Abstract
Emerging evidence suggests that fermentation, historically used for the preservation of perishable foods, may be considered as a useful tool for increasing the nutritional value of fermented products, in terms of increases in bioactive compound content, including short-chain fatty acids (SCFAs), as bacteria end-products, whose beneficial effects on human health are well-established. The purpose of the present manuscript is to summarize studies in this field, providing evidence about this novel potential of fermentation. A limited number of studies directly investigated the increased SCFA levels in fermented foods. All studies, however, agree in confirming that levels of SCFAs in fermented products are higher than in unfermented products, recognizing the key role played by the microorganisms in metabolizing food matrices, producing and releasing bioactive substances. According to the available literature, fermentation might be taken into account by the food industry as a natural strategy with no environmental impacts to produce functional foods and beverages with a higher nutritional value and health-promoting compounds.
1. Introduction
Fermentation has been historically used as a strategy to increase the shelf-life of perishable foods [1]. Over time, boththe production and consumption of fermented foods and beverages have strongly increased, due to their peculiar and appreciated taste, and their recognized health benefits. Over the last few decades, scientific research has focused attention on the beneficial properties of fermented foods and beverages, and their impact on human health [2]. Besides their well-established role on gastrointestinal tract, there is increasing evidence demonstrating that this class of products is able to ameliorate several metabolic outcomes, including glycemia, lipidemia, and oxidative stress, as shown in animal-based in vivo studies [3,4].
Notably, fermentation has been indicated as a tool to enhance the nutritional value of foods and beverages, in terms of both increased bioavailability of bioactive compounds (i.e., polyphenols) and production of health-promoting end-products. Among the latter, short-chain fatty acids (SCFAs) are emerging as some of the most studied compounds in the last decade, due to their proven beneficial impact on human health. SCFAs are small organic monocarboxylic acids with different chain lengths, ranging from two to six carbon atoms. They are prevalently produced by gut microbiota as end-products of fermentation of dietary polysaccharides, including fiber and resistant starch. The most representative SCFAs are acetic acid (C2), propionic acid (C3) and butyric acid (C4) (Figure 1), produced in a molar rate of about 60:20:20, respectively [5].
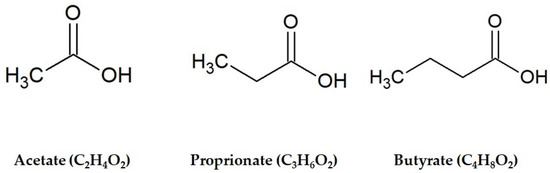
Figure 1.
Chemical structures of the main short-chain fatty acids.
Evidence shows that SCFAs may be efficiently involved inglycemic control [6]. The main putative mechanism would involve G protein-coupled receptors (GPCRs) [7], whose activation by SCFAs leads to secretion of enterohormones, including glucagon-like peptide-1 (GLP-1) and peptide tyrosine–tyrosine (PYY), resulting in improved glucose tolerance and increased insulin release [8,9]. Interestingly, an animal-based study reported that acetate, produced by colonic fermentation, was able to cross the blood–brain barrier and act as an appetite suppressant in hypothalamus [10]. Overall, this evidence suggests that SCFAs might play a relevant role in the management of metabolic diseases, including obesity and diabetes, where the control of food intake is crucial. Moreover, propionate has been shown to reduce both liponeogenesis and cholesterol synthesis [11]. Furthermore, it has been demonstrated that an increased influx of SCFAs into the liver results in decreased levels of angiopoietin-like protein 4 (ANGPTL4), a target gene of peroxisome proliferator-activated receptors (PPARs) [12]. These specific transcription factors regulate the expression of genes [13] involved in various cell functions, including differentiation, the metabolism of carbohydrates, protein and lipids [14], and tumorigenesis [15]. In addition to these metabolic effects, studies demonstrated the positive influence of SFCAs on intestinal functions. In particular, it has been reported that SCFAs are used as an energy source by colonic epithelial cells [16], contributing to the maintenance of colonic mucosa integrity [17]. These data suggest the role of SCFAs in both the prevention and treatment of intestinal diseases [18]. Interestingly, SCFAs, mainly butyrate, have been demonstrated to possess an anticancer potential, due to their ability to regulate proliferation, differentiation and cell apoptosis, via inhibition of histone-D-acetylase [19], and anti-inflammatory activity, via suppression of pro-inflammatory pathways [20]. These mechanisms of action would, at the base, decrease the production of pro-inflammatory cytokines, including tumor necrosis factor (TNF)α, interleukin (Il)-1β, -6 [21], -12 [22], and up-regulate anti-inflammatory cytokines, including IL-10 [21].
