An Artificial Intelligence Characterised Functional Ingredient, Derived from Rice, Inhibits TNF-α and Significantly Improves Physical Strength in an Inflammaging Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Prediction for Bioactivity and Natural Source Identification
2.2. Natural Peptide Network Production
2.3. Sample Preparation and Mass Spectrometry Analysis
2.4. Cell Culture, Differentiation of Monocytes and Inflammation ELISA Assays
2.5. Human Randomised, Double Blinded, Parallel Group, Placebo Controlled Clinical Trial
2.5.1. Participants and Study Design
2.5.1.1. Hand Grip Test
2.5.1.2. Repeated Chair Stand Test
2.5.1.3. Short Physical Performance Battery (SPPB)
2.5.2. Glucose Tolerance Test
2.5.3. Serum Cytokine Concentrations
2.6. Statistics
3. Results
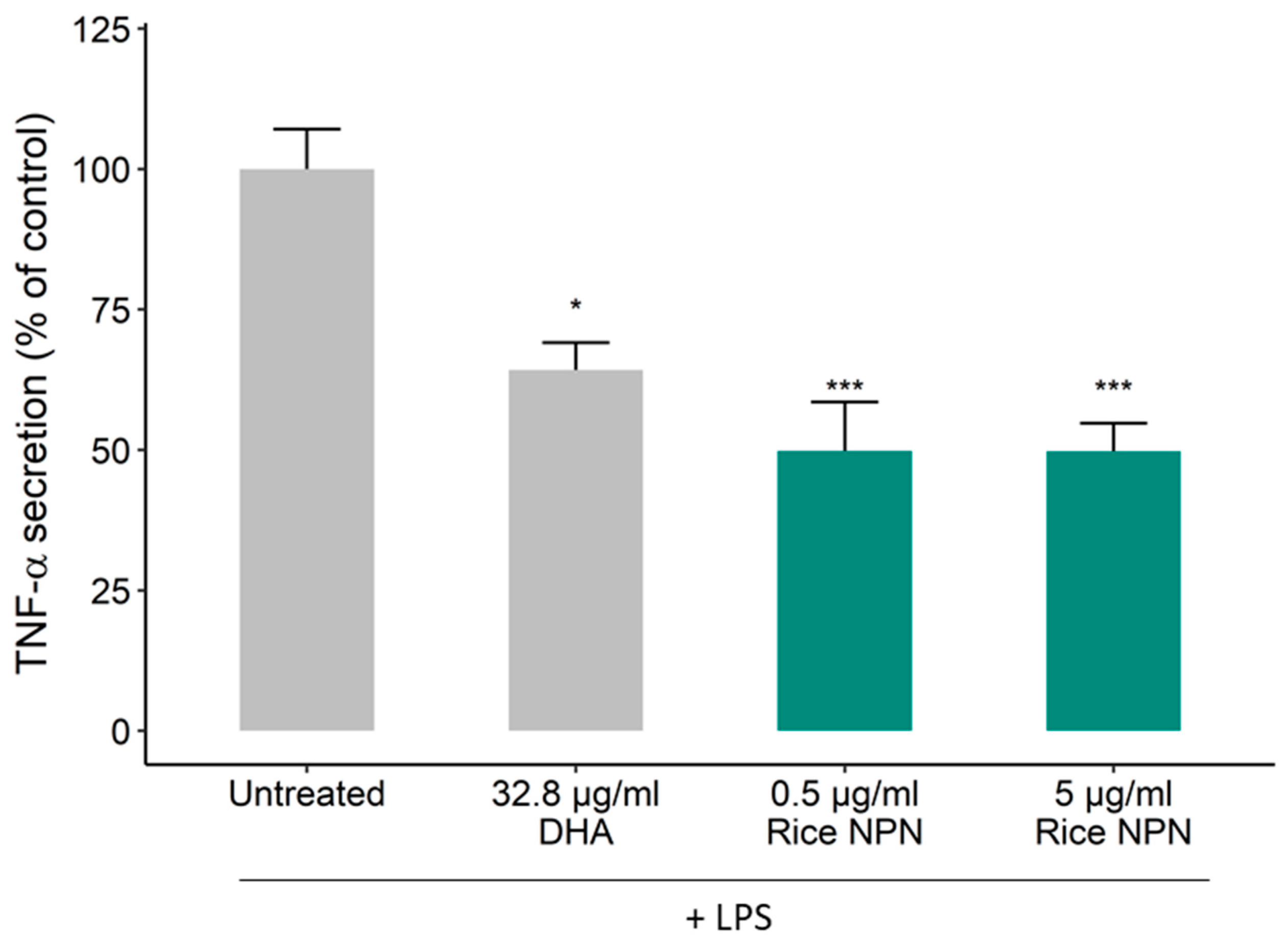
3.1. Bioactivity Screening and Rice NPN Characteristics
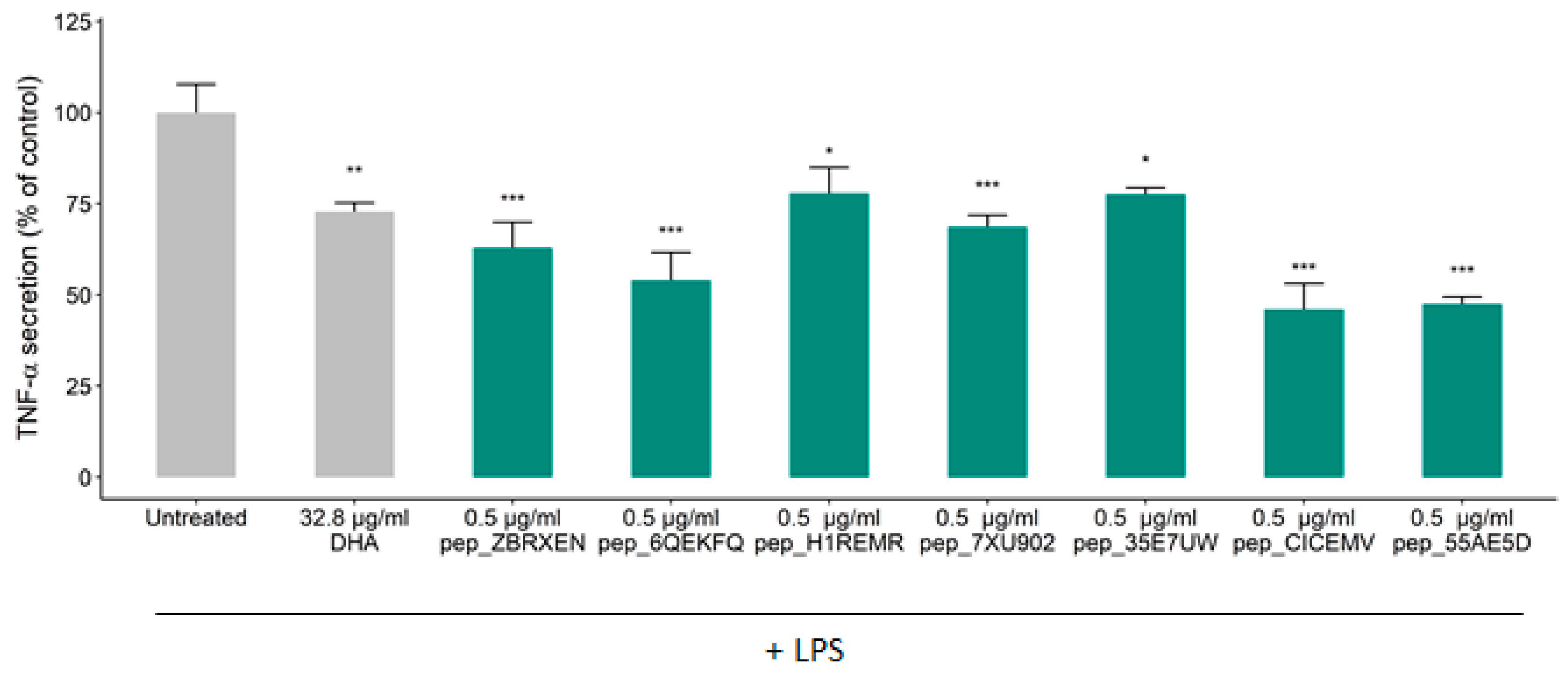
3.2. Effects of Constituent Peptides Predicted with Anti-Inflammatory Effects on TNF-α Secretion
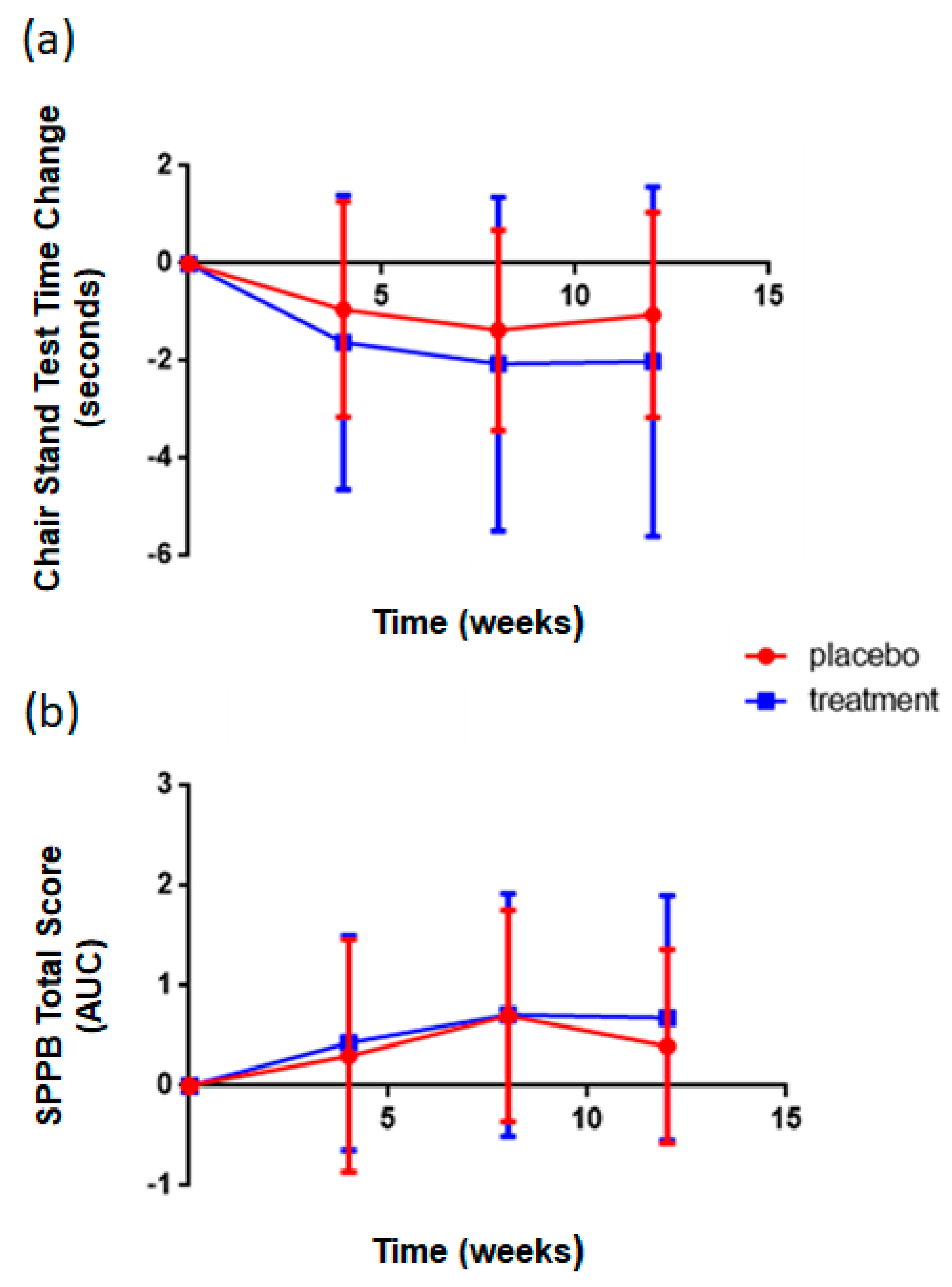
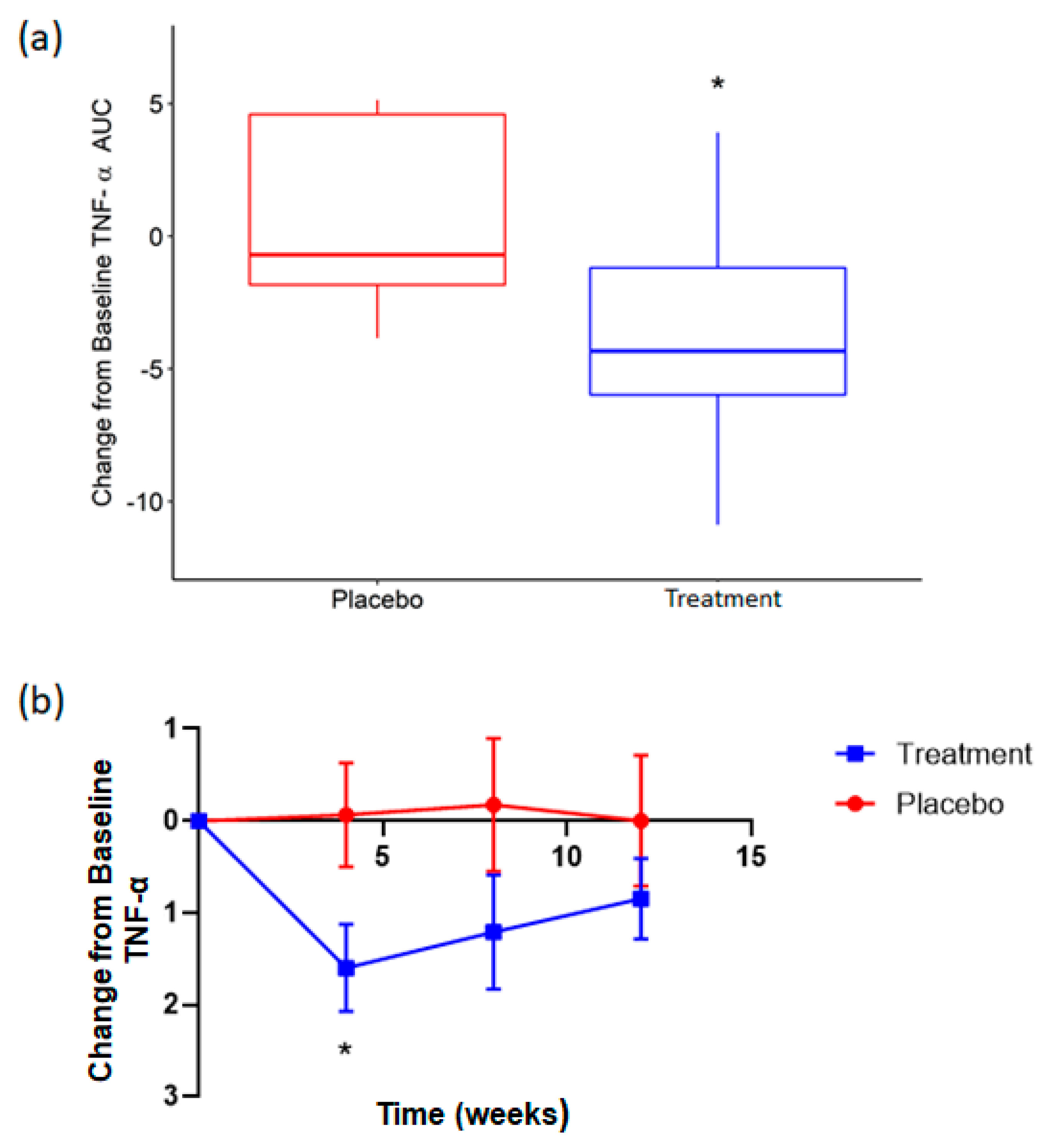
3.3. Supplementation with Rice NPN in an Elderly Cohort Had Beneficial Effects on Physical Activity and Correlated with Markers of Age Associated Inflammation
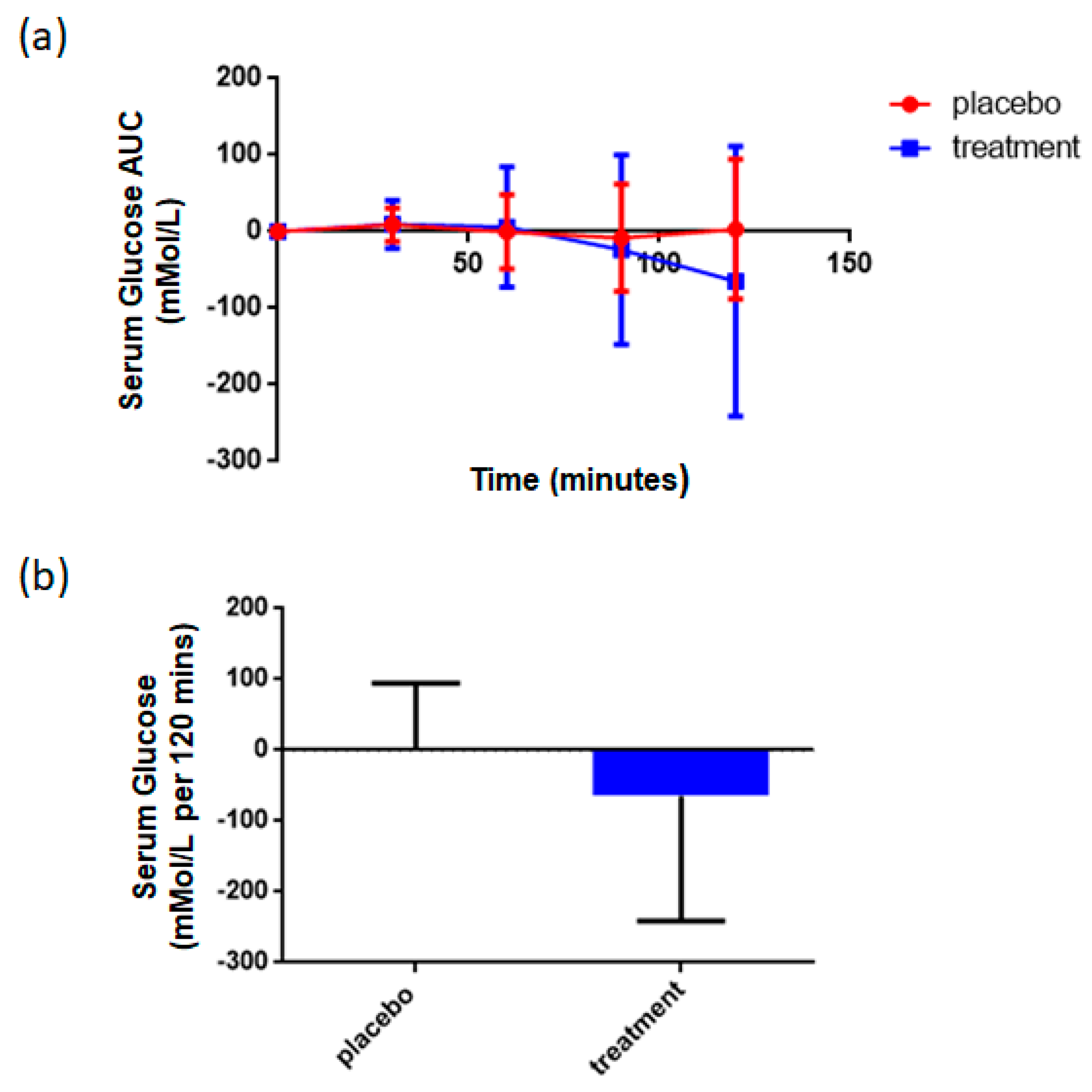
3.4. Supplementation with Rice NPN Increased Glucose Uptake When Challenged with a Glucose Tolerance Test
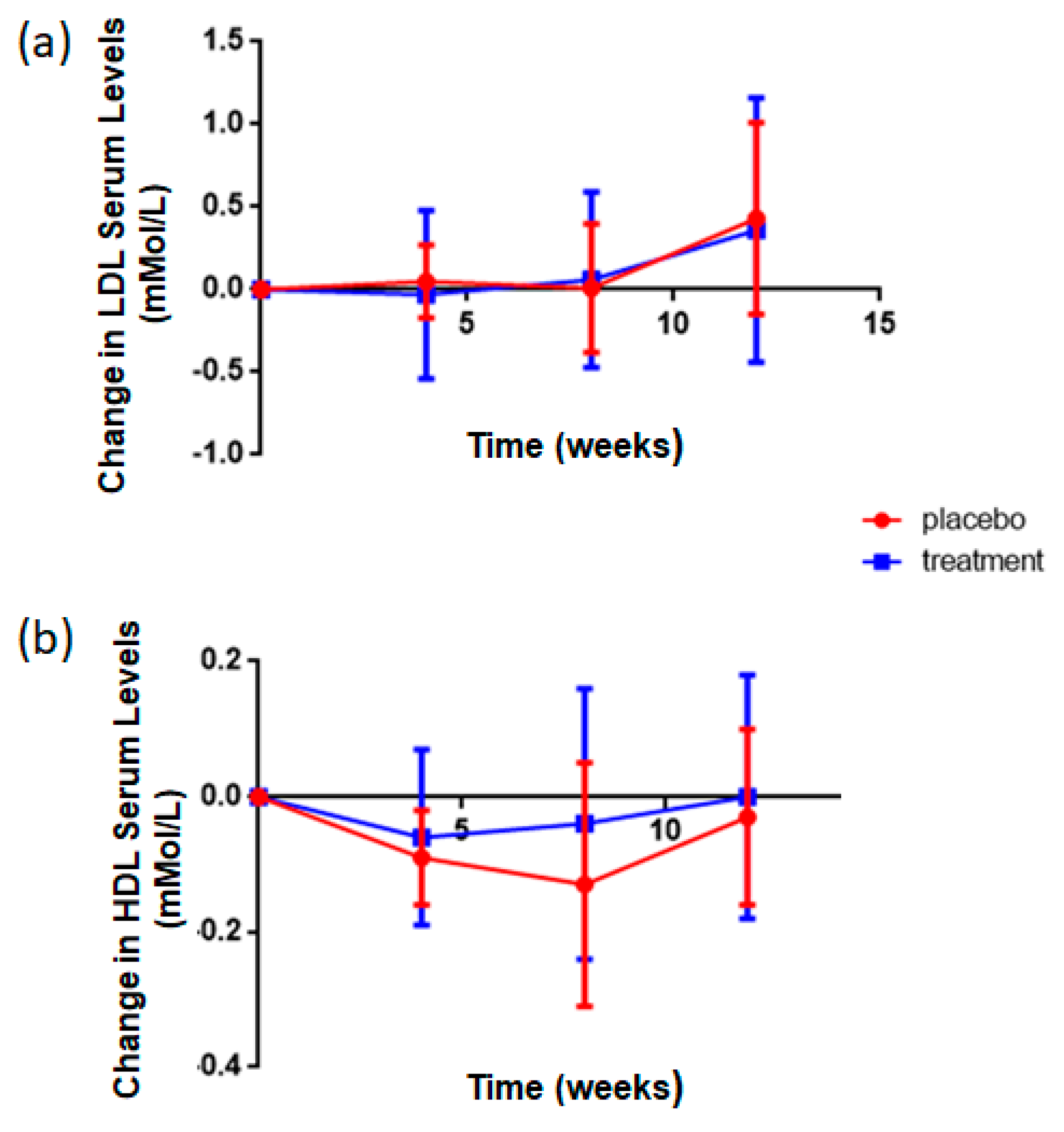
3.5. Rice NPN Supplementation Altered LDL and HDL Serum Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. The inflammation theory of disease: The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- El-Gabalawy, H.; Guenther, L.Y.N.C.; Bernstein, C.N.; El-gabalawy, H.; Guenther, L.Y.N.C.; Bernstein, C.N. Epidemiology of Immune-Mediated Inflammatory Diseases: Incidence, Prevalence, Natural History, and Comorbidities. J. Rheumatol. 