Taurine Stimulates Thermoregulatory Genes in Brown Fat Tissue and Muscle without an Influence on Inguinal White Fat Tissue in a High-Fat Diet-Induced Obese Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Food Uptake, Activity, and Metabolic Parameters
2.3. Body Weight and Composition
2.4. Harvest Tissues from Mice
2.5. Quantitative Real-Time RT-PCR
2.6. Statistical Analysis
3. Results
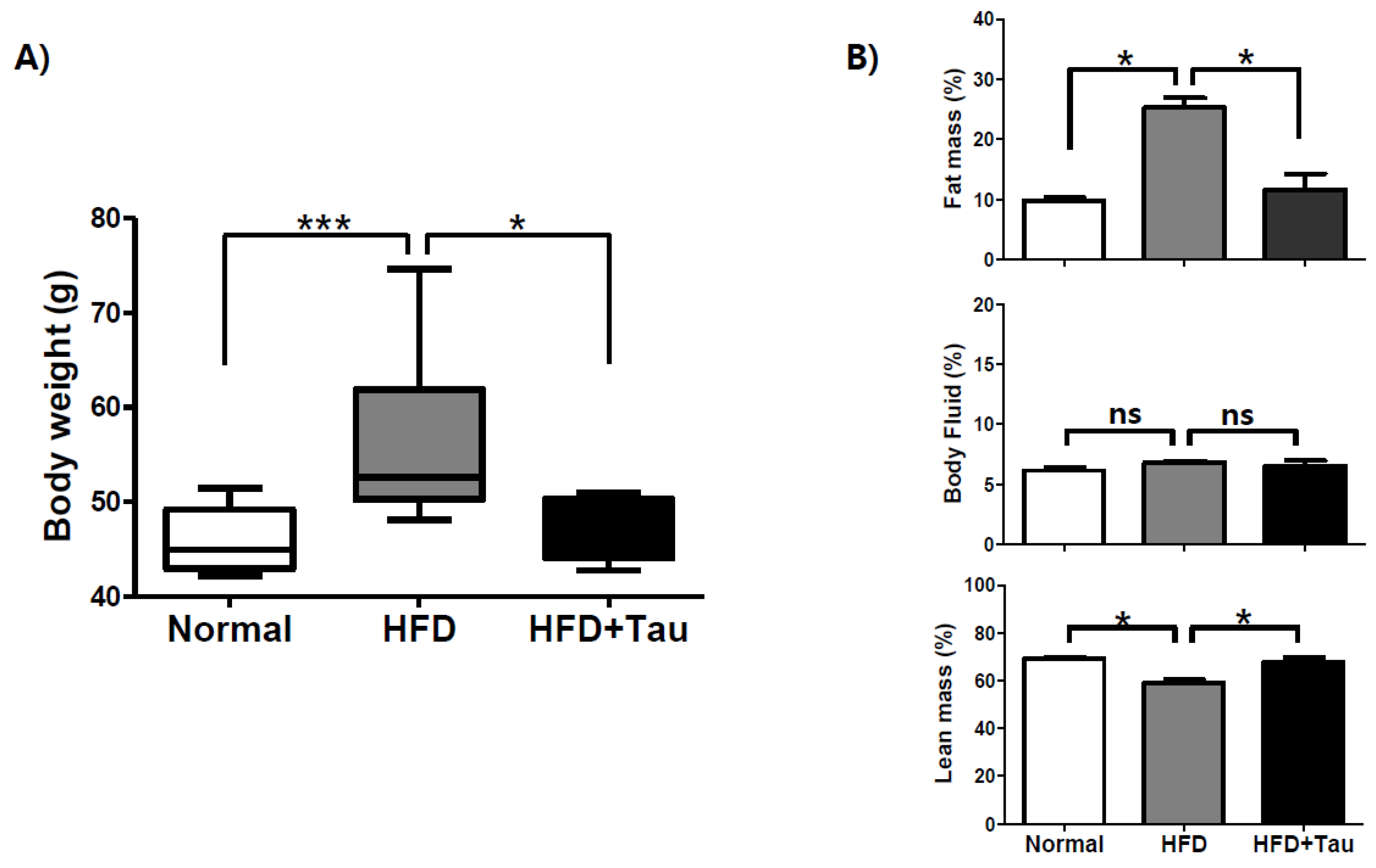
3.1. Effect of Taurine on Anti-Obesity in HFD-Induced Mildly Obese ICR Mice
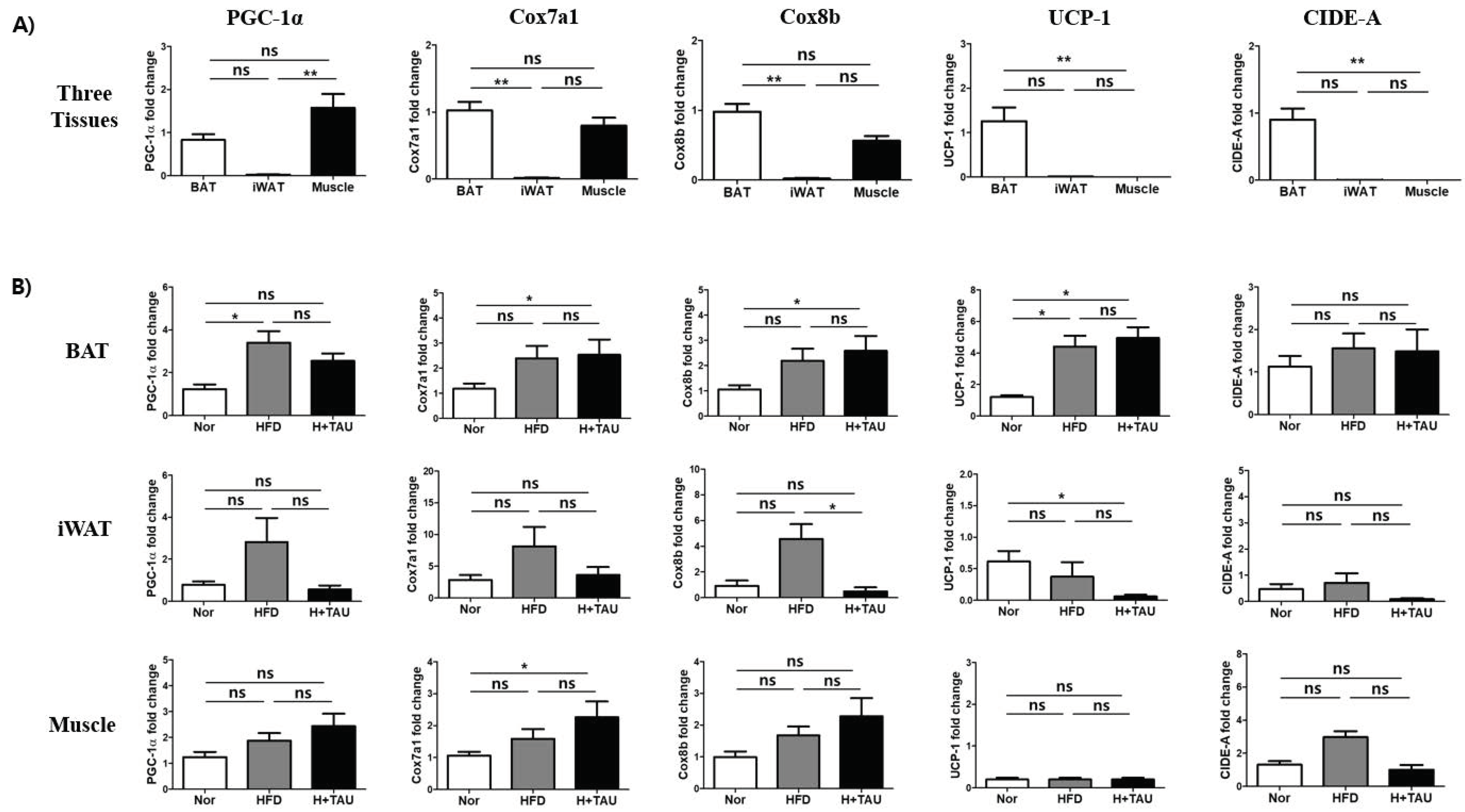
3.2. Effect of Taurine on the Transcriptional Expression of Thermogenesis-Related Genes in Fat Tissue and Muscles of HFD-Induced Mildly Obese ICR Mice
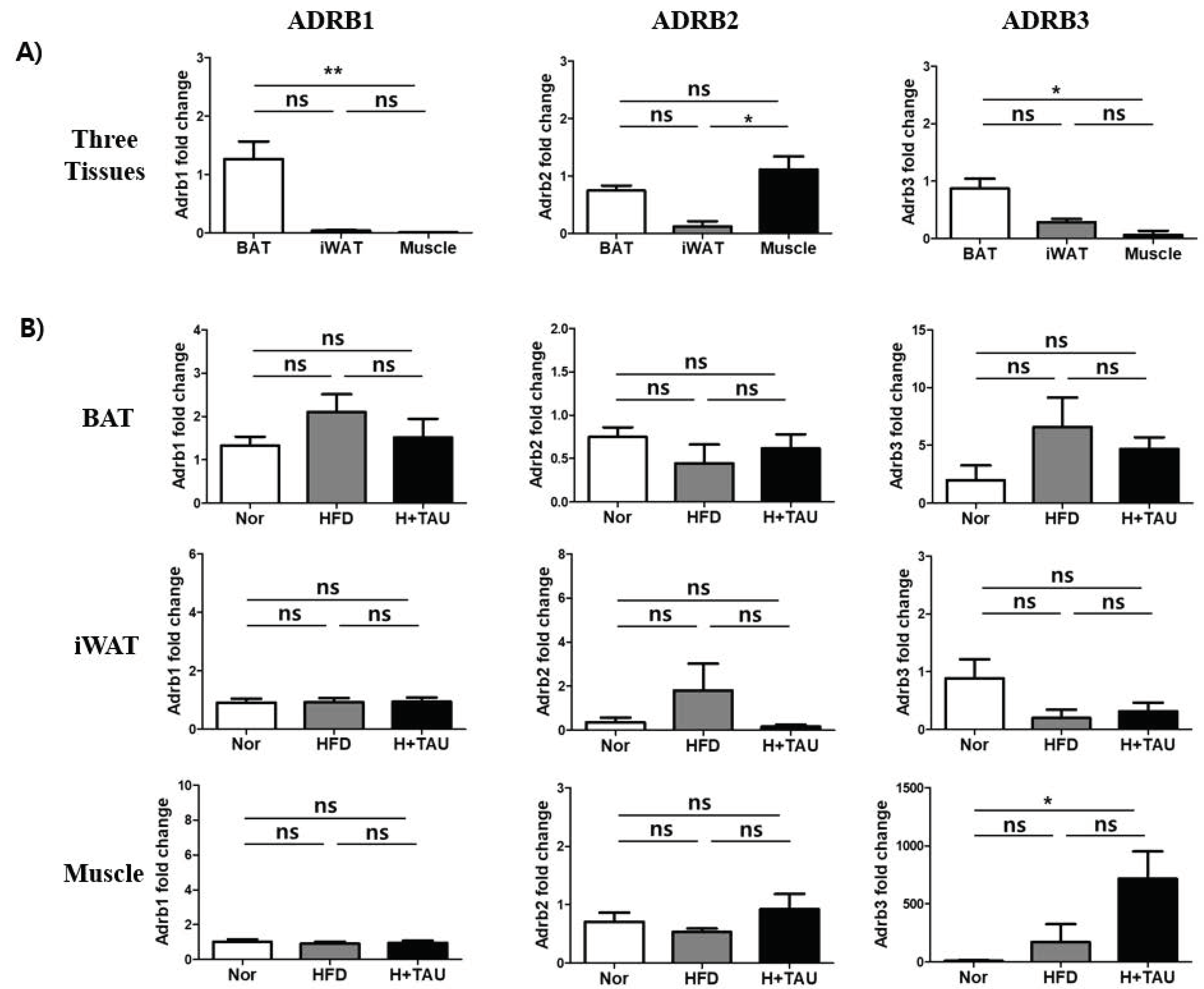
3.3. Effect of Taurine on the Transcriptional Expression of β1, 2, 3-Adrenergic Receptors in Fat Tissue and Muscles of HFD-Induced Mildly Obese ICR Mice
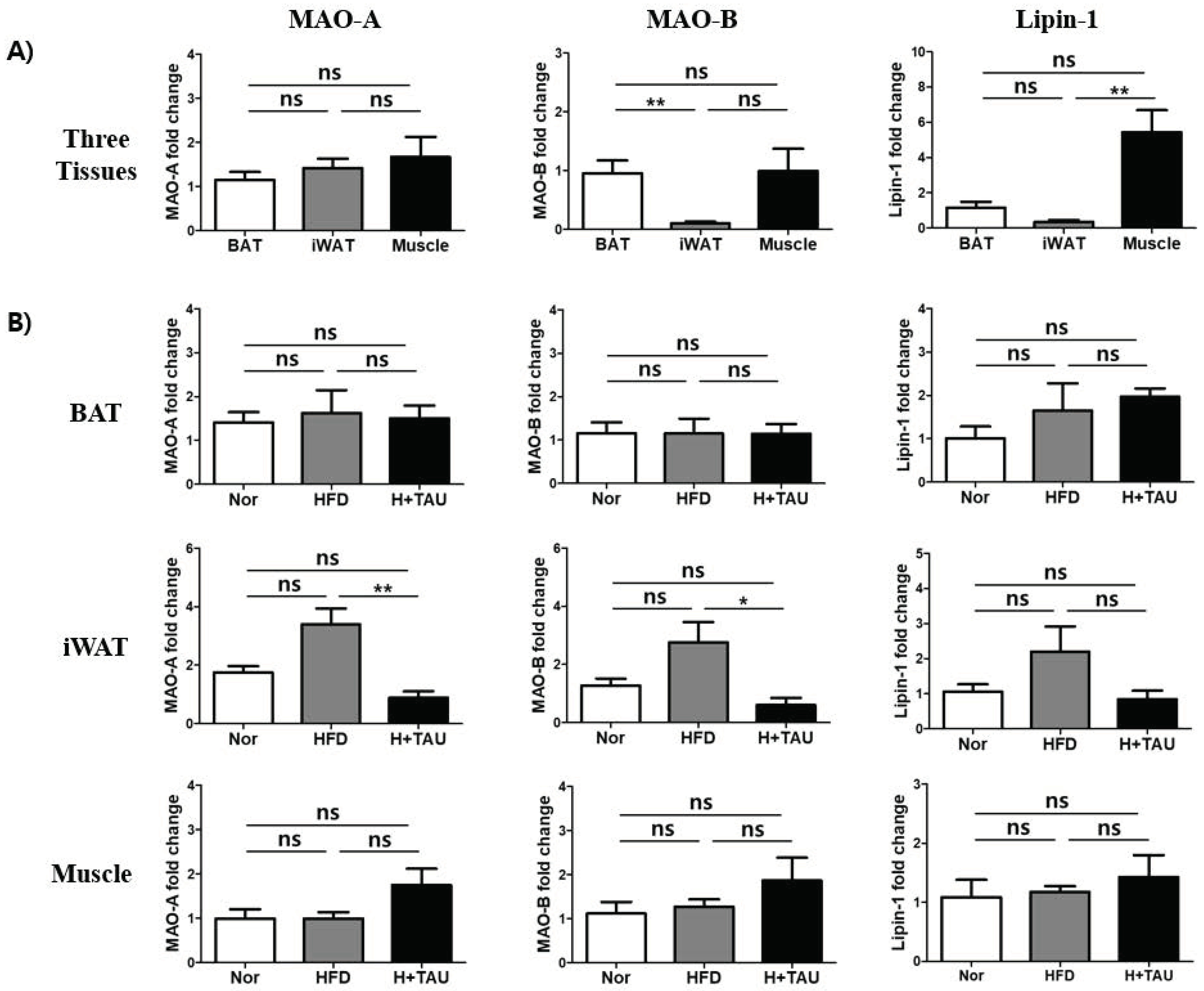
3.4. Effect of Taurine on Transcriptional Expression of Monoamine Oxidases (MAOs) and Lipin-1 in Fat Tissue and Muscles of HFD-Induced Mildly Obese ICR Mice
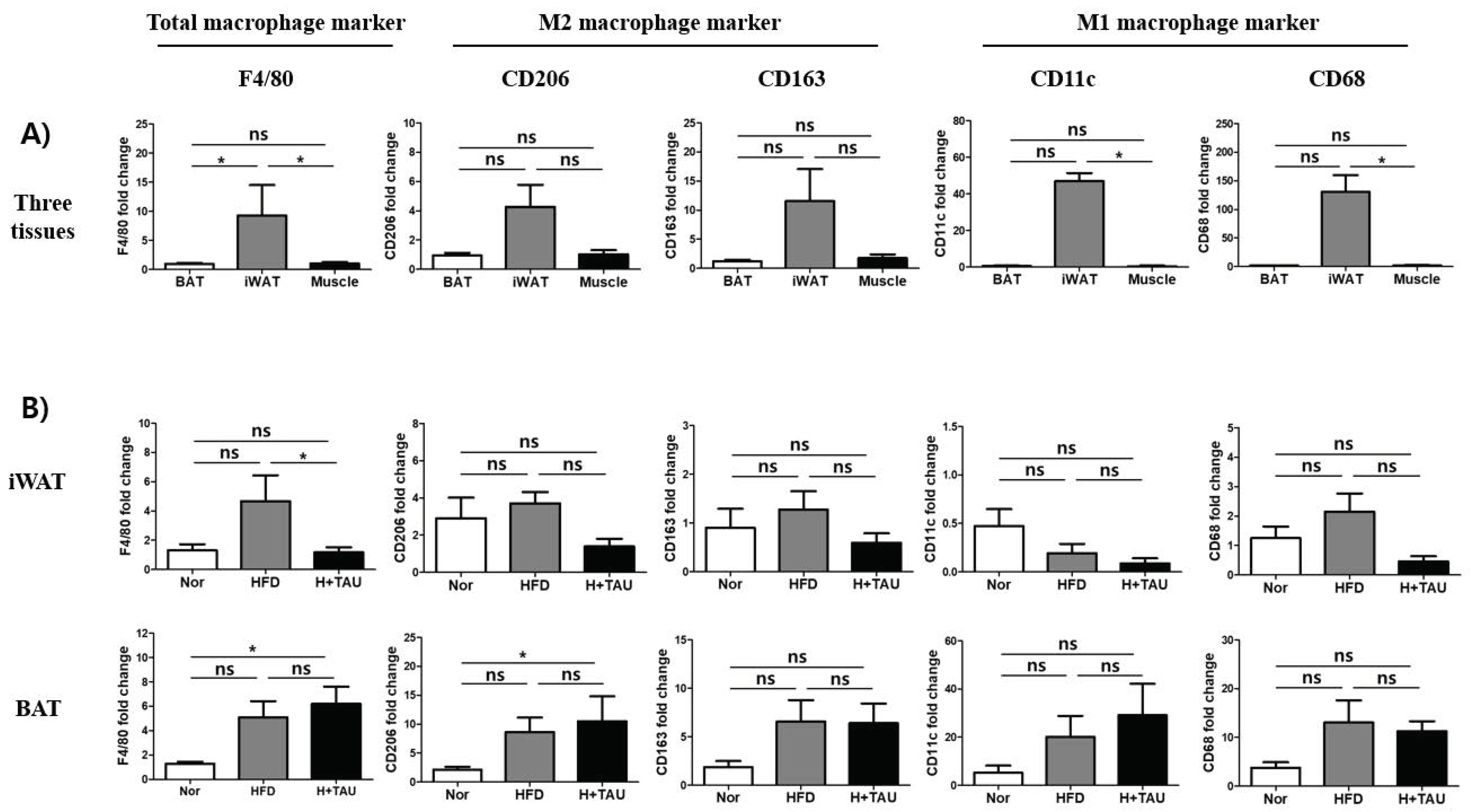
3.5. Effect of Taurine on Infiltration of Macrophages into Fat Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Jong, C.J.; Kc, R.; Azuma, J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010, 17, S2. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Role of the liver in regulation of body cysteine and taurine levels: A brief review. Neurochem. Res. 2004, 29, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W. Taurine: Its biological role and clinical implications. Adv. Pediatr. 1985, 32, 1–42. [Google Scholar]
- Ueki, I.; Stipanuk, M.H. 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J. Nutr. 2008, 139, 207–214. [Google Scholar] [CrossRef]
- Ide, T.; Kushiro, M.; Takahashi, Y.; Shinohara, K.; Cha, S. mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism 2002, 51, 1191–1197. [Google Scholar] [CrossRef]
