Improving the Functional Activities of Curcumin Using Milk Proteins as Nanocarriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures and Methods of Analysis
2.2.1. Preparation and Characterization of Milk Protein–Chitosan Nanocomposite
2.2.2. Preparation of Curcumin-Loaded Milk Proteins
2.3. Characterizations of Nanoparticles
2.3.1. Particle Size and Zeta Potential Measurements
2.3.2. Physicochemical Characterization
2.4. Determination of the Entrapment Efficiency (EE %) and in vitro Release %
2.5. Antioxidant Activity
2.6. Anticancer Activity
2.7. Antimicrobial Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterizations of Nanoparticles
3.1.1. Particle Size
3.1.2. Zeta Potential
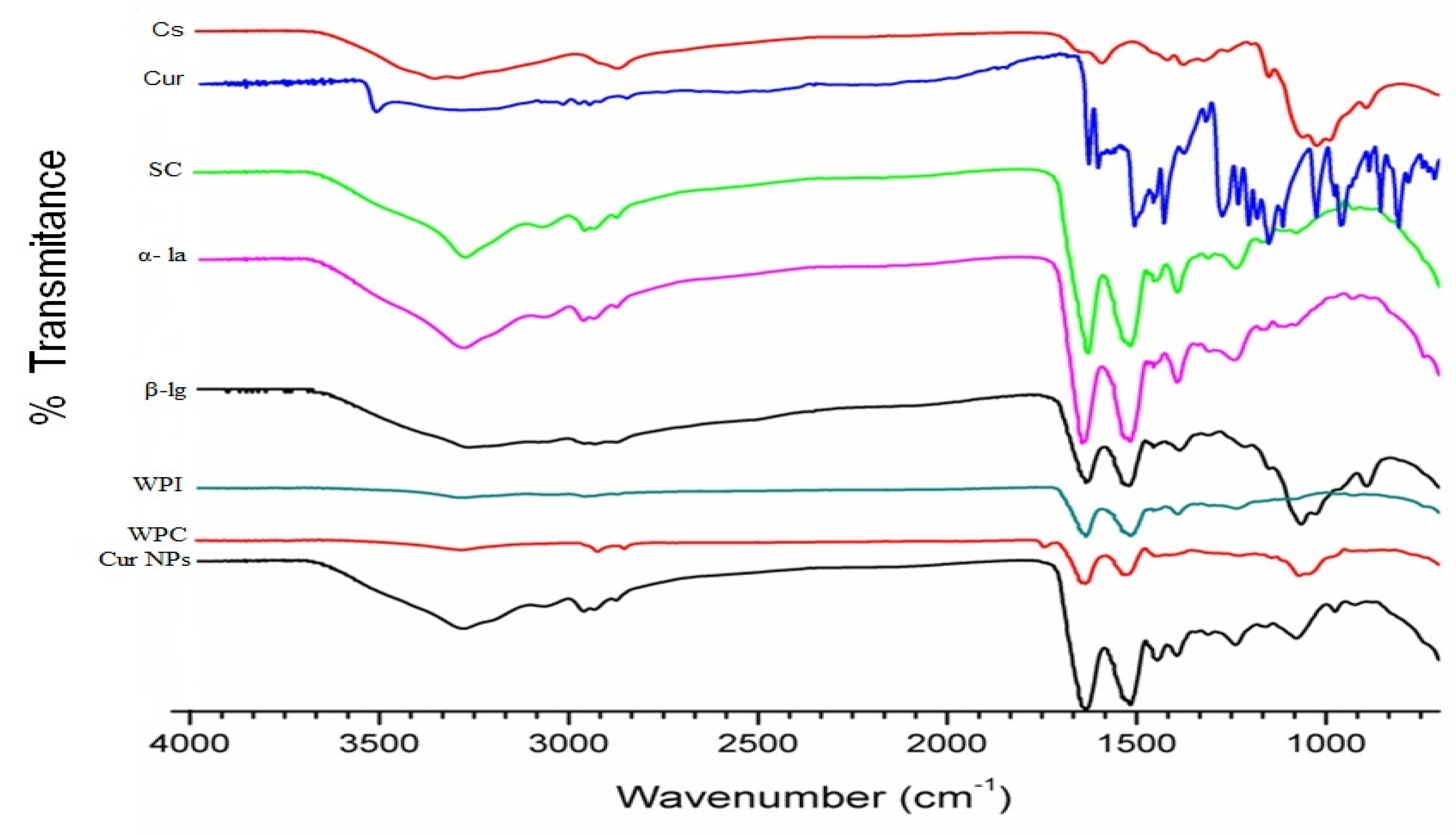
3.1.3. Fourier-Transform Infrared Spectroscopy (FTIR)
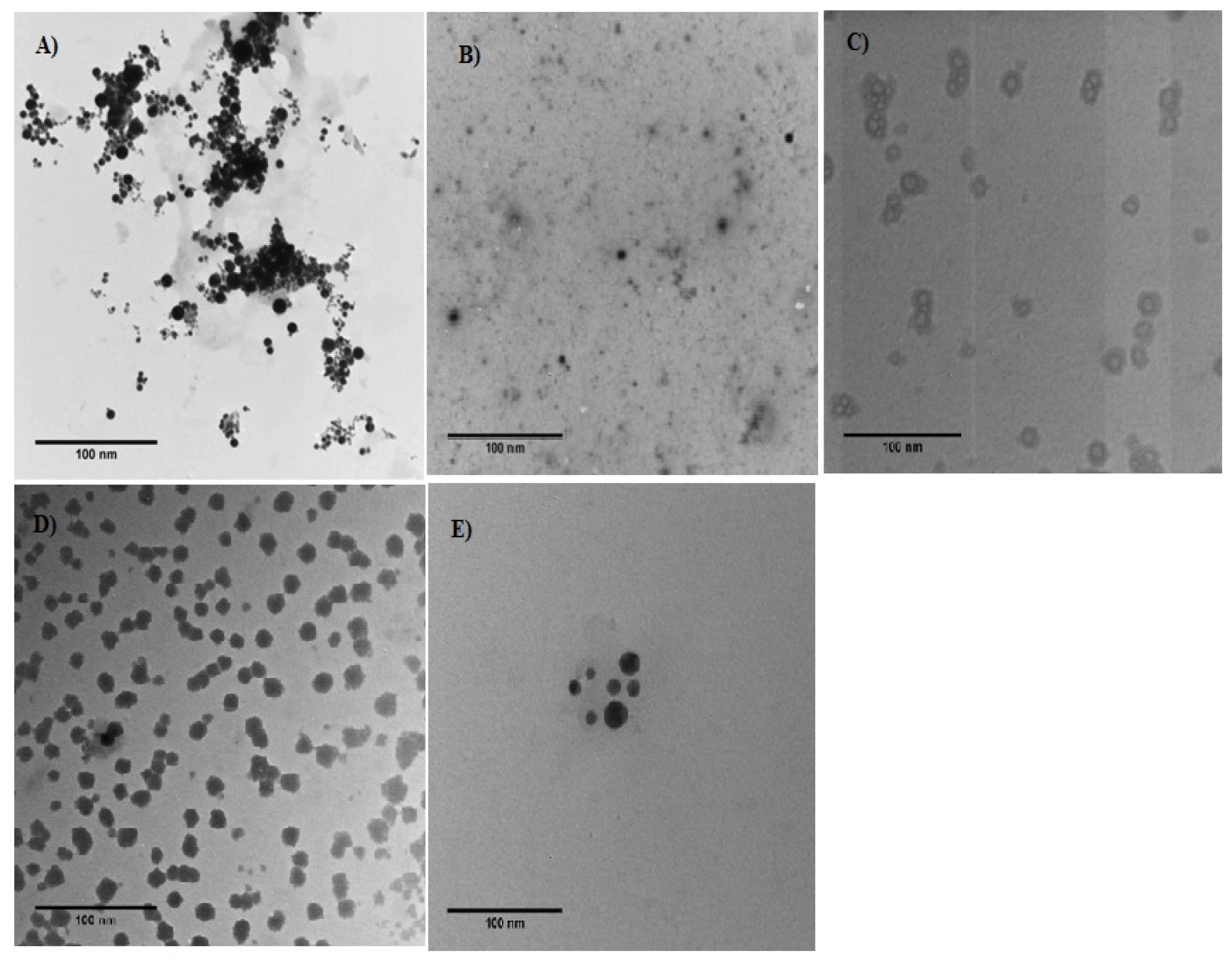
3.1.4. Transmission Electron Microscopy (TEM)
3.2. Entrapment (Encapsulation) Efficiency (EE %)
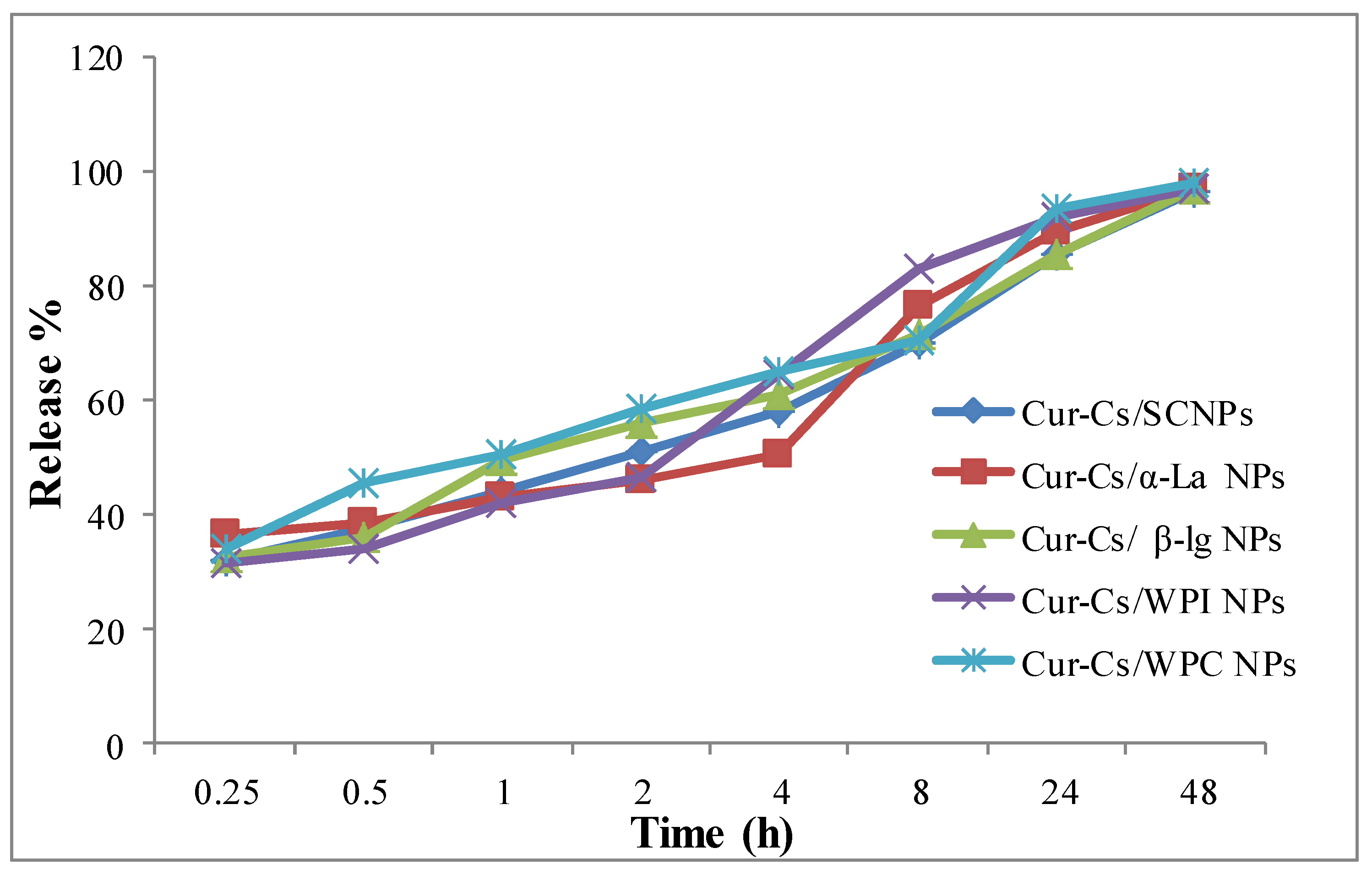
3.3. The In Vitro Behavior Release of Curcumin (%)
3.4. Antioxidant Activity (%)
3.5. Anticancer Activity
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.; De Freitas Santos, P.D.; Do Prado Silva, J.T.; Leimann, F.V.; Bracht, L.; Gonçalves, O.H. Impact of curcumin nanoformulation on its antimicrobial activity. Trends Food Sci. Technol. 2018, 72, 74–82. [Google Scholar] [CrossRef]
- Kumar, D.D.; Mann, B.; Pothuraju, R.; Sharma, R.; Bajaj, R. Formulation and characterization of nanoencapsulated curcumin using sodium caseinate and its incorporation in ice cream. Food Funct. 2016, 7, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential applications of curcumin and curcumin nanoparticles: From traditional therapeutics to modern nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Paralikar, P.; Nagaonkar, D.; Rehman, F.; Dos Santos, C.A. Pharmaceutical applications of curcumin-loaded nanoparticles. In Nanotechnology Applied to Pharmaceutical Technology; Springer: Cham, Siwzerland, 2017; pp. 139–154. [Google Scholar]
