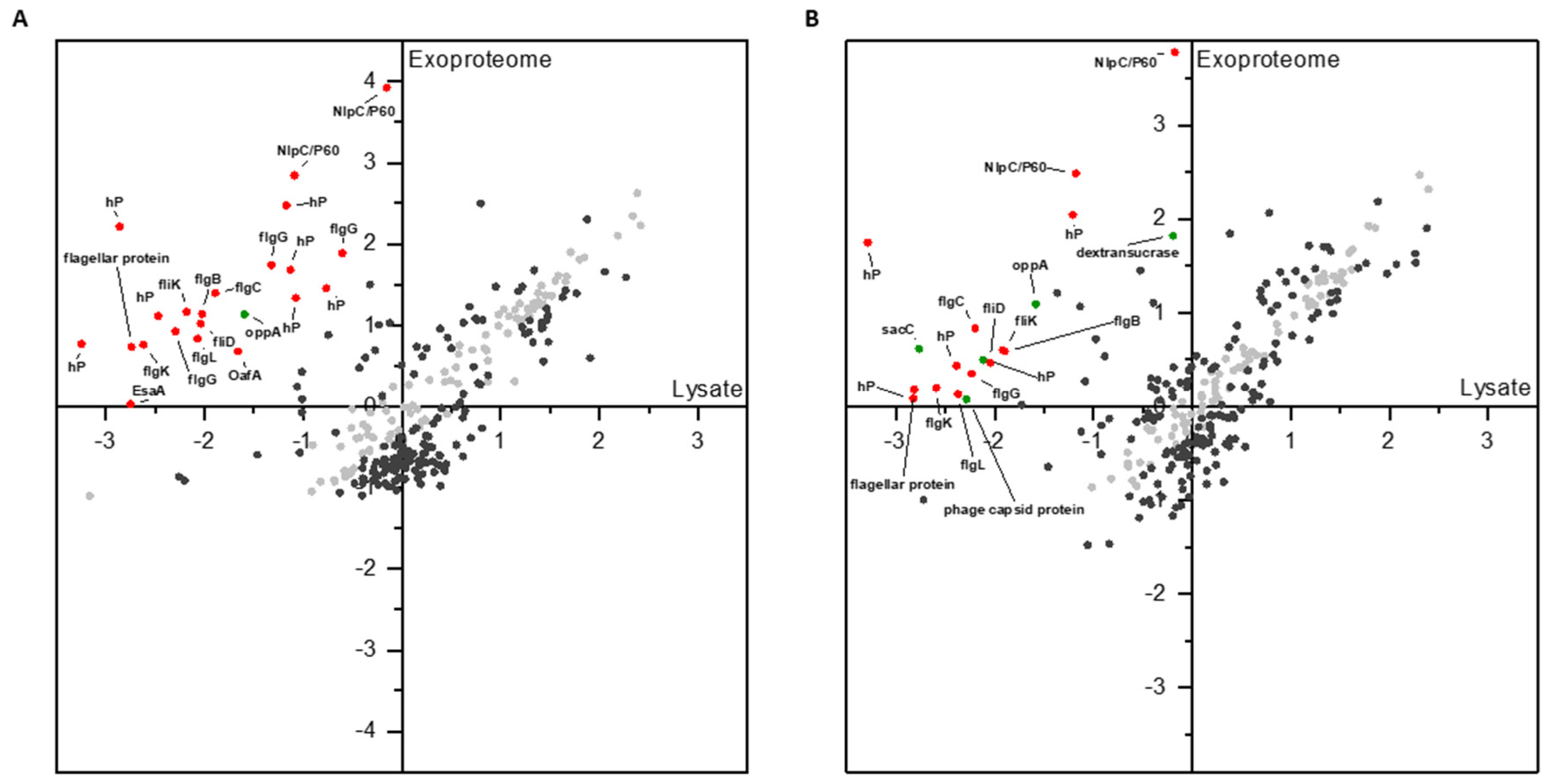
3.1.2. Differential Proteomics of Exoproteomes
3.1.3. Comparison of Proteomic States of Cell Lysates and Exoproteomes
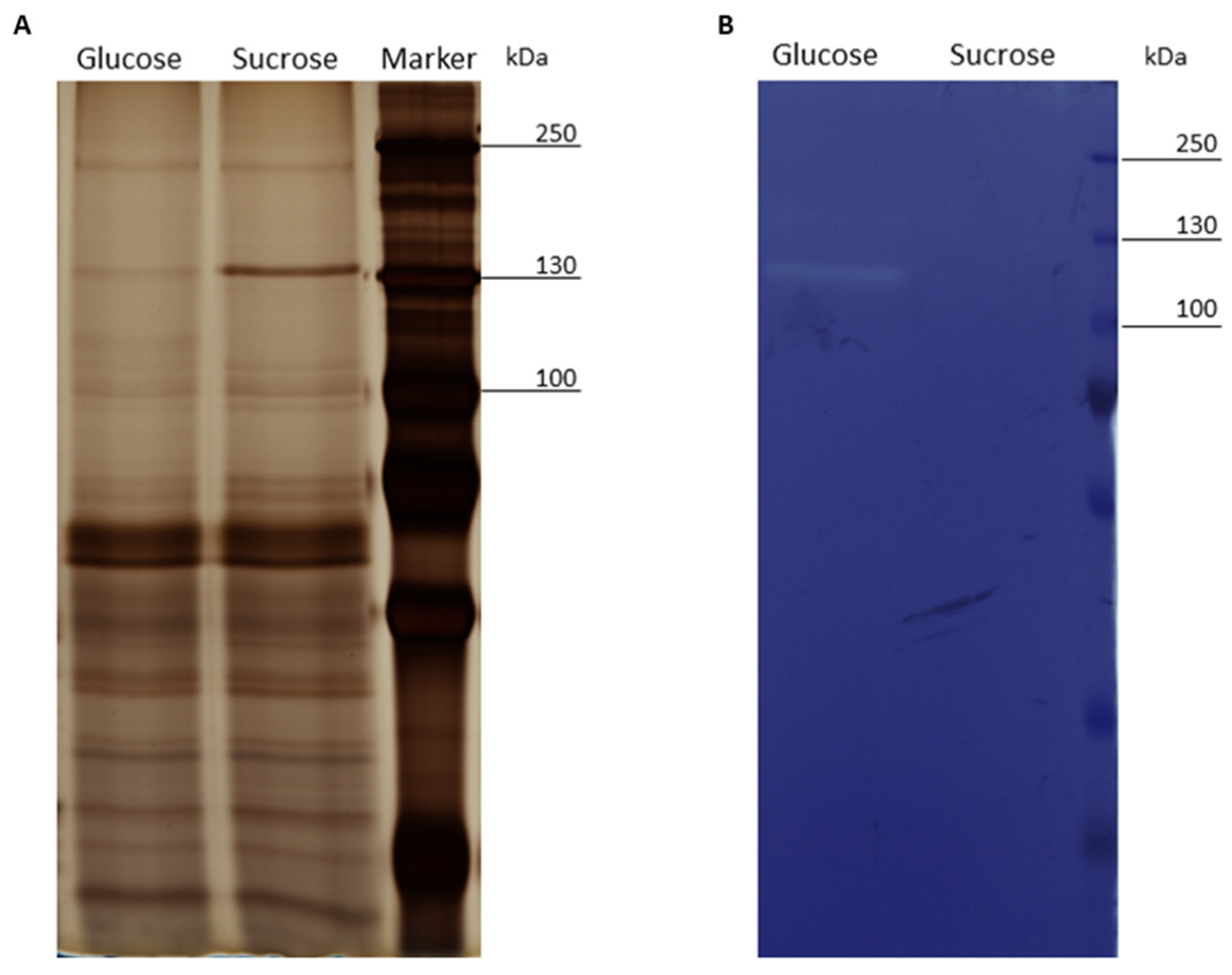
3.2. SDS-PAGE and Zymogram Analysis of the Exoproteomes of Cells Treated with Glucose or Sucrose
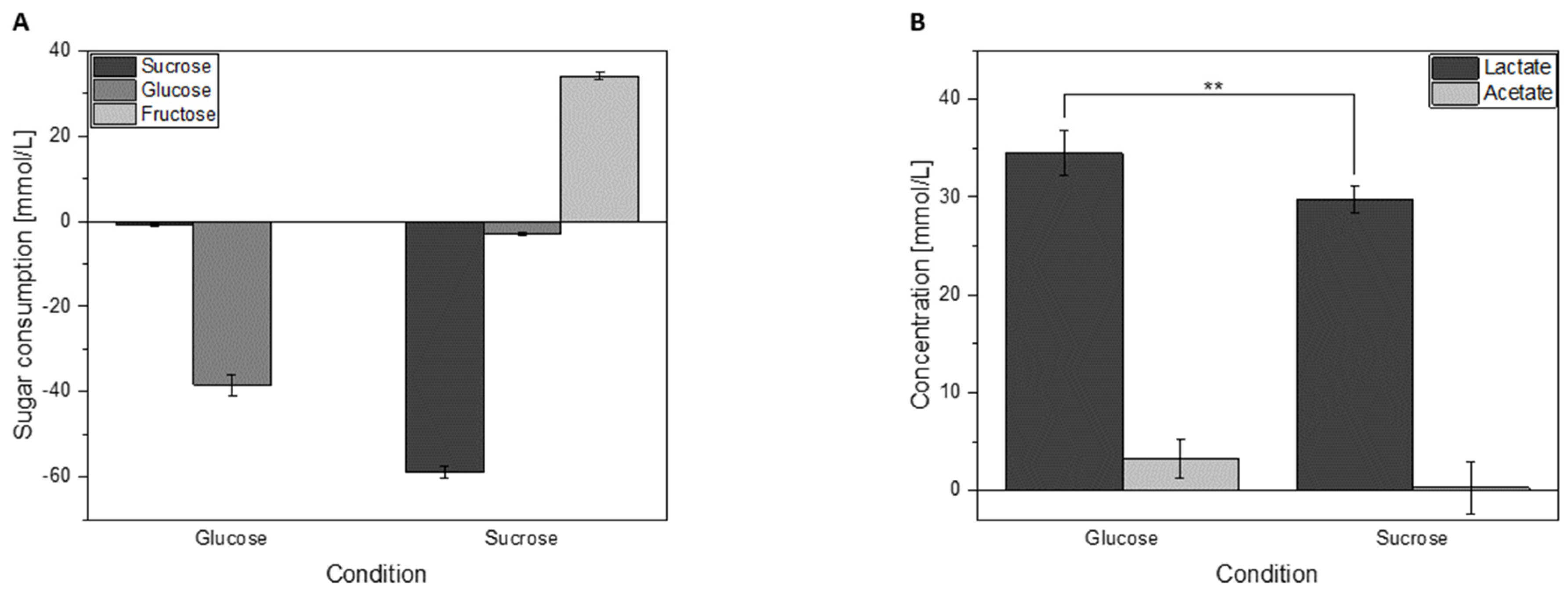
3.3. Sugar Consumption and Acid Formation of Cells Grown in Either Glucose or Sucrose
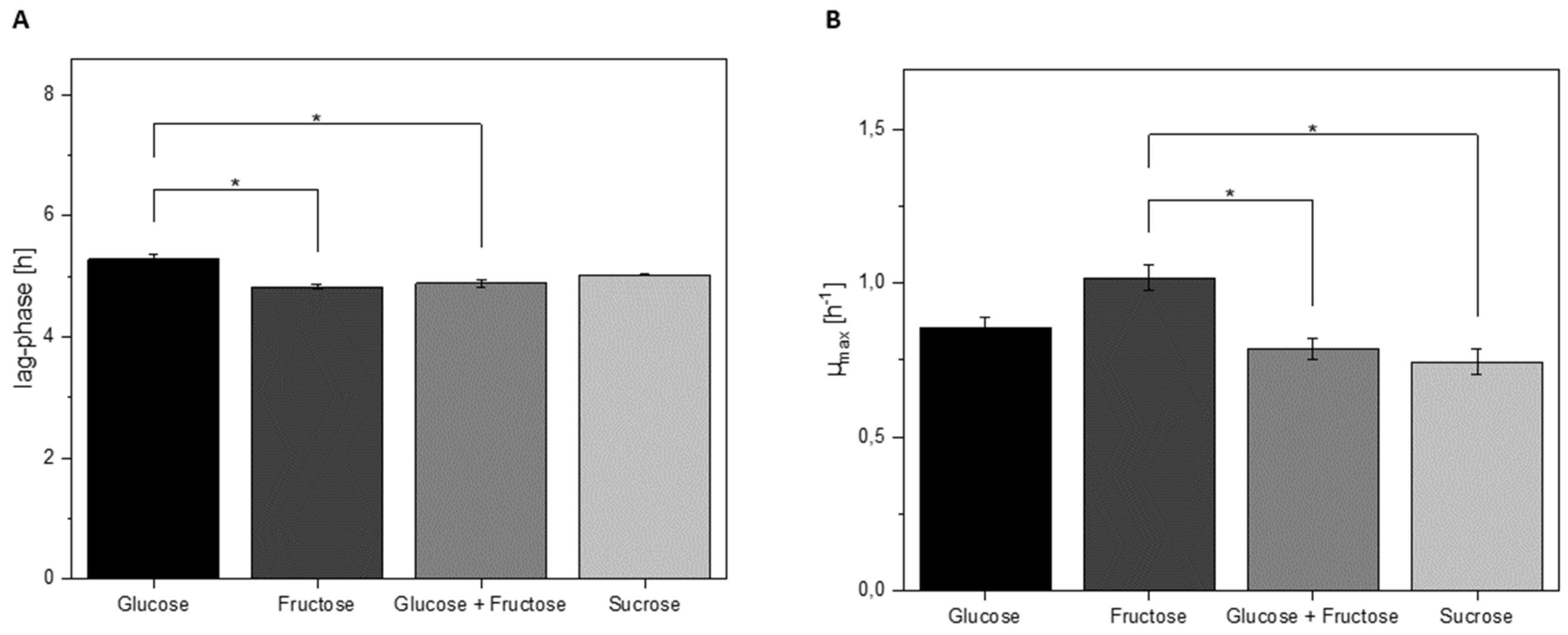
3.4. Growth Characteristics of L. hordei in Different Sugars
3.5. Screening for Dextransucrases in Other L. hordei Isolates
4. Discussion
4.1. Expression and Release of the L. hordei TMW 1.1822 Dextransucrase
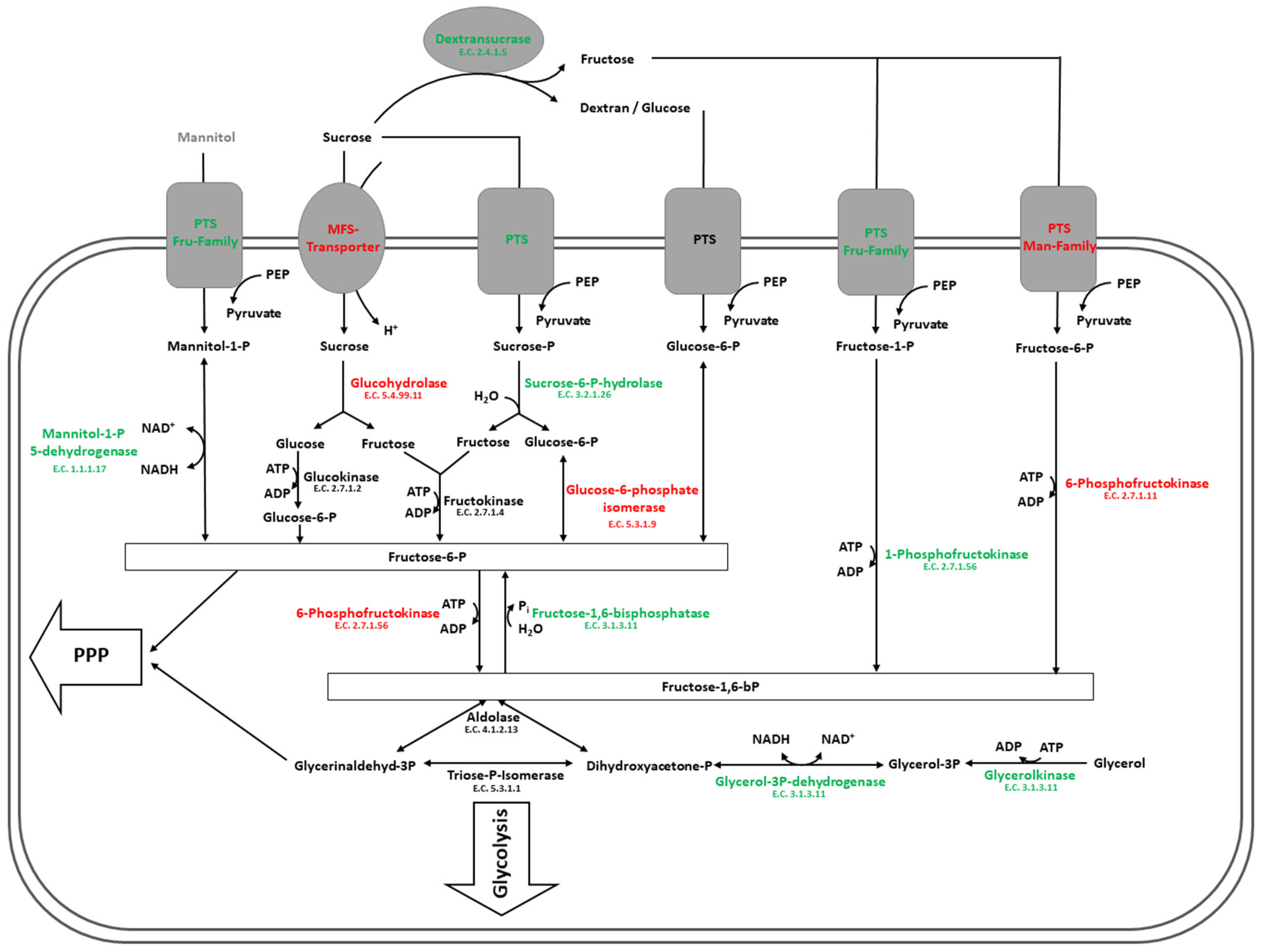
4.2. Carbohydrate Metabolism in Glucose and Sucrose-Treated Cells of L. hordei
4.3. Exoproteomic Features of L. hordei TMW 1.1822 in the Presence of Sucrose
4.4. Expression and Release of Cell Wall Active Enzymes in the Presence of Sucrose Compared to Glucose
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Mollet, B.; Rowland, I. Functional foods: At the frontier between food and pharma. Curr. Opin. Biotechnol. 2002, 13, 483–485. [Google Scholar] [CrossRef]
- Kamiński, M.; Skonieczna-Żydecka, K.; Nowak, J.K.; Stachowska, E. Global and local diet popularity rankings, their secular trends and seasonal variation in Google Trends data. Nutrition 2020. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. Microbial species diversity, community dynamics, and metabolite kinetics of water kefir fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef]
- Stadie, J.; Gulitz, A.; Ehrmann, M.A.; Vogel, R.F. Metabolic activity and symbiotic interactions of lactic acid bacteria and yeasts isolated from water kefir. Food Microbiol. 2013, 35, 92–98. [Google Scholar] [CrossRef]
- Koh, W.Y.; Utra, U.; Ahmad, R.; Rather, I.A.; Park, Y.-H. Evaluation of probiotic potential and anti-hyperglycemic properties of a novel Lactobacillus strain isolated from water kefir grains. Food Sci. Biotechnol. 2018, 27, 1369–1376. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; Vandenberghe, L.P.d.S.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation—A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef]
- Moreira, M.E.C.; Santos, M.H.D.; Zolini, G.P.P.; Wouters, A.T.B.; Carvalho, J.C.T.; Schneedorf, J.M. Anti-Inflammatory and cicatrizing activities of a carbohydrate fraction isolated from sugary kefir. J. Med. Food 2008, 11, 356–361. [Google Scholar] [CrossRef]
- Golowczyc, M.A.; Mobili, P.; Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Protective action of Lactobacillus kefir carrying S-layer protein against Salmonella enterica serovar enteritidis. Int. J. Food Microbiol. 2007, 118, 264–273. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Araújo, T.H.; Schneedorf, J.M.; Ferreira, C.d.S.; Moraes, G.d.O.I.; Coimbra, R.S.; Rodrigues, M.R. A novel beer fermented by kefir enhances anti-inflammatory and anti-ulcerogenic activities found isolated in its constituents. J. Funct. Foods 2016, 21, 58–69. [Google Scholar] [CrossRef]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The microbial diversity of water kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Angelescu, I.-R.; Zamfir, M.; Stancu, M.-M.; Grosu-Tudor, S.-S. Identification and probiotic properties of lactobacilli isolated from two different fermented beverages. Ann. Microbiol. 2019, 69, 1557–1565. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Miguel, M.A.L.; Peixoto, R.S.; Ruas-Madiedo, P.; Paschoalin, V.M.F.; Mayo, B.; Delgado, S. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 2015, 98, 3622–3632. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Eckel, V.P.L.; Vogel, R.F.; Jakob, F. In situ production and characterization of cloud forming dextrans in fruit-juices. Int. J. Food Microbiol. 2019, 306. [Google Scholar] [CrossRef]