2010, 85, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune—metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Dziubek, W.; Wolny-Rokicka, E.; Dabrowska, G.; Wozniewski, M. The relation of inflammaging with skeletal muscle properties in elderly men. Am. J. Mens. Health 2019, 13, 1557988319841934. [Google Scholar] [CrossRef] [Green Version]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.-S.; Youn, J.-C.; Shin, E.-C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Popa, C.; Netea, M.G.; van Riel, P.L.C.M.; van der Meer, J.W.M.; Stalenhoef, A.F.H. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Maini, R.N.; Elliott, M.J.; Brennan, F.M.; Feldmann, M. Beneficial effects of tumour necrosis factor-alpha (TNF-alpha) blockade in rheumatoid arthritis (RA). Clin. Exp. Immunol. 1995, 101, 207–212. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 2003, 9, 1245–1250. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2015, 12, 49. [Google Scholar] [CrossRef]
- Moughan, P.J.; Rutherfurd, S.M.; Montoya, C.A.; Dave, L.A. Food-derived bioactive peptides—A new paradigm. Nutr. Res. Rev. 2014, 27, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of anti-inflammatory proteins/peptides: An insilico approach. J. Transl. Med. 2017, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as therapeutic agents for inflammatory—Related diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaldi, N.; Holton, T.A.; Shields, D.C. Amino acid enrichment and compositional changes among mammalian milk proteins and the resulting nutritional consequences. J. Dairy Sci. 2014, 97, 1248–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udenigwe, C.C. Mohan Aishwarya Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Rein, D.; Ternes, P.; Demin, R.; Gierke, J.; Helgason, T.; Schön, C. Artificial intelligence identified peptides modulate inflammation in healthy adults. Food Funct. 