Despite this promising evidence, most of the studies investigating the beneficial effects of SCFAs on human health focused on the role played by endogenous SCFAs, produced in the lower intestine by microbiota fermentation. On the other hand, limited literature is available on the biological effects of exogenous SCFAs occurring in fermented products.
The purpose of the present mini review is to summarize the available literature of the last 5 years, in order to elucidate the potential role of fermented products as a source of SCFAs. In particular, a literature search was conducted by consulting official scientific databases, including PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Science Direct (http://www.sciencedirect.com). Articles in English were downloaded using specific keywords (“fermented foods”, “fermented beverages”, “short-chain fatty acids”, “butyrate”, “acetate”, “propionate”) and their combinations.
2. Increased Bioaccessibility and Bioavailability of Bioactive Compounds Occurring in Fermented Products: A Prime Example of the Usefulness of Fermentation
Emerging evidence reports that, during fermentation, there is a marked enhancement of the nutritional value of final products [23,24], in terms of increased bioaccessibility and bioavailability of bioactive compounds. As an example, several studies have focused on the increase of polyphenol content in fermented foods.
In fruit and vegetables, polyphenols mainly bind carbohydrate residues in the plant cell wall [25], resulting in variable intestinal absorption. In this context, microorganism fermentation potentially acts by: (i) releasing bioactive compounds from fiber by degrading it, and then (ii) metabolizing them, releasing smaller molecules or their metabolites, which are more efficiently absorbed at the intestinal level. This is probably due to the ability of microorganisms to express amylase, β-glucosidase, decarboxylase, phenolic acid decarboxylases, esterase, phenol reductase, tannase and glucoamylase, which have been described for their pivotal role played in enhancing polyphenol levels in fermented foods [26,27]. The expression of these enzymes is strain-specific [26].
The activity of microorganisms on food matricesduring fermentation is, thus, crucial to obtain high nutritional value of the final products. Previous authors have reported the increase in the antioxidant activity in fermented products compared with non-fermented ones. These observed results might be explained by the above mentioned activities of microorganisms.
Further studies investigated the role of intestinal microbiota in increasing both polyphenol bioaccessibility and antioxidant activity, providing evidence regarding the ability of bacteria to metabolize non-absorbed polyphenols that reach the colon [25,28,29,30,31]. This evidence concerns not only polyphenol-rich foods but also nutraceutical products, as demonstrated in a recent study in which acid-resistant capsules containing tea polyphenolic extract were submitted to a simulated gastrointestinal digestion protocol, including both upper and lower intestinal digestion steps. Following the colonic digestion, researchers noted a significant increase in both polyphenol levels and antioxidant activity, suggesting that gut microbiota is able to further metabolize polyphenols, making them more bioavailable and active [31].
Overall, this evidence suggests that microorganisms used for fermentation are able to metabolize the food matrix and release bioactive compounds. This, in turn, enhances the nutritional value of final fermented products. In this sense, as aforementioned, both bioaccessibility and bioavailability of polyphenols (naturally bound to fibers) are increased in fermented products. These aspects indicate fermentation as a useful strategy for the production of functional foods.