- Fels, L.; Jakob, F.; Vogel, R.F.; Wefers, D. Structural characterization of the exopolysaccharides from water kefir. Carbohydr. Polym. 2018, 189, 296–303. [Google Scholar] [CrossRef]
- Sidebotham, R.L. Dextrans. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1974; Volume 30, pp. 371–444. [Google Scholar]
- Monchois, V.; Willemot, R.-M.; Monsan, P. Glucansucrases: Mechanism of action and structure–function relationships. FEMS Microbiol. Rev. 1999, 23, 131–151. [Google Scholar] [CrossRef]
- Prechtl, R.M.; Wefers, D.; Jakob, F.; Vogel, R.F. Cold and salt stress modulate amount, molecular and macromolecular structure of a Lactobacillus sakei dextran. Food Hydrocoll. 2018, 82, 73–81. [Google Scholar] [CrossRef]
- Schmid, J.; Bechtner, J.; Vogel, R.F.; Jakob, F. A systematic approach to study the pH-dependent release, productivity and product specificity of dextransucrases. Microb. Cell Fact. 2019, 18. [Google Scholar] [CrossRef]
- Falconer, D.J.; Mukerjea, R.; Robyt, J.F. Biosynthesis of dextrans with different molecular weights by selecting the concentration of Leuconostoc mesenteroides B-512FMC dextransucrase, the sucrose concentration, and the temperature. Carbohydr. Res. 2011, 346, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; van Leeuwen, S.S.; Kralj, S.; Dijkstra, B.W.; Dijkhuizen, L. Glucansucrases: Three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J. Biotechnol. 2013, 163, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Bechtner, J.; Wefers, D.; Schmid, J.; Vogel, R.F.; Jakob, F. Identification and comparison of two closely related dextransucrases released by water kefir borne Lactobacillus hordei TMW 1.1822 and Lactobacillus nagelii TMW 1.1827. Microbiology 2019, 165, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Münkel, F.; Bechtner, J.; Eckel, V.; Fischer, A.; Herbi, F.; Jakob, F.; Wefers, D. Detailed structural characterization of glucans produced by glucansucrases from Leuconostoc citreum TMW 2.1194. J. Agric. Food Chem. 2019, 67, 6856–6866. [Google Scholar] [CrossRef] [PubMed]
- Waldherr, F.W.; Doll, V.M.; Meissner, D.; Vogel, R.F. Identification and characterization of a glucan-producing enzyme from Lactobacillus hilgardii TMW 1.828 involved in granule formation of water kefir. Food Microbiol. 2010, 27, 672–678. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Kolida, S.; Naeye, T.; Einerhand, A.W.; Gibson, G.R.; Rastall, R.A. The prebiotic effect of α-1,2 branched, low molecular weight dextran in the batch and continuous faecal fermentation system. J. Funct. Foods 2013, 5, 1938–1946. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Kolida, S.; Deaville, E.R.; Gibson, G.R.; Rastall, R.A. Potential of novel dextran oligosaccharides as prebiotics for obesity management through in vitro experimentation. Br. J. Nutr. 2014, 112, 1303–1314. [Google Scholar] [CrossRef]