- Tsuboyama-Kasaoka, N.; Shozawa, C.; Sano, K.; Kamei, Y.; Kasaoka, S.; Hosokawa, Y.; Ezaki, O. Taurine (2-Aminoethanesulfonic Acid) Deficiency Creates a Vicious Circle Promoting Obesity. Endocrinology 2006, 147, 3276–3284. [Google Scholar] [CrossRef]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine-from organism to organelle. Acta Physiol. 2014, 213, 191–212. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Zhang, M.; Bi, L.F.; Fang, J.H.; Su, X.L.; Da, G.L.; Kuwamori, T.; Kagamimori, S. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids 2003, 26, 267–271. [Google Scholar] [CrossRef]
- Rosa, F.T.; De Freitas, E.C.; Deminice, R.; Jordão, A.A.; Marchini, J.S. Oxidative stress and inflammation in obesity after taurine supplementation: A double-blind, placebo-controlled study. Eur. J. Nutr. 2013, 53, 823–830. [Google Scholar] [CrossRef]
- Lee, M.Y.; Cheong, S.H.; Chang, K.J.; Choi, M.J.; Kim, S.K. Effect of the obesity index on plasma taurine levels in Korean female adolescents. Adv. Exp. Med. Biol. 2003, 526, 285–290. [Google Scholar] [CrossRef]
- Lin, S.; Hirai, S.; Yamaguchi, Y.; Goto, T.; Takahashi, N.; Tani, F.; Mutoh, C.; Sakurai, T.; Murakami, S.; Yu, R.; et al. Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol. Nutr. Food Res. 2013, 57, 2155–2165. [Google Scholar] [CrossRef]
- Batista, T.; Ribeiro, R.A.; Da Silva, P.M.R.; Camargo, R.L.; Lollo, P.C.B.; Boschero, A.C.; Carneiro, E.M. Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol. Nutr. Food Res. 2012, 57, 423–434. [Google Scholar] [CrossRef]
- Cao, P.-J.; Jin, Y.-J.; Li, M.-E.; Zhou, R.; Yang, M.-Z. PGC-1α may associated with the anti-obesity effect of taurine on rats induced by arcuate nucleus lesion. Nutr. Neurosci. 2014, 19, 86–93. [Google Scholar] [CrossRef]