3. Increased Levels of SCFAs in Fermented Foods and Beverages
A limited number of studies investigated the amount of SCFAs in fermented products. Nevertheless, authors generally agree that fermentation significantly increases the levels of this class of bioactive compounds (Table 1). Different raw materials have been studied, including milk [32,33,34], beer wort [35], fruit [36] and vegetables [2], and several bacteria strains have been used for fermentation.

Table 1.
Studies reporting the increased levels of short-chain fatty acids (SCFAs) in fermented foods.
Yoghurt is undoubtedly one of the most common fermented products, obtained from the fermentation of milk by specific bacterial strains capable of fermenting it by breaking down molecular bonds with sugars, mainly lactose, and producing acids (mainly lactic acid), with the consequent coagulation of proteins which thicken the matrix [39,40,41]. Besides the well-established beneficial effects of yoghurt consumption on human health, a limited number of studies focused on the evaluation of SCFA content in fermented milk. Jia et al. [33] demonstrated that in goat milk fermented with Lactobacillus rhamnosus GG, the amount of total SCFAs was significantly higher than that in non-fermented goat milk (p < 0.05). On the other hand, the levels of long-chain fatty acids (LCFAs) progressively decreased, suggesting that lipoprotein lipase might be responsible for hydrolyses of LCFAs, leading to the production of both SCFAs and medium-chain fatty acids (MCFAs). In the same study, goat milk was also fermented with traditional yoghurt starter cultures, including Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus. In these samples, the trend of fatty acid production was inverted, with an increase in LCFAs, and a decrease in SCFA and MCFA levels. According to the authors, the addition of mixed starter cultures may result in a partial inhibition of lipoprotein lipase activity [33], suggesting that the use of a specific bacterial strain, rather than another, may be a useful tool to increase the nutritional value of fermented products. Another research group analyzed different samples of human milk, commercial pure cow milk, infant formula and fermented cow milk, finding highest amount of SCFAs in fermented cow milk [34]. In line with this evidence, a further study demonstrated that the fermentation of skimmed milk with Lactobacilli or Bifidobacteria increased SCFA production with variable trends, based on the type of added prebiotics, including β-glucan, inulin and hi-maize. Generally, after 24 h fermentation, higher amounts of SCFAs (acetate 2.72 mM, propionate 0.92 mM and butyrate 0.41 mM) were found in samples fermented with Bifidobacteriumanimalis subsp. Lactis and inulin as prebiotic, suggesting a strain- and substrate-dependent relationship [32]. It is clear that various microbe strains have different metabolic pathways, and this evidence may guide research toward the choice of the best combination of raw material and microorganisms, in order to optimize the production of SCFAs in the final fermented products. In line with this evidence, an elegant study compared both rate and profile of SCFA production during fermentation of rice fiber with different bacterial strains, including Lactobacillus rhamnosus, Lactobacillus acidophilus and Bifidobacteriumlongum [42]. In general, the amount of SCFAs was higher when samples were treated with an inter-genus than intra-genus combination. In addition, acetate was the most produced metabolite, and butyrate the least. Despite the well-known metabolic pathways of Bifidobacteria, including the Wood–Ljungdahl pathway, the authors suggested that the fructose-6-phosphoketolase-involving pathway, also known as “bifid shunt”, was a further mechanism for carbohydrate metabolism, resulting in SCFA production. In contrast, Lactobacilli mainly produced acetate through the transketolase pathway, in a significantly lower rate than Bifidobacteria. The combination of these two genera, thus, leads to a higher production of SCFAs [42].