- Tingirikari, J.M.R.; Kothari, D.; Goyal, A. Superior prebiotic and physicochemical properties of novel dextran from Weissella cibaria JAG8 for potential food applications. Food Funct. 2014, 5, 2324–2330. [Google Scholar] [CrossRef]
- Kothari, D.; Tingirikari, J.M.R.; Goyal, A. In vitro analysis of dextran from Leuconostoc mesenteroides NRRL B-1426 for functional food application. Bioact. Carbohydr. Diet. Fibre 2015, 6, 55–61. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Kolida, S.; Naeye, T.; Einerhand, A.; Brison, Y.; Remaud-Simeon, M.; Monsan, P.; Gibson, G.R.; Rastall, R.A. In Vitro Fermentation of Linear and α-1,2-Branched Dextrans by the Human Fecal Microbiota. Appl. Environ. Microbiol. 2011, 77, 5307. [Google Scholar] [CrossRef]
- Hilbig, J.; Gisder, J.; Prechtl, R.M.; Herrmann, K.; Weiss, J.; Loeffler, M. Influence of exopolysaccharide-producing lactic acid bacteria on the spreadability of fat-reduced raw fermented sausages (Teewurst). Food Hydrocoll. 2019, 93, 422–431. [Google Scholar] [CrossRef]
- Prechtl, R.M.; Janßen, D.; Behr, J.; Ludwig, C.; Küster, B.; Vogel, R.F.; Jakob, F. Sucrose-induced proteomic response and carbohydrate utilization of Lactobacillus sakei TMW 1.411 during dextran formation. Front. Microbiol. 2018, 9, 2796. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Rouse, S.; Canchaya, C.; van Sinderen, D. Lactobacillus hordei sp. nov., a bacteriocinogenic strain isolated from malted barley. Int. J. Syst. Evol. Microbiol. 2008, 58, 2013–2017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, D.; Bechtner, J.; Behr, J.; Eisenbach, L.; Geißler, A.J.; Vogel, R.F. Lifestyle of Lactobacillus hordei isolated from water kefir based on genomic, proteomic and physiological characterization. Int. J. Food Microbiol. 2019, 290, 141–149. [Google Scholar] [CrossRef] [PubMed]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Schott, A.-S.; Behr, J.; Geißler, A.J.; Kuster, B.; Hahne, H.; Vogel, R.F. Quantitative proteomics for the comprehensive analysis of stress responses of Lactobacillus paracasei subsp. paracasei F19. J. Proteome Res. 2017, 16, 3816–3829. [Google Scholar] [CrossRef]
- Heinze, S.; Zimmermann, K.; Ludwig, C.; Heinzlmeir, S.; Schwarz, W.H.; Zverlov, V.V.; Liebl, W.; Kornberger, P. Evaluation of promoter sequences for the secretory production of a Clostridium thermocellum cellulase in Paenibacillus polymyxa. Appl. Microbiol. Biotechnol. 2018, 102, 10147–10159. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Alexa, A.; Rahnenfuhrer, J. TopGo: Enrichment Analysis for Gene Ontology 2.40.0; Bioconductor: Buffalo, NY, USA, 2020. [Google Scholar]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Lepeuple, A.-S.; Van Gemert, E.; Chapot-Chartier, M.-P. Analysis of the bacteriolytic enzymes of the autolytic lactococcus lactis subsp. cremoris strain am2 by renaturing polyacrylamide gel electrophoresis: Identification of a prophage-encoded enzyme. Appl. Environ. Microbiol. 1998, 64, 4142. [Google Scholar] [CrossRef]
- Kahm, M.; Hasenbrink, G.; Lichtenberg-Frate, H.; Ludwig, J.; Kschischo, M. Grofit: Fitting biological growth curves. Nat. Preced. 2010. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Zhou, M.; Theunissen, D.; Wels, M.; Siezen, R.J. LAB-Secretome: A genome-scale comparative analysis of the predicted extracellular and surface-associated proteins of Lactic Acid Bacteria. BMC Genom. 2010, 11, 651. [Google Scholar] [CrossRef]