2019, 10, 6030–6041. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.-H.; Choi, S.; Jang, J.-E.; Ramalingam, P.; Ko, Y.T.; Kim, S.Y.; Oh, S.H. Wasabia japonica is a potential functional food to prevent colitis via inhibiting the NF-[small kappa]B signaling pathway. Food Funct. 2017, 8, 2865–2874. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional modulation of immune function: Analysis of evidence, mechanisms, and clinical relevance. Front. Immunol. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Wichers, H. Immunomodulation by food: Promising concept for mitigating allergic disease? Anal. Bioanal. Chem. 2009, 395, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Zhavoronkov, A.; Ivanenkov, Y.A.; Aliper, A.; Veselov, M.S.; Aladinskiy, V.A.; Aladinskaya, A.V.; Terentiev, V.A.; Polykovskiy, D.A.; Kuznetsov, M.D.; Asadulaev, A.; et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat. Biotechnol. 2019, 37, 1038–1040. [Google Scholar] [CrossRef]
- Kemp, G.J.; Birrell, F.; Clegg, P.D.; Cuthbertson, D.J.; De Vito, G.; Van Dieën, J.H.; Del Din, S.; Eastell, R.; Garnero, P.; Goljanek-Whysall, K.; et al. Developing a toolkit for the assessment and monitoring of musculoskeletal ageing. Age Ageing 2018, 47, iv1–iv19. [Google Scholar] [CrossRef]