Utoiu et al. [38] performed a laboratory experiment, proving that fermentation of Kombucha beverage with pollen led to a significant increase in SCFAs. In particular, they used a mixture of microorganisms called symbiotic culture of bacteria and yeast (SCOBY), which included lactic acid bacteria, acetobacteria, and yeasts from various genera. Both laboratory-level and large-scale experiments were performed with different fermentation duration periods (0–17 days and 18 days, respectively). Pollen addition contributed to a significant enhancement in SCFA production in both experiments. In particular, SCFAs progressively increased during fermentation days (in laboratory level: acetate from 0.415 ± 0.005 g/L to 3.51 ± 0.11 g/L, propionate from 0.095 ± 0.012 g/L to 0.56 ± 0.041 g/L, butyrate from 0.12 ± 0.038 g/L to 1.78 ± 0.054 g/L), reaching their highest amount in 18 days (large-scale: acetate 19.56 ± 0.18 g/L, propionate 0.66 ± 0.037 g/L, butyrate 1.92 ± 0.033 g/L) [38]. The large microbial diversity in SCOBY, and, in particular, the high content of lactic acid bacteria, might explain the high SCFA production. Similarly, it has been noted that butyrate levels significantly increased in short-time tea infusion fermented with lactic acid bacteria (including Lactobacillus bulgaricus, L. acidophilus, L. rhamnosus, Lactobacillus plantarum), in a time-dependent manner. In particular, the highest concentrations of butyrate were detected after (i) 15-min fermentation with L. rhamnosus (1.332 ± 0.065 µg/g), (ii) 30-min fermentation in samples treated with L. bulgaricus and L. plantarum (3.642 ± 0.058 µg/g and 2.157 ± 0.364 µg/g, respectively), and (iii) 120-min fermentation with L. acidophilus (2.479 ± 0.137 µg/g) [37]. The fermentation of fiber occurring in herb samples might be considered as the main mechanism for the observed increase in SCFA levels, despite the short fermentation time (from 7 to 180 min). In non-fermented samples, butyrate was not detected.
Besides the well-known Kombucha, many other fermented beverages are traditionally produced and consumed worldwide, mainly alcoholic beverages. Among these, beer holds a prominent place. It has been demonstrated that during fermentation, SCFA concentrations significantly increase, from 1.2–2.2 mg/L to 2.3–8.1 mg/L. It seems that the initial steps during the brewing process are also able to influence the amount of SCFAs in the final product. In particular, when infusion mashing is used, the total amount of SCFAs, mainly, butyrate, is higher when compared with decoction [35].
Due to their high content in sugar and non-digestible carbohydrates, fruits may be considered one of the best raw material for fermented products [43]. It has been reported that the fermentation of raw guava fruits with L. plantarum significantly increase the content of butyrate compared to the unfermented control (unfermented: 1.30 ± 0.39 ng/100 mL; fermented: 17.85 ± 0.68 ng/100 mL) [36]. This would suggest that fruit carbohydrate may represent a useful substrate for bacterial fermentation, leading to the increased production of bioactive end-products, including SCFAs. Similar to fruits, vegetables can also be considered a good raw material for fermented products. Recently, a study reported a significant increase in SCFAs in L. rhamnosus GG-fermented carrot juice, compared with non-fermented ones (unfermented carrot juice: acetate 0.16 ± 0.02 mg/mL, propionate 0.51 ± 0.07 mg/mL, butyrate 0.64 ± 0.11 mg/mL; fermented carrot juice: acetate 0.42 ± 0.05 mg/mL, propionate 0.72 ± 0.09 mg/mL, butyrate 0.995 ± 0.09 mg/mL;p<0.05). Additionallyin this case, the fermentation of dietary fiber was considered as the main mechanism for the increased production of SCFAs [2].
4. Conclusions and Future Perspectives
In this manuscript, we reported evidence from various studies monitoring the levels of SCFAs in various fermented foods and beverages, reaching the conclusion that fermentation is a useful strategy to increase the levels of SCFAs in fermented foods and beverages. More specifically, microorganisms produce SCFAs as end-products via the metabolism of food components, such as fiber and other carbohydrates. In this sense, fermentation should be taken into account by the food industry as a possible process with neither additional impact on the environment, nor further food processing for the production of functional foods and beverages (Figure 2).