- Moulis, C.; Joucla, G.; Harrison, D.; Fabre, E.; Potocki-Veronese, G.; Monsan, P.; Remaud-Simeon, M. Understanding the polymerization mechanism of glycoside-hydrolase family 70 glucansucrases. J. Biol. Chem. 2006, 281, 31254–31267. [Google Scholar] [CrossRef]
- Arskold, E.; Svensson, M.; Grage, H.; Roos, S.; Radstrom, P.; van Niel, E.W. Environmental influences on exopolysaccharide formation in Lactobacillus reuteri ATCC 55730. Int. J. Food Microbiol. 2007, 116, 159–167. [Google Scholar] [CrossRef]
- Bounaix, M.S.; Robert, H.; Gabriel, V.; Morel, S.; Remaud-Simeon, M.; Gabriel, B.; Fontagne-Faucher, C. Characterization of dextran-producing Weissella strains isolated from sourdoughs and evidence of constitutive dextransucrase expression. FEMS Microbiol. Lett. 2010, 311, 18–26. [Google Scholar] [CrossRef]
- Schwab, C.; Walter, J.; Tannock, G.W.; Vogel, R.F.; Gänzle, M.G. Sucrose utilization and impact of sucrose on glycosyltransferase expression in Lactobacillus reuteri. Syst. Appl. Microbiol. 2007, 30, 433–443. [Google Scholar] [CrossRef]
- Quirasco, M.; López-Munguía, A.; Remaud-Simeon, M.; Monsan, P.; Farrés, A. Induction and transcription studies of the dextransucrase gene in Leuconostoc mesenteroides NRRL B-512F. Appl. Environ. Microbiol. 1999, 65, 5504. [Google Scholar] [CrossRef]
- Kralj, S.; van Geel-Schutten, G.H.; Dondorff, M.M.G.; Kirsanovs, S.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Glucan synthesis in the genus Lactobacillus: Isolation and characterization of glucansucrase genes, enzymes and glucan products from six different strains. Microbiology 2004, 150, 3681–3690. [Google Scholar] [CrossRef]
- Carr, J.G.; Davies, P.A. Homofermentative lactobacilli of ciders including Lactobacillus mali nov. spec. J. Appl. Bacteriol. 1970, 33, 768–774. [Google Scholar] [CrossRef]
- Irisawa, T.; Okada, S. Lactobacillus sucicola sp. nov., a motile lactic acid bacterium isolated from oak tree (Quercus sp.) sap. Int. J. Syst. Evol. Microbiol. 2009, 59, 2662–2665. [Google Scholar] [CrossRef]
- Schmid, J.; Wefers, D.; Vogel, R.F.; Jakob, F. Analysis of structural and functional differences of glucans produced by the natively released dextransucrase of Liquorilactobacillus hordei TMW 1.1822. Appl. Biochem. Biotechnol. 2020, in press. [Google Scholar]
- Yebra, M.J.; Monedero, V.; Zúñiga, M.; Deutscher, J.; Pérez-Martínez, G. Molecular analysis of the glucose-specific phosphoenolpyruvate: Sugar phosphotransferase system from Lactobacillus casei and its links with the control of sugar metabolism. Microbiology 2006, 152, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.S.; Fredslund, F.; Majumder, A.; Nakai, H.; Poulsen, J.-C.N.; Lo Leggio, L.; Svensson, B.; Abou Hachem, M. Enzymology and structure of the GH13_31 glucan 1,6-α-glucosidase that confers isomaltooligosaccharide utilization in the probiotic Lactobacillus acidophilus NCFM. J. Bacteriol. 2012, 194, 4249–4259. [Google Scholar] [CrossRef]
- Paludan-Müller, C.; Gram, L.; Rattray, F.P. Purification and characterisation of an extracellular fructan β-fructosidase from a lactobacillus pentosus strain isolated from fermented fish. Syst. Appl. Microbiol. 2002, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.M.A.; Molenaar, D.; de Vos, W.M.; Gibson, G.R.; Kolida, S. Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl. Environ. Microbiol. 2007, 73, 1753–1765. [Google Scholar] [CrossRef]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Kets, E.P.; Galinski, E.A.; de Wit, M.; de Bont, J.A.; Heipieper, H.J. Mannitol, a novel bacterial compatible solute in Pseudomonas putida S12. J. Bacteriol. 1996, 178, 6665–6670. [Google Scholar] [CrossRef] [PubMed]