- Freiberger, E.; De vreede, P.; Schoene, D.; Rydwik, E.; Mueller, V.; Frändin, K.; Hopman-Rock, M. Performance-based physical function in older community-dwelling persons: A systematic review of instruments. Age Ageing 2012, 41, 712–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, N.; Bolzetta, F.; Toffanello, E.D.; Zambon, S.; De Rui, M.; Perissinotto, E.; Coin, A.; Corti, M.C.; Baggio, G.; Crepaldi, G.; et al. Association between short physical performance battery and falls in older people: The progetto veneto anziani study. Rejuvenation Res. 2014, 17, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatic, M.; von Haehling, S.; Ebner, N. Inflammatory biomarkers of frailty. Exp. Gerontol. 2020, 133, 110858. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef]
- Hovland, I.H.; Leikanger, I.S.; Stokkeland, O.; Waage, K.H.; Mjøs, S.A.; Brokstad, K.A.; McCann, A.; Ueland, P.M.; Slizyte, R.; Carvajal, A.; et al. Effects of low doses of fish and milk proteins on glucose regulation and markers of insulin sensitivity in overweight adults: A randomised, double blind study. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3319242776. [Google Scholar]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Kiewiet, M.; Faas, M.; de Vos, P. Immunomodulatory Protein Hydrolysates and Their Application. Nutrients 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive protein hydrolysates in the functional food ingredient industry: Overcoming current challenges. Food Rev. Int. 2017, 33, 217–246. [Google Scholar] [CrossRef]
- Fagerström, C.; Borglin, G. Mobility, functional ability and health-related quality of life among people of 60 years or older Aging Clinical and Experimental Research. Aging Clin. Exp. Res. 2010, 22, 387–394. [Google Scholar] [CrossRef]