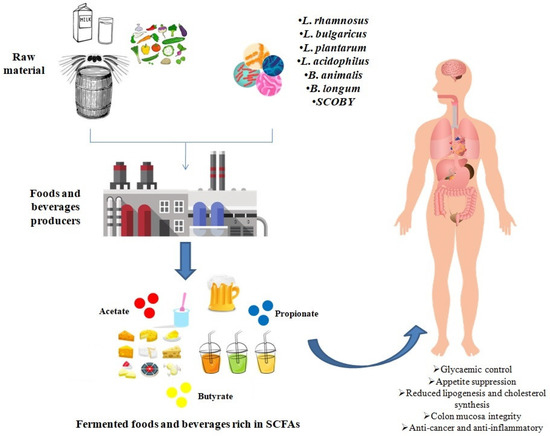
Figure 2.
Fermentation with different microorganisms might be used by food industry to produce functional foods and beverages enriched in short-chain fatty acids that can exert several beneficial effects on human health.
The available studies herein reported, however, led to individuate various concerns. Firstly, different microorganisms and prebiotics were used in these studies. This makes establishing the best criteria for the fermentation process in order to optimize SCFAs production difficult. Then, although promising, these studies are limited in number and, to our knowledge, based exclusively on in vitro evidence. No studies were conducted in humans, chronically administered with fermented products in order to test the effects of exogenous SCFAs. These are weak points hindering the use of fermentation by the food industry for the production of functional foods. In this sense, further studies are needed to individuate the best combination of raw materials, microorganisms, prebiotics, and fermentation times. In parallel, clinical trials, aimed to confirm the health-promoting effects of SCFAs, administered with fermented foods and beverages, should be performed.
Author Contributions
Conceptualization, G.A., A.A. and R.C.; Writing—original draft preparation, G.A., A.A. and R.C.; Validation, G.C.T. and E.N.; Supervision, G.C.T. and E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Roos, J.; De Vuyst, L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Marazza, J.A.; LeBlanc, J.G.; de Giori, G.S.; Garro, M.S. Soymilk fermented with Lactobacillus rhamnosus CRL981 ameliorates hyperglycemia, lipid profiles and increases antioxidant enzyme activities in diabetic mice. J. Funct. Foods 2013, 5, 1848–1853. [Google Scholar] [CrossRef]
- Li, C.; Nie, S.P.; Zhu, K.X.; Xiong, T.; Xie, M.Y. Lactobacillus plantarum NCU116 fermented carrot juice evokes changes of metabolites in serum from type 2 diabetic rats. Food Res. Int. 2016, 80, 36–40. [Google Scholar] [CrossRef]
- Rosa Pérez, A.; Knauf, C.; Morrison, D.; Sprenger, N.; Luiz Frozza, R.; Silva, Y.P.; Bernardi, A. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef]
- Cox, H.M.; Tough, I.R.; Woolston, A.M.; Zhang, L.; Nguyen, A.D.; Sainsbury, A.; Herzog, H. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010, 11, 532–542. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Rieckhoff, D.; Erbersdobler, H.F. Dietary Inulin Lowers Plasma Cholesterol and Triacylglycerol and Alters Biliary Bile Acid Profile in Hamsters. J. Nutr. 1998, 128, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 48, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE 2014, 9, e87327. [Google Scholar] [CrossRef]
- Belfiore, A.; Genua, M.; Malaguarnera, R. PPAR-γ agonists and their effects on igf-i receptor signaling: Implications for cancer. PPAR Res. 2009, 2009, 830501. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-chain fatty acids: Ready for prime time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-Chain fatty acids suppress lipopolysaccharide-Induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-?B Pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef]
- Säemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A.D.W. An introduction to the traditional fermented foods and beverages of Turkey. Crit. Rev. Food Sci. Nutr. 2011, 51, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Behera, S.K.; Witness Qaku, X.; Sekar, S.; Ndinteh, D.T.; Nanjundaswamy, H.M.; Ray, R.C.; Kayitesi, E. Quality enhancement of prickly pears (Opuntia sp.) juice through probiotic fermentation using Lactobacillus fermentum-ATCC 9338. LWT 2017, 75, 453–459. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexican “Ataulfo” mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef]