- Efiuvwevwere, B.J.O.; Gorris, L.G.M.; Smid, E.J.; Kets, E.P.W. Mannitol-enhanced survival of Lactococcus lactis subjected to drying. Appl. Microbiol. Biotechnol. 1999, 51, 100–104. [Google Scholar] [CrossRef]
- Neves, A.R.; Ramos, A.; Shearman, C.; Gasson, M.J.; Almeida, J.S.; Santos, H. Metabolic characterization of Lactococcus lactis deficient in lactate dehydrogenase using in vivo 13C-NMR. Eur. J. Biochem. 2000, 267, 3859–3868. [Google Scholar] [CrossRef] [PubMed]
- Henstra, S.A.; Tuinhof, M.; Duurkens, R.H.; Robillard, G.T. The Bacillus stearothermophilus Mannitol Regulator, MtlR, of the Phosphotransferase System. J. Biol. Chem. 1999, 274, 4754–4763. [Google Scholar] [CrossRef]
- Henstra, S.A.; Duurkens, R.H.; Robillard, G.T. Multiple phosphorylation events regulate the activity of the mannitol transcriptional regulator MtlR of the Bacillus stearothermophilus phosphoenolpyruvate-dependent mannitol phosphotransferase system. J. Biol. Chem. 2000, 275, 7037–7044. [Google Scholar] [CrossRef]
- Xu, D.; Behr, J.; Geißler, A.J.; Bechtner, J.; Ludwig, C.; Vogel, R.F. Label-free quantitative proteomic analysis reveals the lifestyle of Lactobacillus hordei in the presence of Sacchromyces cerevisiae. Int. J. Food Microbiol. 2019, 294, 18–26. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837. [Google Scholar] [CrossRef]
- Sunny-Roberts, E.O.; Knorr, D. Evaluation of the response of Lactobacillus rhamnosus VTT E-97800 to sucrose-induced osmotic stress. Food Microbiol. 2008, 25, 183–189. [Google Scholar] [CrossRef]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell wall hydrolases in bacteria: Insight on the diversity of cell wall amidases, glycosidases and peptidases toward peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef]
- Cousin, F.J.; Lynch, S.M.; Harris, H.M.B.; McCann, A.; Lynch, D.B.; Neville, B.A.; Irisawa, T.; Okada, S.; Endo, A.; Toole, P.W. Detection and genomic characterization of motility in Lactobacillus curvatus: Confirmation of motility in a species outside the Lactobacillus salivarius clade. Appl. Environ. Microbiol. 2015, 81, 1297. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.A.; Forde, B.M.; Claesson, M.J.; Darby, T.; Coghlan, A.; Nally, K.; Ross, R.P.; O’Toole, P.W. Characterization of pro-inflammatory flagellin proteins produced by Lactobacillus ruminis and related motile Lactobacilli. PLoS ONE 2012, 7, e40592. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir: A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 183. [Google Scholar] [CrossRef]
- Waśko, A.; Polak-Berecka, M.; Paduch, R.; Jóźwiak, K. The effect of moonlighting proteins on the adhesion and aggregation ability of Lactobacillus helveticus. Anaerobe 2014, 30, 161–168. [Google Scholar] [CrossRef]
- Peng, Z.; Vogel, R.F.; Ehrmann, M.A.; Xiong, T. Identification and characterization of adhesion proteins in lactobacilli targeting actin as receptor. Mol. Cell. Probes 2018, 37, 60–63. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Olesen, S.V.; Prehn, K.; Lahtinen, S.J.; Brix, S.; Abou Hachem, M.; Svensson, B. Mucin- and carbohydrate-stimulated adhesion and subproteome changes of the probiotic bacterium Lactobacillus acidophilus NCFM. J. Proteomics 2017, 163, 102–110. [Google Scholar] [CrossRef]