- Liu, W.; Chen, X.D.; Cheng, Z.; Selomulya, C. On enhancing the solubility of curcumin by microencapsulation in whey protein isolate via spray drying. J. Food Eng. 2016, 169, 189–195. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Zahednasab, H.; Hafez, A.A. Nanocarriers usage for drug delivery in cancer therapy. Iran. J. Cancer Prev. 2016, 9, e3966. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Li, M.; Cui, J.; Ngadi, M.O.; Ma, Y. Absorption mechanism of whey-protein-delivered curcumin using caco-2 cell monolayers. Food Chem. 2015, 180, 48–54. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z. Fabrication of curcumin-loaded whey protein microgels: Structural properties, antioxidant activity, and in vitro release behavior. LWT 2019, 103, 94–100. [Google Scholar] [CrossRef]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.-M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. Lwt-Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (bsa)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Sangeetha, K.S.S.; Umamaheswari, S.; Reddy, C.U.M.; Kalkura, S.N. Chrysin loaded chitosan nanoparticle: Formulation and in-vitro characterization. Int. J. Pharm. Sci. Res. 2017, 8, 1102–1109. [Google Scholar]
- Chopra, M.; Jain, R.; Dewangan, A.K.; Varkey, S.; Mazumder, S. Design of curcumin loaded polymeric nanoparticles-optimization, formulation and characterization. J. Nanosci. Nanotechnol. 2016, 16, 9432–9442. [Google Scholar] [CrossRef]
- Swed, A.; Cordonnier, T.; Fleury, F.; Boury, F. Protein encapsulation into plga nanoparticles by a novel phase separation method using non-toxic solvents. J. Nanomed. Nanotechnol. 2014, 5, 241. [Google Scholar]
- Sadeghi, R.; Moosavi-Movahedi, A.A.; Emam-Jomeh, Z.; Kalbasi, A.; Razavi, S.H.; Karimi, M.; Kokini, J. The effect of different desolvating agents on bsa nanoparticle properties and encapsulation of curcumin. J. Nanopart Res. 2014, 16, 2565. [Google Scholar] [CrossRef]
- Teng, Z.; Li, Y.; Wang, Q. Insight into curcumin-loaded β-lactoglobulin nanoparticles: Incorporation, particle disintegration, and releasing profiles. J. Agric. Food Chem. 2014, 62, 8837–8847. [Google Scholar] [CrossRef]
- Asouri, M.; Ataee, R.; Ahmadi, A.A.; Amini, A.; Moshaei, M.R. Antioxidant and free radical scavenging activities of curcumin. Asian J. Chem. 2013, 25, 7593–7595. [Google Scholar] [CrossRef]
