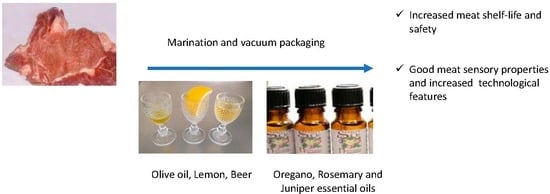
Use of Essential Oils to Increase the Safety and the Quality of Marinated Pork Loin
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminary Tests: Selection of the Marinade Solution’s Composition and Essential Oils Mixture
2.2. Ingredients and Microorganisms Used
2.3. Preparation of the Samples and Shelf-Life Trials
- Control group (non-marinated) added with 1% NaCl (C);
- Marinade solution beer/olive oil/concentrated lemon juice (2/1/1; 10% w/w) (M);
- Marinade solution beer/olive oil/concentrated lemon juice (2/1/1; 10% w/w) added with a mixture of essential oils (oregano 0.02%, rosemary 0.03% and juniper 0.03% essential oils) (M + E).
2.3.1. pH
2.3.2. Color
2.3.3. Marinade Uptake
2.3.4. Cooking Loss
2.3.5. Shear Force
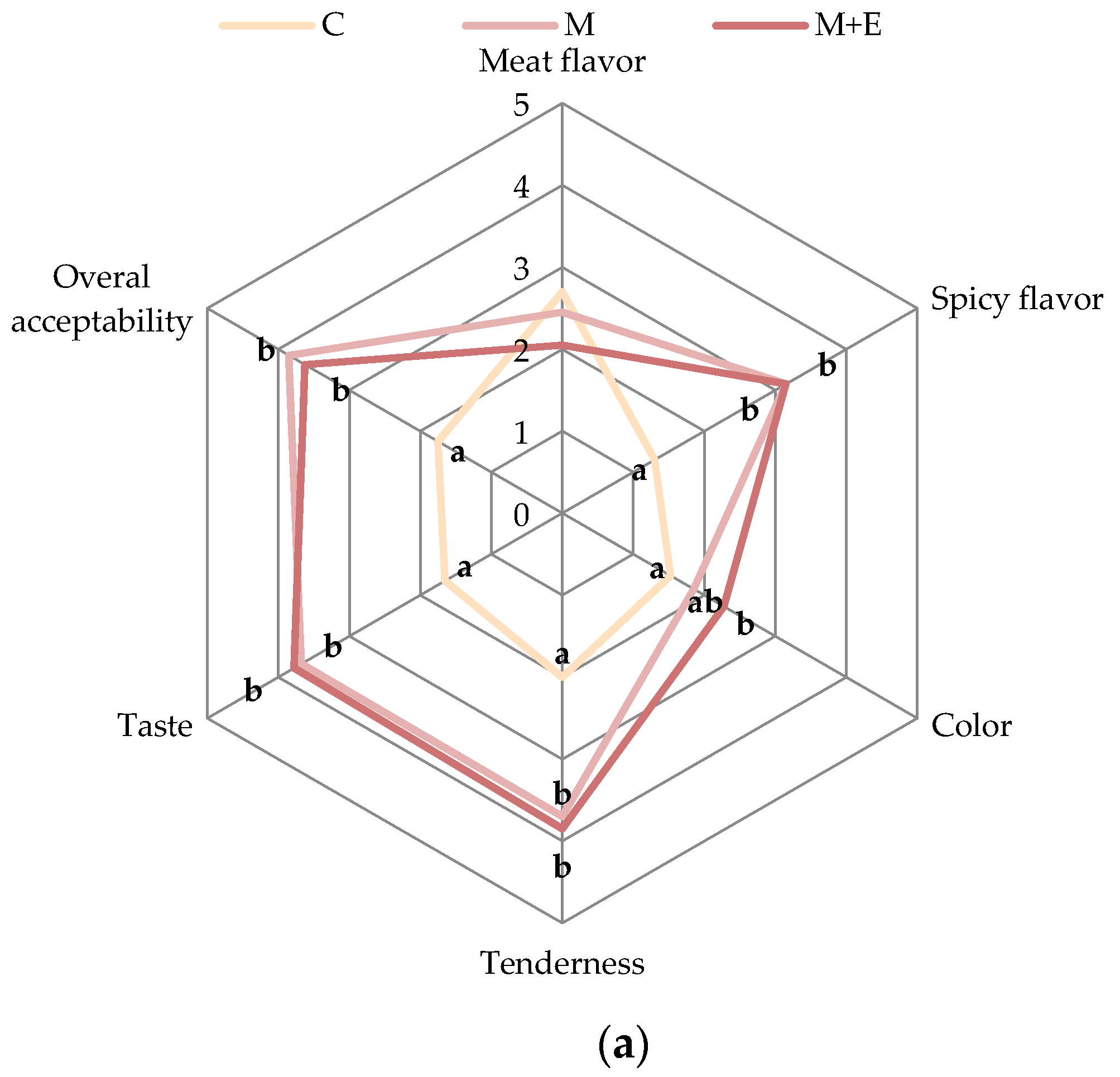
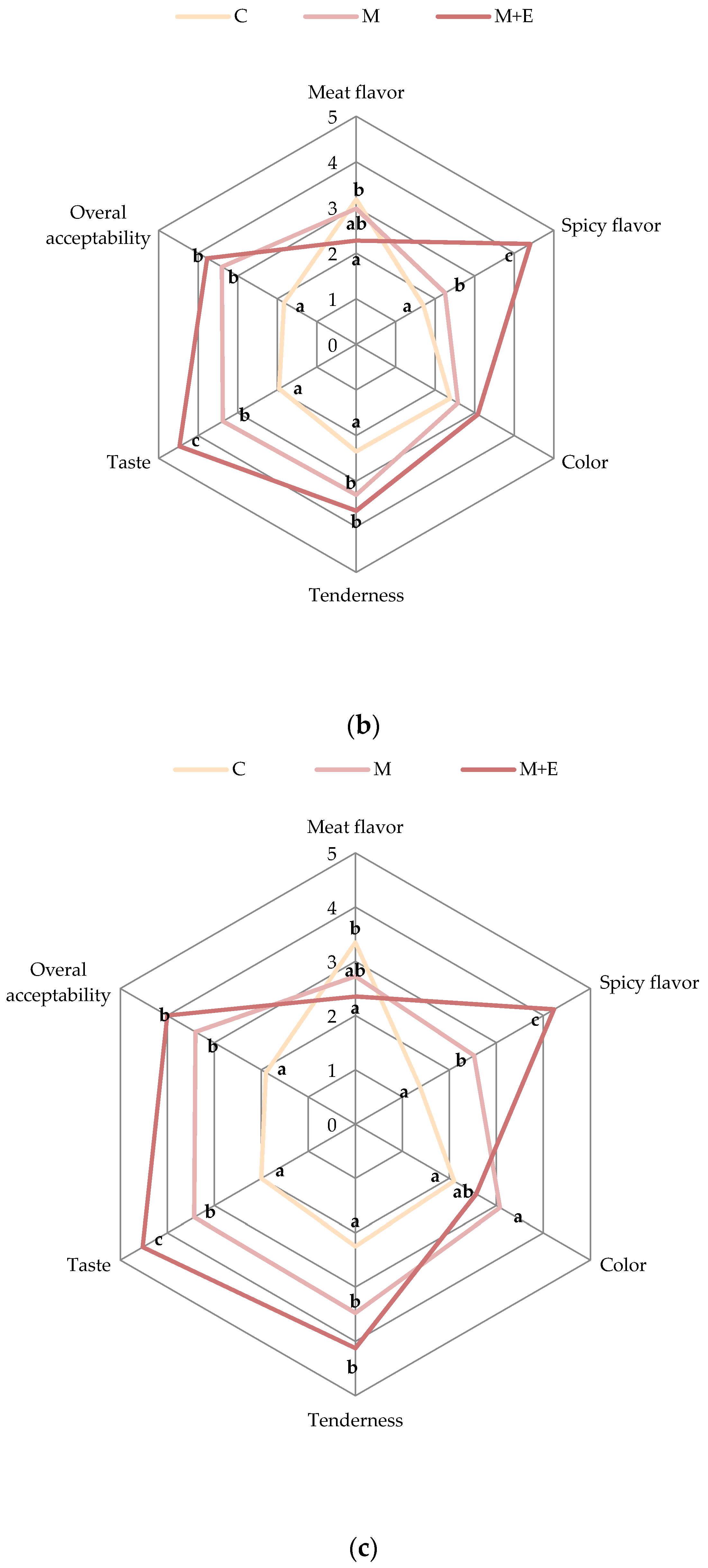
2.3.6. Sensory Analysis
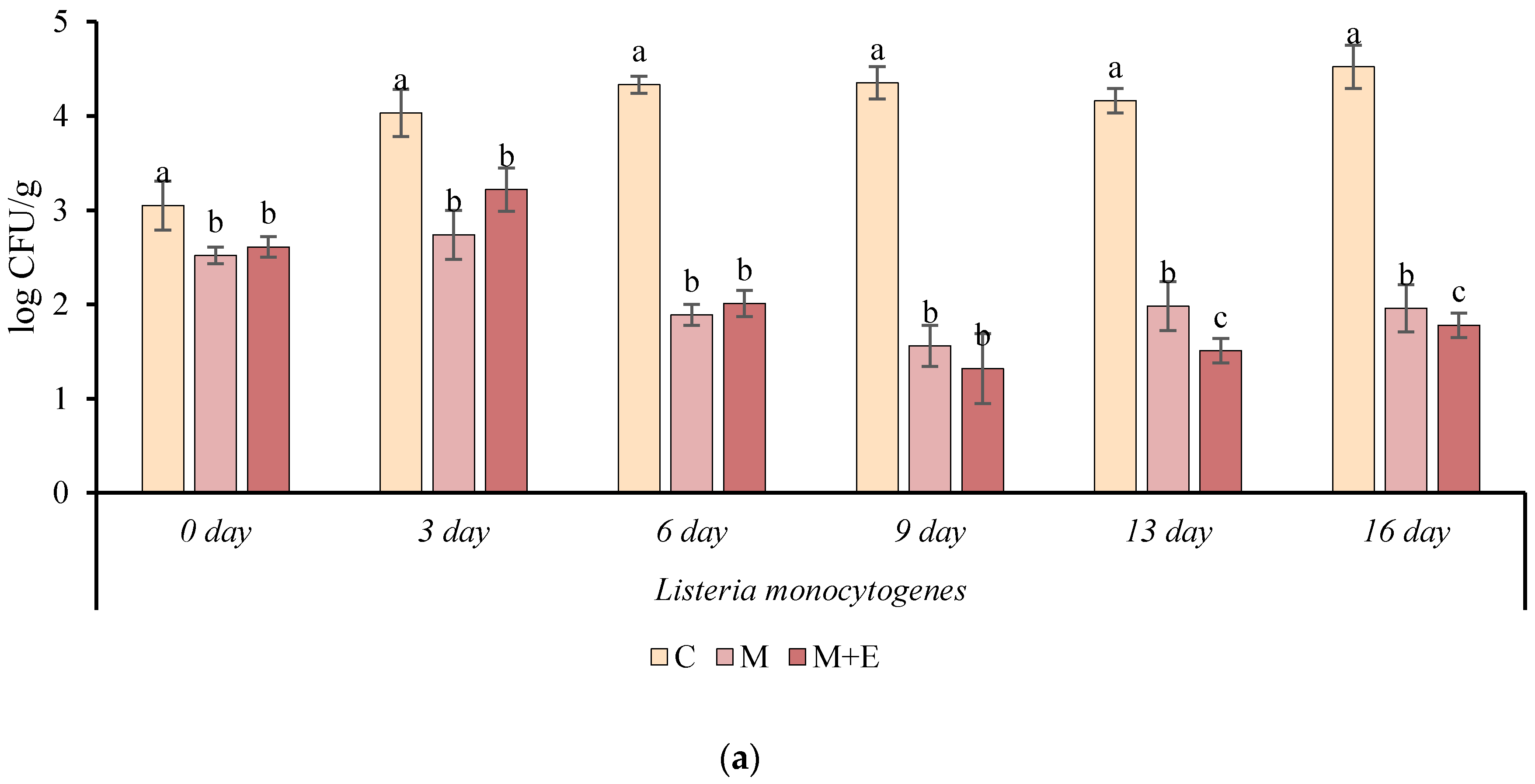
2.3.7. Microbiological Analysis
2.4. Challenge-Test Trials
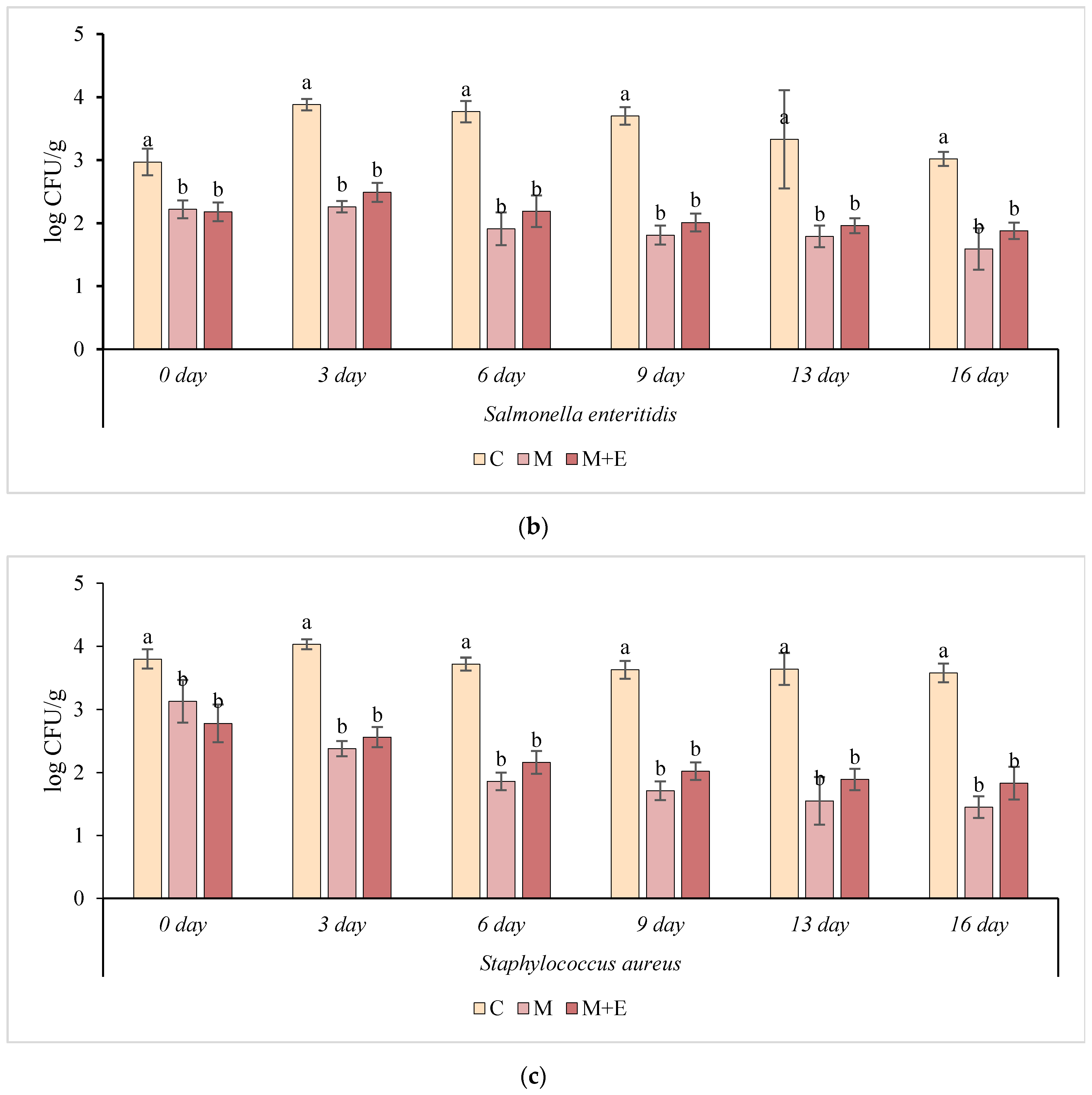
- Control group (non-marinated) + pathogens (L. monocytogenes, S. enteritidis and S. aureus), (C+P) inoculated at a level of 4.0 log CFU/g;