- Kim, K.S.; Oh, D.H.; Kim, J.Y.; Lee, B.G.; You, J.S.; Chang, K.J.; Chung, H.; Yoo, M.C.; Yang, H.-I.; Kang, J.-H.; et al. Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Exp. Mol. Med. 2012, 44, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Bennardini, F.; Mattana, A.; Miceli, M.; Ciuti, M.; Mian, M.; Gironi, A.; Anichini, R.; Seghieri, G. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: Effects of taurine supplementation. Am. J. Clin. Nutr. 1995, 61, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Schaffer, S.; Azuma, J. The effect of taurine on chronic heart failure: Actions of taurine against catecholamine and angiotensin II. Amino Acids 2013, 46, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Mahdavi, R.; Hajizadeh-Sharafabad, F.; Alizadeh, M. The effects of taurine supplementation on oxidative stress indices and inflammation biomarkers in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Diabetol. Metab. Syndr. 2020, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S. Role of taurine in the pathogenesis of obesity. Mol. Nutr. Food Res. 2015, 59, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.L.; Hummel, K.P. The influence of genetic background on the expression of the obese (ob) gene in the mouse. Diabetologia 1973, 9, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.-J.; Song, H.-K.; Kim, K.-S.; Jung, Y.-S.; Hwang, D.-Y.; Cho, J.Y. Comparative analysis of basal locomotor activity-related metabolic phenotypes between C57BL/6 mice and ICR mice substrains derived from three different sources. Lab. Anim. Res. 2017, 33, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Jang, M.J.; Fang, S.; Yoon, S.G.; Kim, I.Y.; Seong, J.K.; Yang, H.-I.; Hahm, D.-H. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids 2018, 51, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. Uncoupling protein—A useful energy dissipator. J. Bioenerg. Biomembr. 1999, 31, 419–430. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Srivastava, S.; Veech, R. Brown and Brite: The Fat Soldiers in the Anti-obesity Fight. Front. Physiol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Kim, S.H.; Plutzky, J. Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes Metab. J. 2016, 40, 12–21. [Google Scholar] [CrossRef]