- Vidhyalakshmi, R.; Vallinachiyar, C. Apoptosis of human breast cancer cells (mcf-7) induced by polysacccharides produced by bacteria. J. Cancer Sci. Ther. 2013, 5, 031–034. [Google Scholar]
- Cappuccino, J.G.; Sherman, N. Microbiology. A Laboratory Manual; Pearson Education, Inc.: New Delhi, India, 2004; pp. 282–283. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Ames, Iowa, Iowa State College Press: Iowa City, IA, USA, 1976. [Google Scholar]
- Athira, G.K.; Jyothi, A.N. Preparation and characterization of curcumin loaded cassava starch nanoparticles with improved cellular absorption. Int. J. Pharm. Pharm. Sci. 2014, 6, 171–176. [Google Scholar]
- Singh, H.D.; Wang, G.; Uludağ, H.; Unsworth, L.D. Poly-l-lysine-coated albumin nanoparticles: Stability, mechanism for increasing in vitro enzymatic resilience, and sirna release characteristics. Acta Biomater. 2010, 6, 4277–4284. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; Rodiles-López, J.; Cornejo-Mazon, M.; Gutierrez-Lopez, G.F.; Hernández-Arana, A.; Toledo-Núñez, C.; Barbosa-Cánovas, G.; Flores-Flores, J.; Hernandez-Sanchez, H. Effect of different treatments on the ability of α-lactalbumin to form nanoparticles. J. Dairy Sci. 2012, 95, 6204–6214. [Google Scholar] [CrossRef]
- Awad, R.; Hassan, Z.; Farrag, A.; El-Sayed, M.; Soliman, T. The use of whey portien isolate in microencapsulation of curcumin. Int. J. Food Nutr. Sci. 2015, 4, 125–131. [Google Scholar]
- Udompornmongkol, P.; Chiang, B.H. Curcumin-loaded polymeric nanoparticles for enhanced anti-colorectal cancer applications. J. Biomater. Appl. 2015, 30, 537–546. [Google Scholar] [CrossRef]
- Chen, L.; Subirade, M. Chitosan/β-lactoglobulin core–shell nanoparticles as nutraceutical carriers. Biomaterials 2005, 26, 6041–6053. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- El-Sayed, M.; Hassan, Z.; Awad, M.; Salama, H. Chitosan-whey protein complex (cs-wp) as delivery systems to improve bioavailability of iron. Int. J. Appl. Pure Sci. Agric. 2015, 1, 34–46. [Google Scholar]