- Marinade solution beer/olive oil/concentrated lemon juice (2/1/1) used at 10% + pathogens (L. monocytogenes, S. enteritidis and S. aureus), (M + P) inoculated at a level of 4.0 log CFU/g;
- Marinade solution beer/olive oil/concentrated lemon juice (2/1/1) used at 10%; added with essential oils (oregano 0.02%, rosemary 0.03% and juniper 0.03% essential oils) + pathogens (L. monocytogenes, S. enteritidis and S. aureus), (M + E + P) inoculated at a level of 4.0 log CFU/g.
2.5. Microbiological Analysis
2.6. Statistical Analysis
3. Results
3.1. Shelf-Life Trials
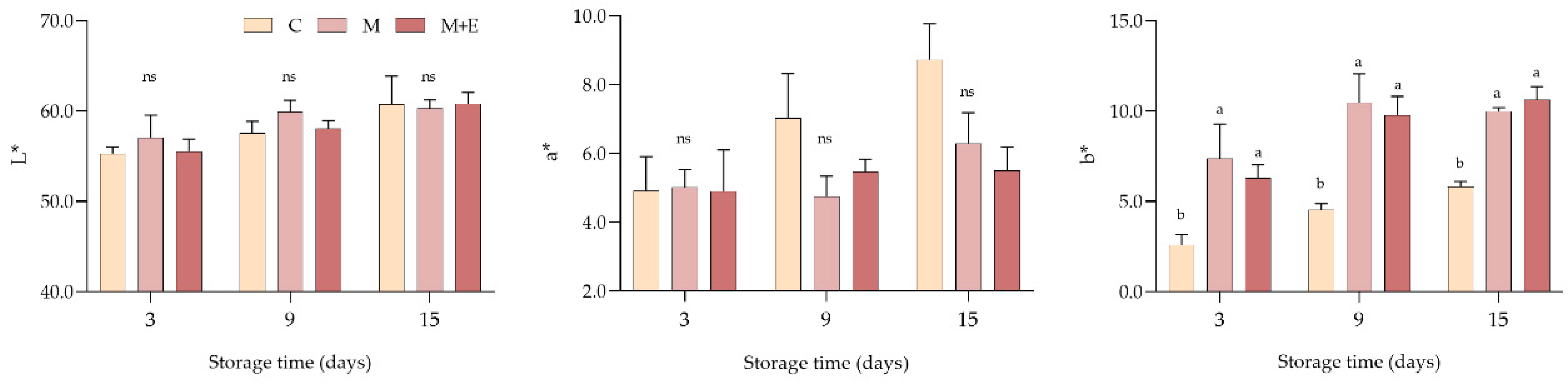
3.1.1. pH and Color
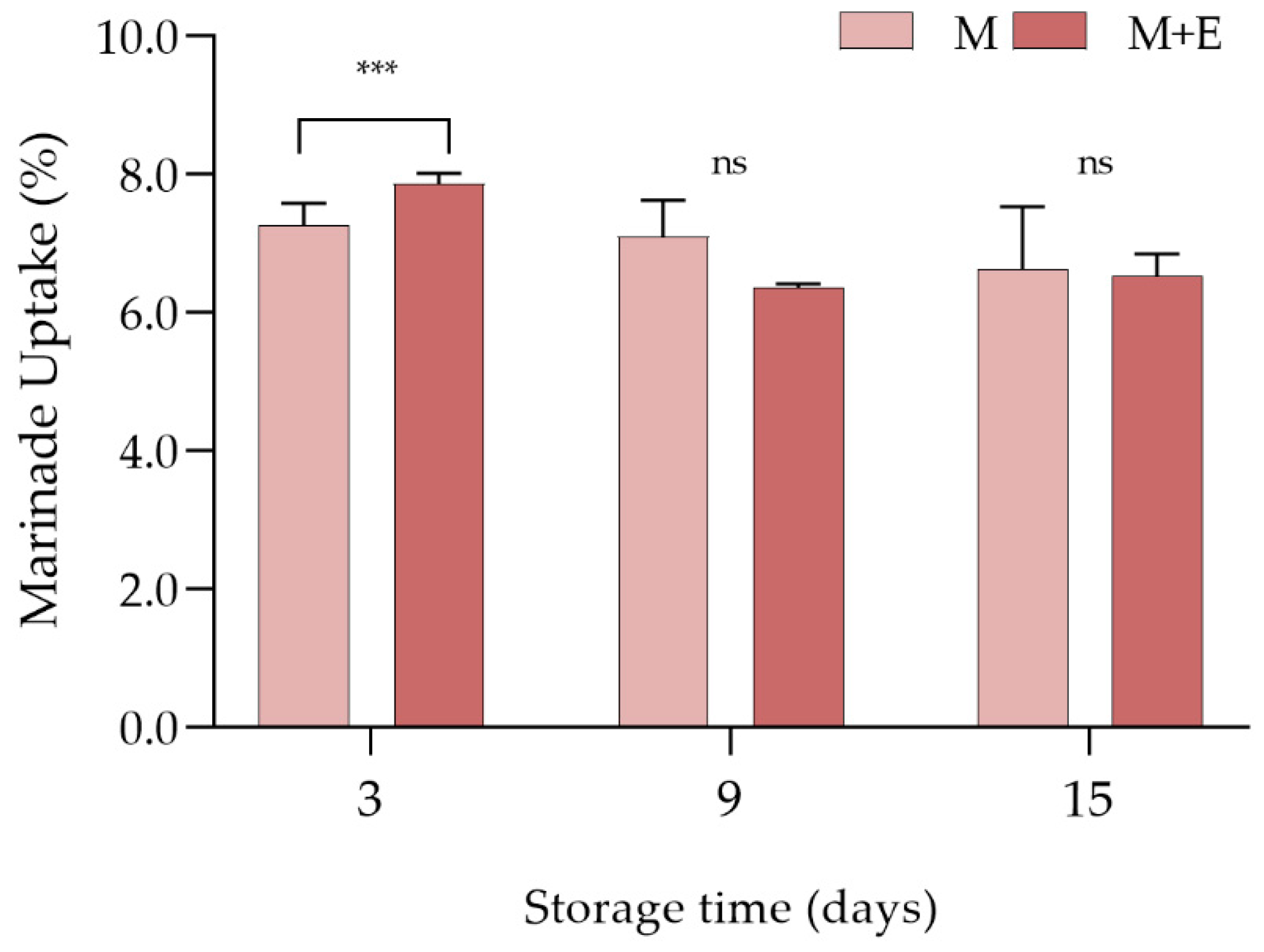
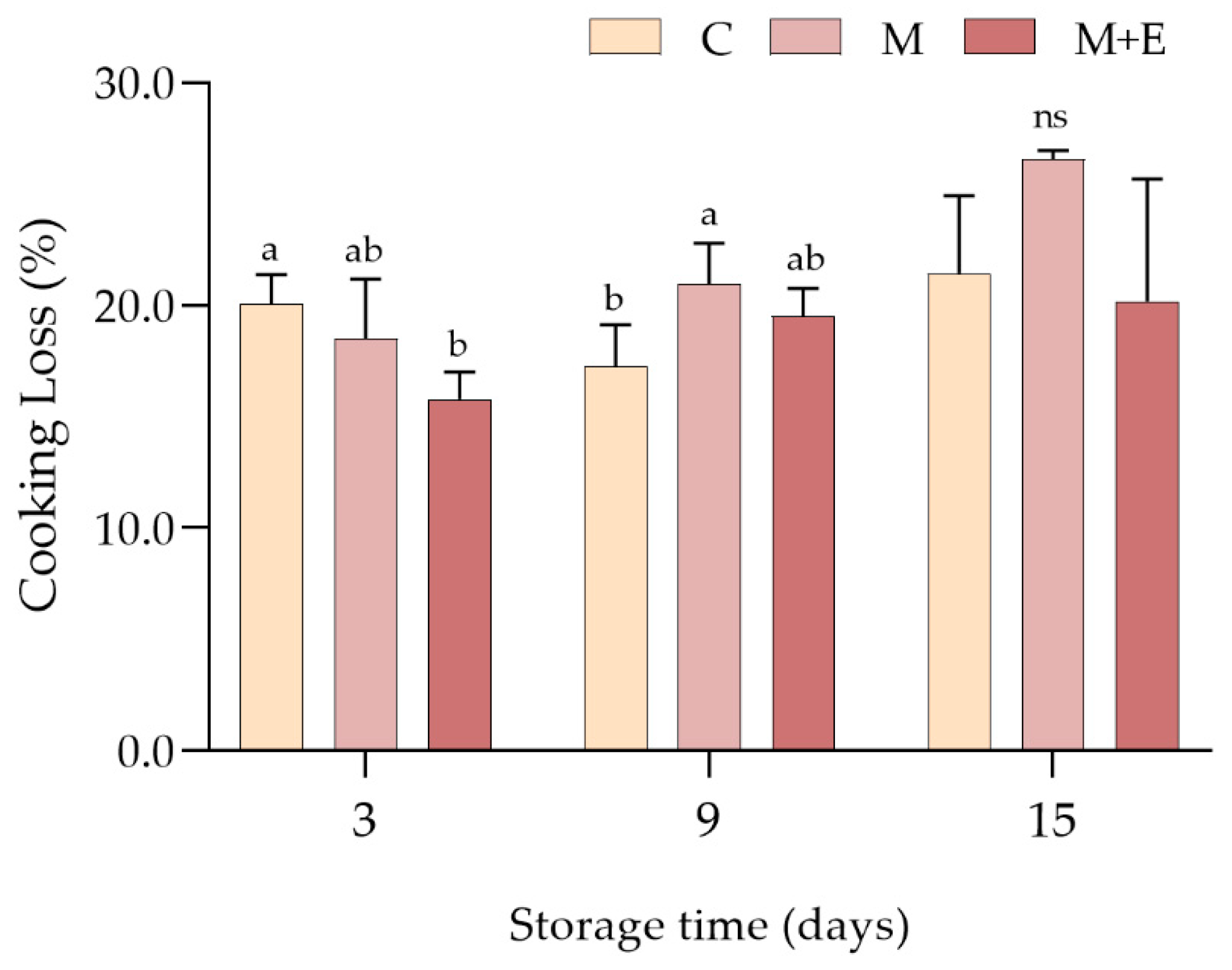
3.1.2. Marinade Uptake and Cooking Loss
3.1.3. Shear Force
3.1.4. Sensory Analysis
3.1.5. Microbiological Analysis
3.2. Challenge Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barbut, S.; Sosnicki, A.A.; Lonergan, S.M.; Knapp, T.; Ciobanu, D.C.; Gatcliffe, L.J.; Huff-Lonergan, E.; Wilson, E.W. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 2008, 79, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Yusop, S.M.; O’Sullivan, M.G.; Kerry, J.P. Marinating and enhancement of the nutritional content of processed meat products. In Processed Meats: Improving Safety, Nutrition and Quality; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; pp. 421–449. ISBN 9781845694661. [Google Scholar]