- Sakamoto, T.; Takahashi, N.; Goto, T.; Kawada, T. Dietary factors evoke thermogenesis in adipose tissues. Obes. Res. Clin. Pr. 2014, 8, e533–e539. [Google Scholar] [CrossRef]
- Kang, H.W.; Gil Lee, S.; Otieno, D.; Ha, K. Flavonoids, Potential Bioactive Compounds, and Non-Shivering Thermogenesis. Nutrients 2018, 10, 1168. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Wu, J.; Su, T.; Chao, X.-J.; Liu, B.; Fu, X.; Chan, C.L.; Lau, R.H.Y.; Tse, A.K.W.; Han, Q.-B.; et al. Cinnamon induces browning in subcutaneous adipocytes. Sci. Rep. 2017, 7, 2447. [Google Scholar] [CrossRef]
- Cohen, P.; Spiegelman, B.M. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes 2015, 64, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Catecholamine-induced lipolysis in obesity. Int. J. Obes. 1999, 23, S10–S13. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Abello, V.; Iffiú-Soltész, Z.; Mercier, N.; Feve, B.; Valet, P. Limitation of adipose tissue enlargement in rats chronically treated with semicarbazide-sensitive amine oxidase and monoamine oxidase inhibitors. Pharmacol. Res. 2008, 57, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bour, S.; Daviaud, D.; Grès, S.; Lefort, C.; Prévot, D.; Zorzano, A.; Wabitsch, M.; Saulnier-Blache, J.S.; Valet, P.; Carpéné, C. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie 2007, 89, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rui, B.-B.; Tang, L.-Y.; Hu, C.-M. Lipin Family Proteins—Key Regulators in Lipid Metabolism. Ann. Nutr. Metab. 2014, 66, 10–18. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 11. [Google Scholar] [CrossRef]
- De Jong, J.M.A.; Wouters, R.T.F.; Boulet, N.; Cannon, B.; Nedergaard, J.; Petrovic, N. The beta3-adrenergic receptor is dispensable for browning of adipose tissues. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E508–E518. [Google Scholar] [CrossRef]