- Farnia, P.; Mollaei, S.; Bahrami, A.; Ghassempour, A.; Velayati, A.A.; Ghanavi, J. Improvement of curcumin solubility by polyethylene glycol/chitosan-gelatin nanoparticles (cur-peg/cs-g-nps). Biomed. Res. 2016, 27, 659–665. [Google Scholar]
- Yi, J.; Fan, Y.; Zhang, Y.; Wen, Z.; Zhao, L.; Lu, Y. Glycosylated α-lactalbumin-based nanocomplex for curcumin: Physicochemical stability and dpph-scavenging activity. Food Hydrocoll. 2016, 61, 369–377. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle c2c12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef]
- Akalya, V.; Rajeshwari, M.; Sivasurya, R.V.; Manivasagan, N.G.; Ramesh, B.; Karthikeyan, S. Antimicrobial and anticancer activity of curcumin on hepg2 cells. Int. J. Res. Appl. Sci. Eng. Technol. (Ijraset) 2017, 5, 1188–1194. [Google Scholar]
- Senft, C.; Polacin, M.; Priester, M.; Seifert, V.; Kögel, D.; Weissenberger, J. The nontoxic natural compound curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. Bmc Cancer 2010, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a component of turmeric: From farm to pharmacy. Biofactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Bouhenna, M.; Salah, R.; Bakour, R.; Drouiche, N.; Abdi, N.; Grib, H.; Lounici, H.; Mameri, N. Effects of chitin and its derivatives on human cancer cells lines. Environ. Sci. Pollut. Res. 2015, 22, 15579–15586. [Google Scholar] [CrossRef]
- Bounous, G.; Batist, G.; Gold, P. Whey proteins in cancer prevention. Cancer Lett. 1991, 57, 91–94. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. Ph-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Krissansen, G.W. Emerging health properties of whey proteins and their clinical implications. J. Am. Coll. Nutr. 2007, 26, 713S–723S. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Tsakiridis, K.; Karapantzou, C.; Lampaki, S.; Kioumis, I.; Pitsiou, G.; Papaiwannou, A.; Hohenforst-Schmidt, W.; Huang, H.; Kesisis, G.; et al. Use of proteins as biomarkers and their role in carcinogenesis. J. Cancer 2015, 6, 9–18. [Google Scholar] [CrossRef]