- Liu, A.B.; Tao, S.; Lee, M.J.; Hu, Q.; Meng, X.; Lin, Y.; Yang, C.S. Effects of gut microbiota and time of treatment on tissue levels of green tea polyphenols in mice. BioFactors 2018, 44, 348–360. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Singh, R.; Westfall, S.; Herman, F.; Faith, J.; Ho, L. The Role of the Gut Microbiota in the Metabolism of Polyphenols as Characterized by Gnotobiotic Mice. J. Alzheimer’s Dis. 2018, 63, 409–421. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Daliu, P.; Narciso, V.; Tenore, G.C.; Novellino, E. Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 2018, 10, 1711. [Google Scholar] [CrossRef] [PubMed]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-chain fatty acids produced by synbiotic mixtures in skim milk differentially regulate proliferation and cytokine production in peripheral blood mononuclear cells. Int. J. Food Sci. Nutr. 2015, 66, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Chen, H.; Chen, H.; Ding, W. Effects of fermentation with Lactobacillus rhamnosus GG on product quality and fatty acids of goat milk yogurt. J. Dairy Sci. 2016, 99, 221–227. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Zhu, Y.; Yang, J.; Sun, L.; Chai, X.; Wang, Y. Characteristic chromatographic fingerprint study of short-chain fatty acids in human milk, infant formula, pure milk and fermented milk by gas chromatography–mass spectrometry. Int. J. Food Sci. Nutr. 2016, 67, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The chemical profiling of fatty acids during the brewing process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Suryanarayana, L.C.; Chandrashekara, K.A.; Krishnan, P.; Kush, A.; Ravikumar, P. Lactobacillus plantarum mediated fermentation of Psidium guajava L. fruit extract. J. Biosci. Bioeng. 2015, 119, 430–432. [Google Scholar] [CrossRef]
- Annunziata, G.; Tenore, G.C.; Ciampaglia, R.; Schisano, C.; Narciso, V.; Maisto, M.; Novellino, E. Short-time lactic-acid fermentation improves the nutraceutical value of black tea beverage. In Proceedings of the CHIMALI 2018, Italian Food Chemistry Congress, Camerino, Italy, 24–27 September 2018. [Google Scholar]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.F.; Ștefan, L.M.; Mănoiu, S.; Vrăjmașu, V.V.; Moraru, I.; Oancea, A.; Israel-Roming, F.; et al. Bee collected pollen with enhanced health benefits, produced by fermentation with a Kombucha Consortium. Nutrients 2018, 10, 1365. [Google Scholar] [CrossRef]
- Savaiano, D.A. Lactose digestion from yogurt: Mechanism and relevance. Am. J. Clin. Nutr. 2014, 99, 1251S–1255S. [Google Scholar] [CrossRef]
- Barros, R.F.; Cutrim, C.S.; da Costa, M.P.; Conte Junior, C.A.; Cortez, M.A.S. Lactose hydrolysis and organic acids production in yogurt prepared with different onset temperatures of enzymatic action and fermentation. Ciência Anim. Bras. 2019, 20. [Google Scholar] [CrossRef]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef]
- Fernando, W.M.A.D.B.; Flint, S.H.; Ranaweera, K.K.D.S.; Bamunuarachchi, A.; Johnson, S.K.; Brennan, C.S. The potential synergistic behaviour of inter- and intra-genus probiotic combinations in the pattern and rate of short chain fatty acids formation during fibre fermentation. Int. J. Food Sci. Nutr. 2018, 69, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).