- Blaak, E.E.; Van Baak, M.; Kempen, K.P.; Saris, W.H. Role of alpha- and beta-adrenoceptors in sympathetically mediated thermogenesis. Am. J. Physiol. Metab. 1993, 264, E11–E17. [Google Scholar] [CrossRef]
- Nguyen, K.; Qiu, Y.; Cui, X.; Goh, Y.P.S.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef]
- Garcia, R.A.; Roemmich, J.N.; Claycombe, K.J. Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. Nutr. Metab. 2016, 13, 24. [Google Scholar] [CrossRef]
- Lin, S.-C.; Li, P. CIDE-A, a novel link between brown adipose tissue and obesity. Trends Mol. Med. 2004, 10, 434–439. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, E.; Reynés, B.; Diaz-Rua, R.; Ceresi, E.; Oliver, P.; Palou, A. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int. J. Obes. 2015, 39, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Mercader, J.; Palou, A. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie 2017, 134, 99–117. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Li, B.-Y.; Peng, W.-Q.; Guo, L.; Tang, Q.-Q. Taurine-mediated browning of white adipose tissue is involved in its anti-obesity effect in mice. J. Boil. Chem. 2019, 294, 15014–15024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bolze, F.; Fromme, T.; Klingenspor, M. Intrinsic differences in BRITE adipogenesis of primary adipocytes from two different mouse strains. Biochim. Biophys. Acta (BBA) Mol. Cell Boil. Lipids 2014, 1841, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, M.; Mensink, M.; Schrauwen, P. Human Uncoupling Protein-3 and Obesity: An Update. Obes. Res. 2003, 11, 1429–1443. [Google Scholar] [CrossRef]
- Maurer, S.F.; Fromme, T.; Grossman, L.I.; Hüttemann, M.; Klingenspor, M. The brown and brite adipocyte marker Cox7a1 is not required for non-shivering thermogenesis in mice. Sci. Rep. 2015, 5, 17704. [Google Scholar] [CrossRef]