- Sartor-Glittenberg, C.; Lehmann, S.; Okada, M.; Rosen, D.; Brewer, K.; Bay, R.C. Variables explaining health-related quality of life in community-dwelling older adults. J. Geriatr. Phys. Ther. 2014, 37, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Groessl, E.J.; Kaplan, R.M.; Rejeski, W.J.; Katula, J.A.; King, A.C.; Frierson, G.; Glynn, N.W.; Hsu, F.C.; Walkup, M.; Pahor, M. Health-Related Quality of Life in Older Adults at Risk for Disability. Am. J. Prev. Med. 2007, 33, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Lencel, P.; Magne, D. Inflammaging: The driving force in osteoporosis? Med. Hypotheses 2011, 76, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Patel, A.V.; Newton, C.C.; Jacobs, E.J.; Gapstur, S.M. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J. Clin. Oncol. 2013, 31, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Giovannucci, E.L.; Holmes, M.D.; Chan, A.T.; Chan, J.A.; Colditz, G.A.; Fuchs, C.S. Physical activity and survival after colorectal cancer diagnosis. J. Clin. Oncol. 2006, 24, 3527–3534. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Bernstein, L.; Deka, A.; Feigelson, H.S.; Campbell, P.T.; Gapstur, S.M.; Colditz, G.A.; Thun, M.J. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am. J. Epidemiol. 2010, 172, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, D.R.; Reynolds, K.; Sidell, M.; Brar, S.; Ghai, N.R.; Sternfeld, B.; Jacobsen, S.J.; Slezak, J.M.; Caan, B.; Quinn, V.P. Effects of physical activity and sedentary time on the risk of heart failure. Circ. Heart Fail. 2014, 7, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feskanich, D.; Willett, W.; Colditz, G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. J. Am. Med. Assoc. 2002, 288, 2300–2306. [Google Scholar] [CrossRef] [Green Version]
- Khalaj, N.; Osman, N.A.A.; Mokhtar, A.H.; Mehdikhani, M.; Abas, W.A.B.W. Balance and risk of fall in individuals with bilateral mild and moderate knee osteoarthritis. PLoS ONE 2014, 9, e92270. [Google Scholar] [CrossRef] [Green Version]