- Adahoun, M.A.; Al-Akhras, M.-A.H.; Jaafar, M.S.; Bououdina, M. Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artif. CellsNanomed. Biotechnol. 2017, 45, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei Mirakabad, F.S.; Akbarzadeh, A.; Milani, M.; Zarghami, N.; Taheri-Anganeh, M.; Zeighamian, V.; Badrzadeh, F.; Rahmati-Yamchi, M. A comparison between the cytotoxic effects of pure curcumin and curcumin-loaded plga-peg nanoparticles on the mcf-7 human breast cancer cell line. Artif. CellsNanomed. Biotechnol. 2016, 44, 423–430. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin i is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive biological activities of chitosan and its derivatives: Antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int. J. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Deka, C.; Aidew, L.; Devi, N.; Buragohain, A.K.; Kakati, D.K. Synthesis of curcumin-loaded chitosan phosphate nanoparticle and study of its cytotoxicity and antimicrobial activity. J. Biomater. Sci. Polym. Ed. 2016, 27, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Diekema, D. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D. An antifungal mechanism of curcumin lies in membrane-targeted action within candida albicans. Iubmb Life 2014, 66, 780–785. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.; Yépiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Paul, S.; Mohanram, K.; Kannan, I. Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of candida species. Ayu 2018, 39, 182. [Google Scholar] [CrossRef]
| Treatments | Particle Size (nm) | Zeta Potential | EE% | pH |
|---|---|---|---|---|
| Cs/SCNPs | 328.67 b ± 47.55 | −12.73 cd ± 0.87 | - | 5.00 |
| Cs/α-LaNPs | 334.90 b ± 5.84 | −12.70 cd ± 0.44 | - | 5.21 |
| Cs/β-lgNPs | 310.53 bc ±13.17 | −11.13 c ± 0.64 | - | 5.00 |
| Cs/WPI NPs | 275.33 c ± 30.89 | 17.30 b ± 1.90 | - | 4.30 |
| Cs/WPC NPs | 318.17 bc ± 33.98 | −14.03 d ± 1.21 | - | 5.30 |
| Cur-Cs/SCNPs | 278.10 c ± 16.89 | −12.63 cd ± 2.11 | 72.27 e ± 0.13 | 5.20 |
| Cur-Cs/α-La NPs | 290.83 bc ± 47.17 | −19.50 e ± 0.17 | 74.67 c ± 0.06 | 5.31 |
| Cur-Cs/β-lg NPs | 274.80 c ± 8.84 | −14.83 d ± 2.58 | 73.97 d ± 0.12 | 5.30 |
| Cur-Cs/WPI NPs | 462.80 a ± 6.15 | −13.57 d ± 3.23 | 76.30 b ± 0.10 | 5.42 |
| Cur-Cs/WPC NPs | 439.90 a ± 2.95 | 27.73 a ± 0.81 | 77.27 a ± 0.06 | 4.20 |