- Espino, E.; Koskenniemi, K.; Mato-Rodriguez, L.; Nyman, T.A.; Reunanen, J.; Koponen, J.; Öhman, T.; Siljamäki, P.; Alatossava, T.; Varmanen, P.; et al. Uncovering surface-exposed antigens of Lactobacillus rhamnosus by cell shaving proteomics and two-dimensional immunoblotting. J. Proteome Res. 2015, 14, 1010–1024. [Google Scholar] [CrossRef]
- De Angelis, M.; Calasso, M.; Cavallo, N.; Di Cagno, R.; Gobbetti, M. Functional proteomics within the genus Lactobacillus. Proteomics 2016, 16, 946–962. [Google Scholar] [CrossRef]
- Walter, J.; Schwab, C.; Loach, D.M.; Gänzle, M.G.; Tannock, G.W. Glucosyltransferase a (GtfA) and inulosucrase (Inu) of Lactobacillus reuteri TMW1.106 contribute to cell aggregation, in vitro biofilm formation, and colonization of the mouse gastrointestinal tract. Microbiology 2008, 154, 72–80. [Google Scholar] [CrossRef]
- Anzengruber, J.; Courtin, P.; Claes, I.J.J.; Debreczeny, M.; Hofbauer, S.; Obinger, C.; Chapot-Chartier, M.-P.; Vanderleyden, J.; Messner, P.; Schäffer, C. Biochemical characterization of the major N-acetylmuramidase from Lactobacillus buchneri. Microbiology 2014, 160, 1807–1819. [Google Scholar] [CrossRef]
- Herlihey, F.A.; Moynihan, P.J.; Clarke, A.J. The essential protein for bacterial flagella formation FlgJ functions as a β-N-acetylglucosaminidase. J. Biol. Chem. 2014, 289, 31029–31042. [Google Scholar] [CrossRef]
- Claes, I.J.J.; Schoofs, G.; Regulski, K.; Courtin, P.; Chapot-Chartier, M.-P.; Rolain, T.; Hols, P.; von Ossowski, I.; Reunanen, J.; de Vos, W.M.; et al. Genetic and biochemical characterization of the cell wall hydrolase activity of the major secreted protein of Lactobacillus rhamnosus GG. PLoS ONE 2012, 7, e31588. [Google Scholar] [CrossRef] [PubMed]
- Regulski, K.; Courtin, P.; Meyrand, M.; Claes, I.J.J.; Lebeer, S.; Vanderleyden, J.; Hols, P.; Guillot, A.; Chapot-Chartier, M.-P. Analysis of the peptidoglycan hydrolase complement of Lactobacillus casei and characterization of the major γ-D-glutamyl-L-lysyl-endopeptidase. PLoS ONE 2012, 7, e32301. [Google Scholar] [CrossRef] [PubMed]
- Rolain, T.; Bernard, E.; Courtin, P.; Bron, P.A.; Kleerebezem, M.; Chapot-Chartier, M.-P.; Hols, P. Identification of key peptidoglycan hydrolases for morphogenesis, autolysis, and peptidoglycan composition of Lactobacillus plantarum WCFS1. Microb. Cell Factories 2012, 11, 137. [Google Scholar] [CrossRef] [PubMed]

| # | Log2 FC | −Log10 (p-Value) | Function | SEED Category | FIG Identifier | Gene Loci |
|---|---|---|---|---|---|---|
| 1 | 0.32 | 4.75 | Adenylosuccinate synthase | Nucleosides and Nucleotides | fig|468911.3.peg.15 | BSQ49_00075 |
| 2 | 0.30 | 4.46 | Pyruvate dehydrogenase E1 subunit alpha | Carbohydrates | fig|468911.3.peg.54 | BSQ49_00265 |
| 3 | 0.36 | 4.57 | Pyruvate dehydrogenase E1 subunit beta | Carbohydrates | fig|468911.3.peg.55 | BSQ49_00270 |
| 4 | 1.64 | 2.33 | Transcriptional regulator DeoR | Carbohydrates | fig|468911.3.peg.65 | BSQ49_00320 |
| 5 | 1.22 | 8.37 | 1-phosphofructokinase | Carbohydrates | fig|468911.3.peg.66 | BSQ49_00325 |
| 6 | 1.17 | 9.08 | PTS system, fructose-specific IIC component | Carbohydrates | fig|468911.3.peg.67 | BSQ49_00330 |