- Matthews, C.E.; George, S.M.; Moore, S.C.; Bowles, H.R.; Blair, A.; Park, Y.; Troiano, R.P.; Hollenbeck, A.; Schatzkin, A. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am. J. Clin. Nutr. 2012, 95, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Ghanim, H.; Sia, C.L.; Abuaysheh, S.; Korzeniewski, K.; Patnaik, P.; Marumganti, A.; Chaudhuri, A.; Dandona, P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J. Clin. Endocrinol. Metab. 2010, 95, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Stone, M.A.; Payne, U.; Pacheco-Tena, C.; Inman, R.D. Cytokine correlates of clinical response patterns to infliximab treatment of ankylosing spondylitis. Ann. Rheum. Dis. 2004, 63, 84–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluess, T.-T.; Hayoz, D.; Berger, M.M.; Tappy, L.; Revelly, J.-P.; Michaeli, B.; Carpentier, Y.A.; Chioléro, R.L. Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med. 2007, 33, 789–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Cao, S.; Nagamani, M.; Anderson, K.E.; Grady, J.J.; Lu, L.J.W. Decreased circulating levels of tumor necrosis factor-α in postmenopausal women during consumption of soy-containing isoflavones. J. Clin. Endocrinol. Metab. 2005, 90, 3956–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahzad, H.; Aghdashi, M.A.; Asghari Jafarabadi, M.; Alipour, B. Effects of Coenzyme Q10 Supplementation on Inflammatory Cytokines (TNF-α, IL-6) and Oxidative Stress in Rheumatoid Arthritis Patients: A Randomized Controlled Trial. Arch. Med. Res. 2015, 46, 527–533. [Google Scholar] [CrossRef]