| Treatments | Concentration (mg/mL) | Mean | ||
|---|---|---|---|---|
| 2.5 | 5 | 10 | ||
| Chitosan | 46.12 ± 2.9 | 54.08 ± 3.9 | 60.32 ± 6.7 | 53.51 ± 4.5 |
| Curcumin | 41.60 ± 3.5 | 54.02 ± 4.0 | 62.13 ± 2.9 | 52.58 ± 3.5 |
| SC | 39.18 ± 3.0 | 42.75 ± 3.3 | 52.28 ± 3.7 | 44.74 ± 3.3 |
| CS/SCNPs | 39.97 ± 2.7 | 50.48 ± 2.7 | 66.90 ± 3.5 | 52.45 ± 3.0 |
| Cur-Cs/SCNPs | 49.17 ± 4.4 | 60.61 ± 4.4 | 67.54 ± 3.5 | 59.11 ± 4.1 |
| α-La | 36.15 ± 1.7 | 48.38 ± 3.6 | 56.55 ± 2.1 | 47.03 ± 2.5 |
| CS/α-LaNPs | 47.06 ± 2.8 | 56.90 ± 2.5 | 62.51 ± 3.0 | 55.49 ± 2.7 |
| Cur-CS/α-La NPs | 53.82 ± 2.0 | 59.35 ± 2.9 | 66.44 ± 1.9 | 59.87 ± 2.3 |
| β-lg | 43.70 ± 2.9 | 53.51 ± 2.0 | 60.49 ± 2.8 | 52.57 ± 2.6 |
| CS/βlgNPs | 46.86 ± 3.1 | 54.14 ± 3.3 | 65.90 ± 1.2 | 55.63 ± 2.5 |
| Cur-Cs/βlg NPs | 48.08 ± 2.8 | 61.62 ± 2.9 | 69.05 ± 3.5 | 59.58 ± 3.1 |
| WPI | 43.26 ± 3.1 | 50.52 ± 6.8 | 60.92 ± 1.9 | 51.57 ± 3.9 |
| Cs/WPI NPs | 51.10 ± 3.8 | 55.10 ± 2.4 | 66.49 ± 2.5 | 57.56 ± 2.9 |
| Cur-Cs/WPINPs | 50.11 ± 3.3 | 55.08 ± 1.6 | 68.57 ± 2.5 | 57.92 ± 2.5 |
| WPC | 36.30 ± 2.0 | 52.44 ± 2.4 | 61.03 ± 4.8 | 49.92 ± 3.1 |
| Cs/WPC NPs | 47.03 ± 3.9 | 57.26 ± 2.9 | 65.40 ± 2.7 | 56.56 ± 3.2 |
| Cur-Cs/WPCNPs | 49.03 ± 4.2 | 56.20 ± 3.1 | 63.30 ± 2.6 | 56.18 ± 3.3 |
| Mean | 45.21 ± 3.1 | 54.26 ± 3.2 | 63.28 ± 3.0 | |
| Treatments | Anticancer Activity %/Concentrations (mg/mL) | Mean | ||
|---|---|---|---|---|
| 2.5 | 5 | 10 | ||
| Chitosan | 34.97 ± 1.03 | 73.35 ± 2.25 | 91.79 ± 1.96 | 66.70 ± 1.75 |
| Curcumin | 54.06 ± 1.94 | 81.08 ± 2.32 | 95.07 ± 2.93 | 76.74 ± 2.40 |
| SC | 33.71 ± 1.29 | 71.33 ± 2.12 | 93.05 ± 2.95 | 66.03 ± 2.12 |
| CS/SCNPs | 40.4±3.35 | 82.95±3.35 | 95.29±1.71 | 72.88±2.80 |
| Cur-Cs/SCNPs | 46.49±2.64 | 86.36±2.64 | 97.31±2.04 | 76.72±2.44 |
| α-La | 36.75±1.75 | 70.70±2.40 | 92.04±1.36 | 66.50±1.84 |
| CS/α-LaNPs | 38.23±1.77 | 76.67 ± 1.43 | 94.11 ± 1.89 | 69.67 ± 1.70 |
| Cur-Cs/α-La NPs | 45.9 ± 1.40 | 81.79 ± 1.19 | 96.80 ± 3.30 | 74.83 ± 1.96 |
| βlg | 17.55 ± 2.55 | 48.36 ± 4.64 | 83.58 ± 2.42 | 49.83 ± 3.20 |
| CS/βlgNPs | 40.88 ± 2.12 | 70.84 ± 2.56 | 92.55 ± 2.45 | 68.09 ± 2.38 |