- Schiffelers, S.L.H.; Van Harmelen, V.J.; A De Grauw, H.; Saris, W.H.; A Van Baak, M. Dobutamine as selective beta(1)-adrenoceptor agonist in in vivo studies on human thermogenesis and lipid utilization. J. Appl. Physiol. 1999, 87, 977–981. [Google Scholar] [CrossRef]
- Bachman, E.S.; Dhillon, H.; Zhang, C.-Y.; Cinti, S.; Bianco, A.; Kobilka, B.K.; Lowell, B.B. beta AR Signaling Required for Diet-Induced Thermogenesis and Obesity Resistance. Science 2002, 297, 843–845. [Google Scholar] [CrossRef]
- Da Silva, A.A.; Carmo, J.D.; Dubinion, J.; Hall, J. The role of the sympathetic nervous system in obesity-related hypertension. Curr. Hypertens. Rep. 2009, 11, 206–211. [Google Scholar] [CrossRef]
- Collins, S.; Daniel, K.W.; Rohlfs, E.M.; Ramkumar, V.; Taylor, I.L.; Gettys, T.W. Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol. Endocrinol. 1994, 8, 518–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rohlfs, E.M.; Daniel, K.W.; Premont, R.T.; Kozak, L.P.; Collins, S. Regulation of the uncoupling protein gene (Ucp) by beta 1, beta 2, and beta 3-adrenergic receptor subtypes in immortalized brown adipose cell lines. J. Boil. Chem. 1995, 270, 10723–10732. [Google Scholar] [CrossRef] [PubMed]
- Preite, N.Z.; Nascimento, B.P.P.D.; Muller, C.R.; Américo, A.L.V.; Higa, T.S.; Evangelista, F.S.; Lancellotti, C.L.; Henriques, F.; Batista, M.L.; Bianco, A.; et al. Disruption of beta3 adrenergic receptor increases susceptibility to DIO in mouse. J. Endocrinol. 2016, 231, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Chikazawa, M.; Sato, R. Identification of Functional Food Factors as beta2-Adrenergic Receptor Agonists and Their Potential Roles in Skeletal Muscle. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 68–74. [Google Scholar] [CrossRef]
- Muramatsu, M.; Kakita, K.; Nakagawa, K.; Kuriyama, K. A modulating role of taurine on release of acetylcholine and norepinephrine from neuronal tissues. Jpn. J. Pharmacol. 1978, 28, 259–268. [Google Scholar] [CrossRef][Green Version]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. 6), 7–11. [Google Scholar]