- Daliri, E.; Oh, D.; Lee, B. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Kiemer, A.K. Inhibition of p38 MAPK Activation via Induction of MKP-1: Atrial Natriuretic Peptide Reduces TNF-alpha-Induced Actin Polymerization and Endothelial Permeability. Circ. Res. 2002, 90, 874–881. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Anti-inflammatory neuropeptides: A new class of endogenous immunoregulatory agents. Brain. Behav. Immun. 2008, 22, 1146–1151. [Google Scholar] [CrossRef] [Green Version]
- Luzi, S.; Kondo, Y.; Bernard, E.; Stadler, L.K.J.; Vaysburd, M.; Winter, G.; Holliger, P. Subunit disassembly and inhibition of TNFα by a semi-synthetic bicyclic peptide. Protein Eng. Des. Sel. 2015, 28, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Kruszynski, M.; Shealy, D.J.; Leone, A.O.; Heavner, G.A. Identification of Tnf-a binding peptides from ad-amino acid hexapeptide library that specifically inhibit tnf-α binding to recombinant p55 receptor. Cytokine 1999, 11, 37–44. [Google Scholar] [CrossRef]
- Qin, W.; Feng, J.; Li, Y.; Lin, Z.; Shen, B. De novo design TNF-α antagonistic peptide based on the complex structure of TNF-α with its neutralizing monoclonal antibody Z12. J. Biotechnol. 2006, 125, 57–63. [Google Scholar] [CrossRef]

| ID | Sequence | Length | Molecular Weight | Charge | Uniprot ID |
|---|---|---|---|---|---|
| pep_ZBRXEN | TVFDGVLRPGQL | 12 | 1301.49 | 0 | P14323 |
| pep_6QEKFQ | FYNEGDAPVVAL | 12 | 1294.37 | −2 | Q0E261 |
| pep_H1REMR | IYGPDTGVDYKDNQMR | 16 | 1871.97 | −1 | Q0DEV5 |
| pep_7XU902 | GYYGEQQQQPGMTR | 14 | 1642.75 | 0 | P29835 |
| pep_35E7UW | IDGYDTPVEGR | 11 | 1221.27 | −2 | Q0DEV5 |
| pep_CICEMV | NGVLRPGQL | 9 | 953.1 | 1 | P14614 |
| pep_55AE5D | SEEGYYGEQQQQPGMTR | 17 | 1988.05 | −2 | P29835 |
| Readout | Measured By | Effect | p Value |
|---|---|---|---|
| Gut Discomfort | Questionnaire | None | NS |
| TNF-α | ELISA | ↓ | 0.03 * |