| Cur-Cs/βlg NPs | 43.27 ± 1.73 | 85.54 ± 1.37 | 95.80 ± 2.20 | 74.87 ± 1.77 |
| WPI | 18.59 ± 1.41 | 53.91 ± 1.19 | 76.64 ± 1.66 | 49.71 ± 1.42 |
| CS/WPINPs | 43.03 ± 1.53 | 81.69 ± 1.91 | 93.81 ± 1.19 | 72.84 ± 1.54 |
| Cur-Cs/WPINPs | 49.9 ± 1.10 | 82.20 ± 2.79 | 96.31 ± 2.81 | 76.14 ± 2.23 |
| WPC | 30.63 ± 2.37 | 81.29 ± 3.71 | 93.68 ± 2.18 | 68.53 ± 2.75 |
| Cs/WPC NPs | 42.74 ± 2.26 | 82.53 ± 1.07 | 95.27 ± 3.23 | 73.51 ± 2.19 |
| Cur-Cs/WPCNPs | 48.68 ± 4.32 | 80.36 ± 3.06 | 98.07 ± 3.07 | 75.70 ± 3.48 |
| Mean | 39.16 ± 2.03 | 75.94 ± 2.35 | 93.01 ± 2.31 | |
| Treatments | Anticancer Activity (%)/Concentrations (mg/mL) | |||
|---|---|---|---|---|
| 2.5 | 5 | 10 | Mean | |
| Chitosan | 33.22 ± 1.7 | 67.21 ± 1.6 | 92.21 ± 2.8 | 64.21 ± 2.0 |
| Curcumin | 33.4 ± 1.7 | 73.27 ± 2.0 | 93.52 ± 2.0 | 66.73 ± 1.9 |
| SC | 25.25 ± 2.1 | 71.27 ± 2.6 | 92.93 ± 2.1 | 63.15 ± 2.3 |
| CS/SCNPs | 35.86 ± 3.9 | 75.91 ± 3.9 | 93.33 ± 1.8 | 68.37 ± 3.7 |
| Cur-Cs/SCNPs | 50.84 ± 2.9 | 78.15 ± 2.9 | 96.88 ± 5.5 | 75.29 ± 3.7 |
| α-La | 30.27 ± 2.8 | 71.27 ± 2.0 | 93.25 ± 1.8 | 64.93 ± 2.2 |
| CS/α-La NPs | 41.61 ± 3.1 | 75.13 ± 4.1 | 94.78 ± 3.7 | 70.51 ± 3.6 |
| Cur-Cs/α-La NPs | 51.26 ± 2.3 | 77.18 ± 2.2 | 98.31 ± 1.8 | 75.58 ± 2.1 |
| βlg | 19.24 ± 1.8 | 50.98 ± 3.1 | 85.37 ± 4.2 | 51.86 ± 3.0 |
| CS/βlgNPs | 44.08 ± 2.9 | 61.58 ± 2.5 | 91.93 ± 2.1 | 65.86 ± 2.5 |
| Cur-Cs/βlg NPs | 53.63 ± 2.6 | 74.61 ± 3.4 | 98.47 ± 3.5 | 75.57 ± 3.5 |
| WPI | 16.63 ± 2.5 | 48.80 ± 4.1 | 83.17 ± 2.8 | 49.53 ± 3.1 |
| CS/WPINPs | 41.65 ± 2.4 | 74.78 ± 3.8 | 94.2 ± 1.3 | 70.21 ± 2.5 |
| Cur-Cs/WPINPs | 52.43 ± 2.8 | 77.87 ± 5.9 | 98.48 ± 5.5 | 76.26 ± 4.7 |
| WPC | 17.87 ± 2.1 | 62.24 ± 2.6 | 76.30 ± 3.0 | 52.14 ± 2.6 |
| Cs/WPC NPs | 33.26 ± 2.2 | 74.79 ± 3.3 | 93.86 ± 2.3 | 67.30 ± 2.6 |
| Cur-Cs/WPCNPs | 50.03 ± 1.5 | 75.96 ± 2.9 | 98.06 ± 2.5 | 74.68 ± 2.3 |
| Mean | 37.09 ± 2.4 | 70.06 ± 3.2 | 89.12 ± 2.9 | |
| Treatments | Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|
| E. Coli | Staph. aureus | B. subtilis | P. aeruginoas | C. albicans | |
| Chitosan | 15 g ± 4 | 19 bcd ± 2 | 21 fg ± 3 | 14 f ± 4 | 12 e ± 2 |
| Curcumin | 16 fg ± 3 | 20 bc ± 3 | 23 ef ± 2 | 16 ef ± 3 | 21 bc ± 3 |