- Nutt, D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 2008, 69, 4–7. [Google Scholar]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Wu, G.-F.; Ren, S.; Tang, R.-Y.; Xu, C.; Zhou, J.-Q.; Lin, S.-M.; Feng, Y.; Yang, Q.-H.; Hu, J.; Yang, J.-C. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci. Rep. 2017, 7, 4989. [Google Scholar] [CrossRef]
- Catrysse, L.; Van Loo, G. Adipose tissue macrophages and their polarization in health and obesity. Cell. Immunol. 2018, 330, 114–119. [Google Scholar] [CrossRef]
- Sakamoto, T.; Nitta, T.; Maruno, K.; Yeh, Y.-S.; Kuwata, H.; Tomita, K.; Goto, T.; Takahashi, N.; Kawada, T. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol. Metab. 2016, 310, E676–E687. [Google Scholar] [CrossRef]
- Campbell, B.I.; Zito, G.; Colquhoun, R.; Martinez, N.; Kendall, K.; Buchanan, L.; Lehn, M.; Johnson, M.; Louis, C.S.; Smith, Y.; et al. The effects of a single-dose thermogenic supplement on resting metabolic rate and hemodynamic variables in healthy females-a randomized, double-blind, placebo-controlled, cross-over trial. J. Int. Soc. Sports Nutr. 2016, 13, 13. [Google Scholar] [CrossRef]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary Factors Promoting Brown and Beige Fat Development and Thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef]
| PGC-1α | Forward | AGAAGCGGGAGTCTGAAAGG |
| Backward | TTCTGTCCGCGTTGTGTCAG | |
| Cox7a1 | Forward | CGACAATGACCTCCCAGTACA |
| Backward | AGCCCAAGCAGTATAAGCAGTAG | |
| Cox8b | Forward | AAAGCCCATGTCTCTGCCAA |
| Backward | TGGAACCATGAAGCCAACGA | |
| UCP-1 | Forward | AGTACCCAAGCGTACCAAGC |
| Backward | ACCCGAGTCGCAGAAAAGAA | |
| CIDE-A | Forward | AGACCGCCAGGGACTACG |
| Backward | GAAACTCGAAAAGGGCGAGC | |
| ADRB1 | Forward | ATGGGTGTGTTCACGCTCTG |
| Backward | AGAAGACGAAGAGGCGATCC | |
| ADRB2 | Forward | AATAGCAACGGCAGAACGGA |