- King, D.A.; Wheeler, T.L.; Shackelford, S.D.; Koohmaraie, M. 3 Fresh meat texture and tenderness BT—Improving the Sensory and Nutritional Quality of Fresh Meat. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK; Sawston, UK, 2009; pp. 61–88. ISBN 978-1-84569-343-5. [Google Scholar]
- García-Márquez, I.; Cambero, M.I.; Ordóñez, J.A.; Cabeza, M.C. Shelf-life extension and sanitation of fresh pork loin by e-beam treatment. J. Food Prot. 2012, 75, 2179–2189. [Google Scholar] [CrossRef]
- Kargiotou, C.; Katsanidis, E.; Rhoades, J.; Kontominas, M.; Koutsoumanis, K. Efficacies of soy sauce and wine base marinades for controlling spoilage of raw beef. Food Microbiol. 2011, 28, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S. The Science of Poultry and Meat Processing; University of Guelph: Guelph, ON, Canada, 2015; Self-published online book; ISBN 9780889556256. [Google Scholar]
- Warriss, P.D. Meat Science: An Introductory Text; CABI Publishing: New York, NY, USA, 2000; ISBN 978-0-85199-424-6. [Google Scholar]
- Balestra, F.; Petracci, M. Technofunctional ingredients for meat products. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–68. [Google Scholar]
- Alvarado, C.; McKee, S. Marination to improve functional properties and safety of poultry meat. J. Appl. Poult. Res. 2007, 16, 113–120. [Google Scholar] [CrossRef]
- Vlahova-Vangelova, D.; Dragoev, S. Marination: Effect on meat safety and human health. A review. Bulg. J. Agric. Sci. 2014, 20, 503–509. [Google Scholar]
- Yusop, S.M.; O’Sullivan, M.G.; Kerry, J.F.; Kerry, J.P. Effect of marinating time and low pH on marinade performance and sensory acceptability of poultry meat. Meat Sci. 2010, 85, 657–663. [Google Scholar] [CrossRef]
- Björkroth, J. Microbiological ecology of marinated meat products. In Meat Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; Volume 70, pp. 477–480. [Google Scholar]
- Pathania, A.; McKee, S.R.; Bilgili, S.F.; Singh, M. Antimicrobial activity of commercial marinades against multiple strains of Salmonella spp. Int. J. Food Microbiol. 2010, 139, 214–217. [Google Scholar] [CrossRef]
- Karam, L.; Roustom, R.; Abiad, M.G.; El-Obeid, T.; Savvaidis, I.N. Combined effects of thymol, carvacrol and packaging on the shelf-life of marinated chicken. Int. J. Food Microbiol. 2019, 291, 42–47. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Grant, A.; Parveen, S. All natural and clean-label preservatives and antimicrobial agents used during poultry processing and packaging. J. Food Prot. 2017, 80, 540–544. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Gardini, F.; Lanciotti, R. Effects of sub-lethal concentrations of thyme and oregano essential oils, carvacrol, thymol, citral and trans-2-hexenal on membrane fatty acid composition and volatile molecule profile of Listeria monocytogenes, Escherichia coli and Salmonella enteritidis. Food Chem. 2015, 182, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Williams, S.K.; Djeri, N.; Hinton, A.; Rodrick, G.E. Nisin, rosemary, and ethylenediaminetetraacetic acid affect the growth of Listeria monocytogenes on ready-to-eat turkey ham stored at four degrees Celsius for sixty-three days. Poult. Sci. 2009, 88, 1765–1772. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef]
- Albertsen, L.; Wiedmann, K.P.; Schmidt, S. The impact of innovation-related perception on consumer acceptance of food innovations—Development of an integrated framework of the consumer acceptance process. Food Qual. Prefer. 2020, 84, 103958. [Google Scholar] [CrossRef]
- Jeacocke, R.E. Continuous measurements of the pH of beef muscle in intact beef carcases. Int. J. Food Sci. Technol. 2007, 12, 375–386. [Google Scholar] [CrossRef]
- Pointer, M.R. A comparison of the CIE 1976 colour spaces. Color Res. Appl. 2009, 6, 108–118. [Google Scholar] [CrossRef]
- Aberle, E.; Forrest, J.; Gerrard, D.; Mills, E. Principles of Meat Science; Kendall Hunt Publishing Company: Dubuque, IA, USA, 2012. [Google Scholar]
- Allen, C.D.; Russell, S.M.; Fletcher, D.L. The relationship of raw broiler breast meat color and pH to cooked meat color and pH. Poult. Sci. 1997, 76, 1042–1046. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef]
- Jones, J. Meat science: An introductory text. Br. Poult. Sci. 2000, 41, 521. [Google Scholar]
- Lunde, K.; Egelandsdal, B.; Choinski, J.; Mielnik, M.; Flåtten, A.; Kubberød, E. Marinating as a technology to shift sensory thresholds in ready-to-eat entire male pork meat. Meat Sci. 2008, 80, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Li, J.; Zhang, L.; Jiang, Y.; Yin, M.; Liu, Y.; Gao, F.; Zhou, G. Effect of different tumbling marination methods and time on the water status and protein properties of prepared pork chops. Asian-Australas. J. Anim. Sci. 2015, 28, 1020–1027. [Google Scholar] [CrossRef]