| 7 | −0.35 | 3.79 | Crp/Fnr family transcriptional regulator | fig|468911.3.peg.84 | BSQ49_00410 | |
| 8 | −0.41 | 4.27 | Succinate-semialdehyde dehydrogenase | fig|468911.3.peg.97 | BSQ49_00475 | |
| 9 | 0.95 | 7.13 | Glycerolkinase | Fatty Acids, Lipids and Isoprenoids | fig|468911.3.peg.113 | BSQ49_00555 |
| 10 | 0.75 | 5.78 | Glycerol-3-phosphate dehydrogenase | Fatty Acids, Lipids and Isoprenoids | fig|468911.3.peg.114 | BSQ49_00560 |
| 11 | 1.16 | 5.96 | PTS system, unknown specificity IIB component | Carbohydrates | fig|468911.3.peg.124 | BSQ49_00610 |
| 12 | 1.21 | 7.61 | 6-phospho-beta-glucosidase | Carbohydrates | fig|468911.3.peg.125 | BSQ49_00615 |
| 13 | 0.52 | 6.01 | Sucrose-6-phosphate hydrolase | fig|468911.3.peg.158 | BSQ49_00770 | |
| 14 | 1.04 | 8.10 | PTS system, sucrose-specific EIIBCA components | Carbohydrates | fig|468911.3.peg.159 | BSQ49_00775 |
| 15 | 0.35 | 3.81 | 6-phospho-beta-glucosidase | Carbohydrates | fig|468911.3.peg.251; fig|468911.3.peg.1214 | BSQ49_01215; BSQ49_06130 |
| 16 | 0.38 | 3.94 | PTS system, unknown specificity IIA component | Carbohydrates | fig|468911.3.peg.252 | BSQ49_01220 |
| 17 | −0.43 | 2.87 | Glucohydrolase (putative alpha-glucosidase activity) | fig|468911.3.peg.395 | BSQ49_01980 | |
| 18 | −0.87 | 2.42 | Hypothetical protein | DNA Metabolism | fig|468911.3.peg.566 | BSQ49_02890 |
| 19 | −0.54 | 2.54 | Malonate decarboxylase subunit beta (biotin-independent) | fig|468911.3.peg.814 | BSQ49_04135 | |
| 20 | −0.38 | 3.70 | 6-phospho-beta-glucosidase | Carbohydrates | fig|468911.3.peg.820 | BSQ49_04165 |
| 21 | −0.37 | 4.28 | Beta-phospho-glucomutase | fig|468911.3.peg.916 | BSQ49_04665 | |
| 22 | −0.46 | 6.96 | 6-phosphofructokinase | Carbohydrates | fig|468911.3.peg.1279 | BSQ49_06450 |
| 23 | −0.59 | 3.01 | TIGR00268 family protein | fig|468911.3.peg.1648 | BSQ49_08370 | |
| 24 | −0.45 | 3.23 | Histidinol-phosphatase | Amino Acids and Derivatives | fig|468911.3.peg.1665 | BSQ49_08455 |
| 25 | −0.39 | 3.74 | Alpha/beta hydrolase | fig|468911.3.peg.1666 | BSQ49_08460 | |
| 26 | −0.75 | 8.03 | Glucose-6-phosphate isomerase | Carbohydrates | fig|468911.3.peg.1667 | BSQ49_08465 |
| 27 | −0.32 | 4.61 | Phosphoenolpyruvate-protein-phosphotransferase | Carbohydrates | fig|468911.3.peg.1706 | BSQ49_08745 |
| 28 | −0.39 | 5.24 | 6-phospho-beta-glucosidase | Carbohydrates | fig|468911.3.peg.1810; fig|468911.3.peg.1910 | BSQ49_09735; BSQ49_09265 |
| 29 | −0.41 | 4.17 | PTS system, beta-glucoside specific IIABC components | Carbohydrates | fig|468911.3.peg.1911 | BSQ49_09740 |
| 30 | −0.49 | 2.41 | Transcriptional antiterminator BglB | Carbohydrates | fig|468911.3.peg.1912 | BSQ49_09745 |
| 31 | −0.40 | 3.87 | Transcriptional regulator (LacI family) | fig|468911.3.peg.1949 | BSQ49_09920 | |
| 32 | −1.12 | 3.98 | MFS transporter, sucrose-specific | fig|468911.3.peg.1950 | BSQ49_09925 | |
| 33 | −0.46 | 6.18 | Glucohydrolase (putative alpha-glucosidase activity) | Carbohydrates | fig|468911.3.peg.1951 | BSQ49_09930 |
| 34 | 1.90 | 3.15 | Fructose-1,6-bisphosphatase | Carbohydrates | fig|468911.3.peg.1958 | BSQ49_09960 |
| 35 | 1.11 | 4.08 | hypothetical protein | fig|468911.3.peg.1959 | BSQ49_09965 | |
| 36 | −0.39 | 3.37 | Dihydroneopterin aldolase | fig|468911.3.peg.1969 | BSQ49_10015 | |
| 37 | 3.56 | 9.55 | PTS system, fructose-specific IIA component | fig|468911.3.peg.1998 | BSQ49_10160 | |