| Backward | TCAACGCTAAGGCTAGGCAC | |
| ADRB3 | Forward | AAACTGGTTGCGAACTGTGG |
| Backward | TAACGCAAAGGGTTGGTGAC | |
| MAO-A | Forward | CGGAAAGCTGAACGACTTGC |
| Backward | ACTGCTCCTCACACCAGTTC | |
| MAO-B | Forward | CCCTTGCTGAAGAGTGGGAC |
| Backward | TCACAAAGAGCGTGGCAATC | |
| Lipin-1 | Forward | ACTGGGAAAGGCCACAATAC |
| Backward | GTGCTCTTCATCACTGGAGG | |
| F4/80 | Forward | AAGACTGACAACCAGACGGC |
| Backward | AAGAGCATCACTGCCTCCAC | |
| CD206 | Forward | AGCCTGGAAAGAGCTGTGTG |
| Backward | CATCGCTTGCTGAGGGAATG | |
| CD163 | Forward | ATGCTTCCATCCAGTGCCTC |
| Backward | CTGTCGTCGCTTCAGAGTCC | |
| CD11c | Forward | AGCCTTTCTTCTGCTGTTGG |
| Backward | AAATGTGTCGGCTTCTCTGC | |
| CD68 | Forward | AAAGGCCGTTACTCTCCTGC |
| Backward | ACTCGGGCTCTGATATAGGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.S.; Doss, H.M.; Kim, H.-J.; Yang, H.-I. Taurine Stimulates Thermoregulatory Genes in Brown Fat Tissue and Muscle without an Influence on Inguinal White Fat Tissue in a High-Fat Diet-Induced Obese Mouse Model. Foods 2020, 9, 688. https://doi.org/10.3390/foods9060688
Kim KS, Doss HM, Kim H-J, Yang H-I. Taurine Stimulates Thermoregulatory Genes in Brown Fat Tissue and Muscle without an Influence on Inguinal White Fat Tissue in a High-Fat Diet-Induced Obese Mouse Model. Foods. 2020; 9(6):688. https://doi.org/10.3390/foods9060688
Chicago/Turabian StyleKim, Kyoung Soo, Hari Madhuri Doss, Hee-Jin Kim, and Hyung-In Yang. 2020. "Taurine Stimulates Thermoregulatory Genes in Brown Fat Tissue and Muscle without an Influence on Inguinal White Fat Tissue in a High-Fat Diet-Induced Obese Mouse Model" Foods 9, no. 6: 688. https://doi.org/10.3390/foods9060688
APA StyleKim, K. S., Doss, H. M., Kim, H.-J., & Yang, H.-I. (2020). Taurine Stimulates Thermoregulatory Genes in Brown Fat Tissue and Muscle without an Influence on Inguinal White Fat Tissue in a High-Fat Diet-Induced Obese Mouse Model. Foods, 9(6), 688. https://doi.org/10.3390/foods9060688