| HDL | Blood Chem Panel | ↑ | <0.001 ** |
| LDL | Blood Chem Panel | ↓ | <0.001 ** |
| Oral Glucose Tolerance Test | Glucometer | ↑ | <0.001 # |
| Chair Stand | Physical Test | ↑ | 0.02 |
| Hand Grip | Physical Test | None | NS |
| SPPB | Physical Test | ↑ | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, K.; Keogh, B.; Lopez, C.; Adelfio, A.; Molloy, B.; Kerr, A.; Wall, A.M.; Jalowicki, G.; Holton, T.A.; Khaldi, N. An Artificial Intelligence Characterised Functional Ingredient, Derived from Rice, Inhibits TNF-α and Significantly Improves Physical Strength in an Inflammaging Population. Foods 2020, 9, 1147. https://doi.org/10.3390/foods9091147
Kennedy K, Keogh B, Lopez C, Adelfio A, Molloy B, Kerr A, Wall AM, Jalowicki G, Holton TA, Khaldi N. An Artificial Intelligence Characterised Functional Ingredient, Derived from Rice, Inhibits TNF-α and Significantly Improves Physical Strength in an Inflammaging Population. Foods. 2020; 9(9):1147. https://doi.org/10.3390/foods9091147
Chicago/Turabian StyleKennedy, Kathy, Brian Keogh, Cyril Lopez, Alessandro Adelfio, Brendan Molloy, Alish Kerr, Audrey M. Wall, Gaël Jalowicki, Thérèse A. Holton, and Nora Khaldi. 2020. "An Artificial Intelligence Characterised Functional Ingredient, Derived from Rice, Inhibits TNF-α and Significantly Improves Physical Strength in an Inflammaging Population" Foods 9, no. 9: 1147. https://doi.org/10.3390/foods9091147
APA StyleKennedy, K., Keogh, B., Lopez, C., Adelfio, A., Molloy, B., Kerr, A., Wall, A. M., Jalowicki, G., Holton, T. A., & Khaldi, N. (2020). An Artificial Intelligence Characterised Functional Ingredient, Derived from Rice, Inhibits TNF-α and Significantly Improves Physical Strength in an Inflammaging Population. Foods, 9(9), 1147. https://doi.org/10.3390/foods9091147