- Mozuriene, E.; Bartkiene, E.; Krungleviciute, V.; Zadeike, D.; Juodeikiene, G.; Damasius, J.; Baltusnikiene, A. Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT Food Sci. Technol. 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Fadiloǧlu, E.E.; Serdaroǧlu, M. Effects of pre and post-rigor marinade injection on some quality parameters of Longissimus dorsi muscles. Korean J. Food Sci. Anim. Resour. 2018, 38, 325–337. [Google Scholar]
- Gamage, H.G.C.L.; Mutucumarana, R.K.; Andrew, M.S. Effect of marination method and holding time on physicochemical and sensory characteristics of broiler meat. J. Agric. Sci. 2017, 12, 172–184. [Google Scholar] [CrossRef]
- Gao, T.; Li, J.; Zhang, L.; Jiang, Y.; Ma, R.; Song, L.; Gao, F.; Zhou, G. Effect of different tumbling marination treatments on the quality characteristics of prepared pork chops. Asian-Australas. J. Anim. Sci. 2015, 28, 260–267. [Google Scholar] [CrossRef]
- Miller, R. Functionality Of Non-Meat Ingredients Used In Enhanced Pork—Hogs, Pigs, and Pork. Available online: https://swine.extension.org/functionality-of-non-meat-ingredients-used-in-enhanced-pork/ (accessed on 30 April 2020).