| SC | 19 efg ± 2 | 25 a ± 2 | 26 cde ± 4 | 15 f ± 3 | 14 e ± 2 |
| CS/SCNPs | 22 cde ± 3 | 17 cde ± 3 | 28 bcd ± 3 | 20 cde ± 3 | 20 cd ± 4 |
| Cur-Cs/SCNPs | 27 ab ± 3 | 21 b ± 4 | 31 ab ± 4 | 25 ab ± 4 | 28 a ± 3 |
| α-La | 0 h ± 0 | 0 f ± 0 | 0 h ± 0 | 0 g ± 0 | 20 cd ± 3 |
| CS/α-LaNPs | 20 def ± 3 | 15 e ± 4 | 25 def ± 3 | 25 ab ± 2 | 22 bc ± 3 |
| Cur-Cs/α-La NPs | 24 bcd ± 3 | 20 bc ± 3 | 30 abc ± 3 | 28 a ± 3 | 25 ab ± 2 |
| Βlg | 0 h ± 0 | 0 f ± 0 | 0 h ± 0 | 0 g ± 0 | 0 f ± 0 |
| CS/βlgNPs | 25 bc ± 2 | 19 bcd ± 2 | 30 abc ± 3 | 24 abc ± 3 | 16 de ± 3 |
| Cur-CS/βlgNPs | 30 a ± 3 | 22 ab ± 3 | 33 a ± 2 | 26 ab ± 3 | 21 bc ± 3 |
| WPI | 0 h ± 0 | 0 f ± 0 | 0 h ± 0 | 0 g ± 0 | 0 f ± 0 |
| Cs/WPI NPs | 20 def ± 3 | 14 e ± 3 | 28 bcd ± 3 | 15 f ± 2 | 16 de ± 3 |
| Cur-Cs/WPI NPs | 28 ab ± 3 | 20 bc ± 3 | 32 ab ± 3 | 22 bcd ± 3 | 21 bc ± 3 |
| WPC | 0 h ± 0 | 0 f ± 0 | 16 g ± 1 | 0 g ± 0 | 0 f ± 0 |
| Cs/WPC NPs | 24 b-d ± 3 | 16 de ± 3 | 22 ef ± 3 | 18 def ± 3 | 15 e ± 3 |
| Cur-Cs/WPC NPs | 31 a ± 4 | 22 ab ± 3 | 28 bcd ± 3 | ‘20 cde ± 3 | 23 bc ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, S.; El-Sherbiny, I.; Enomoto, T.; Salem, A.; Nagai, E.; Askar, A.; Abady, G.; Abdel-Hamid, M. Improving the Functional Activities of Curcumin Using Milk Proteins as Nanocarriers. Foods 2020, 9, 986. https://doi.org/10.3390/foods9080986
Taha S, El-Sherbiny I, Enomoto T, Salem A, Nagai E, Askar A, Abady G, Abdel-Hamid M. Improving the Functional Activities of Curcumin Using Milk Proteins as Nanocarriers. Foods. 2020; 9(8):986. https://doi.org/10.3390/foods9080986
Chicago/Turabian StyleTaha, Soad, Ibrahim El-Sherbiny, Toshiki Enomoto, Aida Salem, Emiko Nagai, Ahmed Askar, Ghada Abady, and Mahmoud Abdel-Hamid. 2020. "Improving the Functional Activities of Curcumin Using Milk Proteins as Nanocarriers" Foods 9, no. 8: 986. https://doi.org/10.3390/foods9080986
APA StyleTaha, S., El-Sherbiny, I., Enomoto, T., Salem, A., Nagai, E., Askar, A., Abady, G., & Abdel-Hamid, M. (2020). Improving the Functional Activities of Curcumin Using Milk Proteins as Nanocarriers. Foods, 9(8), 986. https://doi.org/10.3390/foods9080986