| 38 | 3.24 | 12.55 | PTS system, fructose-specific IIB component | fig|468911.3.peg.1999 | BSQ49_10165 | |
| 39 | 3.85 | 4.93 | PTS system, fructose-specific IID component | fig|468911.3.peg.2000 | BSQ49_10170 | |
| 40 | 3.43 | 9.13 | PTS system, fructose-specific IIC/D component | fig|468911.3.peg.2001 | BSQ49_10175 | |
| 41 | 0.35 | 3.41 | Butanediol dehydrogenase | Amino Acids and Derivatives | fig|468911.3.peg.2017 | BSQ49_10255 |
| 42 | 1.42 | 9.64 | ABC-transporter substrate-binding protein, glycerol-3-phosphate specific | Carbohydrates | fig|468911.3.peg.2080 | BSQ49_10570 |
| 43 | 1.12 | 7.83 | ABC-transporter ATP-binding protein | Carbohydrates | fig|468911.3.peg.2084 | BSQ49_10590 |
| 44 | −0.74 | 5.81 | PTS system, fructose-specific IID component | fig|468911.3.peg.2194 | BSQ49_11155 | |
| 45 | −0.70 | 3.13 | PTS system, fructose-specific IIC component | fig|468911.3.peg.2195 | BSQ49_11160 | |
| 46 | −0.76 | 6.01 | PTS system, fructose-specific EIIAB components | Cell Wall and Capsule | fig|468911.3.peg.2196 | BSQ49_11165 |
| 47 | −0.79 | 4.83 | PTS system, fructose-specific EIIB component | Cell Wall and Capsule | fig|468911.3.peg.2197 | BSQ49_11170 |
| 48 | −0.32 | 4.34 | Transcription antiterminator BglG | fig|468911.3.peg.2198 | BSQ49_11175 | |
| 49 | 1.60 | 10.60 | Mannitol-1-phosphate 5-dehydrogenase | Carbohydrates | fig|468911.3.peg.2224 | BSQ49_11290 |
| 50 | 1.64 | 7.24 | PTS system, fructose/mannitol specific IIA component | fig|468911.3.peg.2225 | BSQ49_11295 | |
| 51 | 1.37 | 4.16 | transcriptional regulator | Carbohydrates | fig|468911.3.peg.2226 | BSQ49_11300 |
| 52 | 2.73 | 12.28 | PTS system, fructose/mannitol specific IICBA components | Carbohydrates | fig|468911.3.peg.2227 | BSQ49_11305 |
| 53 | 0.51 | 5.61 | GH25 muramidase (putative) | BSQ49_11795 |
| Regulation | GO ID | GO Term | Terms Annotated | Significant Terms | p-Value |
|---|---|---|---|---|---|
| ↑ | GO:0006412 | translation | 67 | 52 | 0.0375 |
| ↓ | GO:0001539 | cilium or flagellum-dependent cell motility | 7 | 6 | 8.90 × 10−7 |
| ↓ | GO:0030436 | asexual sporulation | 5 | 5 | 2.20 × 10−6 |
| ↓ | GO:0007059 | chromosome segregation | 2 | 2 | 0.0067 |
| ↓ | GO:0030261 | chromosome condensation | 2 | 2 | 0.0067 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechtner, J.; Ludwig, C.; Kiening, M.; Jakob, F.; Vogel, R.F. Living the Sweet Life: How Liquorilactobacillus hordei TMW 1.1822 Changes Its Behavior in the Presence of Sucrose in Comparison to Glucose. Foods 2020, 9, 1150. https://doi.org/10.3390/foods9091150
Bechtner J, Ludwig C, Kiening M, Jakob F, Vogel RF. Living the Sweet Life: How Liquorilactobacillus hordei TMW 1.1822 Changes Its Behavior in the Presence of Sucrose in Comparison to Glucose. Foods. 2020; 9(9):1150. https://doi.org/10.3390/foods9091150
Chicago/Turabian StyleBechtner, Julia, Christina Ludwig, Michael Kiening, Frank Jakob, and Rudi F. Vogel. 2020. "Living the Sweet Life: How Liquorilactobacillus hordei TMW 1.1822 Changes Its Behavior in the Presence of Sucrose in Comparison to Glucose" Foods 9, no. 9: 1150. https://doi.org/10.3390/foods9091150
APA StyleBechtner, J., Ludwig, C., Kiening, M., Jakob, F., & Vogel, R. F. (2020). Living the Sweet Life: How Liquorilactobacillus hordei TMW 1.1822 Changes Its Behavior in the Presence of Sucrose in Comparison to Glucose. Foods, 9(9), 1150. https://doi.org/10.3390/foods9091150