- Berge, P.; Ertbjerg, P.; Larsen, L.M.; Astruc, T.; Vignon, X.; Møller, A.J. Tenderization of beef by lactic acid injected at different times post mortem. Meat Sci. 2001, 57, 347–357. [Google Scholar] [CrossRef]
- Komoltri, P.; Pakdeechanuan, P. Effects of marinating ingredients on physicochemical, microstructural and sensory properties of golek chicken. Int. Food Res. J. 2012, 19, 1449–1455. [Google Scholar]
- Brooks, C. Marinating of Beef for Enhancement; National Cattlemen’s Beef Association: Centennial, CO, USA, 2007. [Google Scholar]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Van der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Hassanzad Azar, H.; Hejazi, J. The use of herbal extracts and essential oils as a potential antimicrobial in meat and meat products: A review. J. Hum. Environ. Health Promot. 2016, 1, 63–74. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Borch, E.; Kant-Muermans, M.L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.-J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Cauchie, E.; Delhalle, L.; Taminiau, B.; Tahiri, A.; Korsak, N.; Burteau, S.; Fall, P.A.; Farnir, F.; Baré, G.; Daube, G. Assessment of spoilage bacterial communities in food wrap and modified atmospheres-packed minced pork meat samples by 16S rDNA metagenetic analysis. Front. Microbiol. 2020, 10, 3074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, G.; Ye, K.; Wang, S.; Xu, X.; Li, C. Microbial changes in vacuum-packed chilled pork during storage. Meat Sci. 2015, 100, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- FCD. Available online: http://www.fcd.fr/media/filer_public/7e/51/7e51261b-e18d-4c9f-be28-5dad72e4cfd0/1200_fcd_criteres_microbiologiques_2016_ateliers_rayons_coupe_28012016.pdf (accessed on 5 April 2020).
- Wickramasinghe, N.N.; Ravensdale, J.; Coorey, R.; Chandry, S.P.; Dykes, G.A. The predominance of psychrotrophic Pseudomonas on aerobically stored chilled red meat. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1622–1635. [Google Scholar] [CrossRef]
- Nychas, G.J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A systematic review of bacterial foodborne outbreaks related to red meat and meat products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef]
- Fegan, N.; Jenson, I. The role of meat in foodborne disease: Is there a coming revolution in risk assessment and management? Meat Sci. 2018, 144, 22–29. [Google Scholar] [CrossRef]
- Mor-Mur, M.; Yuste, J. Emerging bacterial pathogens in meat and poultry: An overview. Food Bioproc. Technol. 2010, 3, 24–35. [Google Scholar] [CrossRef]
- Kamdem, S.S.; Patrignani, F.; Guerzoni, M.E. Shelf-life and safety characteristics of Italian Toscana traditional fresh sausage (Salsiccia) combining two commercial ready-to-use additives and spices. Food Control 2007, 18, 421–429. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Lanciotti, R.; Patrignani, F.; Bagnolini, F.; Guerzoni, M.E.; Gardini, F. Evaluation of diacetyl antimicrobial activity against Escherichia coli, Listeria monocytogenes and Staphylococcus aureus. Food Microbiol. 2003, 20, 537–543. [Google Scholar] [CrossRef]
- Montanari, C.; Serrazanetti, D.I.; Felis, G.; Torriani, S.; Tabanelli, G.; Lanciotti, R.; Gardini, F. New insights in thermal resistance of staphylococcal strains belonging to the species Staphylococcus epidermidis, Staphylococcus lugdunensis and Staphylococcus aureus. Food Control 2015, 50, 605–612. [Google Scholar] [CrossRef]
- Lanciotti, R.; Braschi, G.; Patrignani, F.; Gobbetti, M.; De Angelis, M. How Listeria monocytogenes Shapes Its Proteome in Response to Natural Antimicrobial Compounds. Front. Microbiol. 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Braschi, G.; de Jong, A.; Kok, J.; Patrignani, F.; Lanciotti, R. Transcriptomic approach and membrane fatty acid analysis to study the response mechanisms of Escherichia coli to thyme essential oil, carvacrol, 2-(E)-hexanal and citral exposure. J. Appl. Microbiol. 2018, 125, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Braschi, G.; Serrazanetti, D.I.; Siroli, L.; Patrignani, F.; De Angelis, M.; Lanciotti, R. Gene expression responses of Listeria monocytogenes Scott A exposed to sub-lethal concentrations of natural antimicrobials. Int. J. Food Microbiol. 2018, 286, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Braschi, G.; Patrignani, F.; Siroli, L.; Lanciotti, R.; Schlueter, O.; Froehling, A. Flow cytometric assessment of the morphological and physiological changes of Listeria monocytogenes and Escherichia coli in response to natural antimicrobial exposure. Front. Microbiol. 2018, 9, 2783. [Google Scholar] [CrossRef]
- Lanciotti, R.; Sinigaglia, M.; Gardini, F.; Vannini, L.; Guerzoni, M.E. Growth/no growth interfaces of Bacillus cereus, Staphylococcus aureus and Salmonella enteritidis in model systems based on water activity, pH, temperature and ethanol concentration. Food Microbiol. 2001, 18, 659–668. [Google Scholar] [CrossRef]
- Castellano, P.; Pérez Ibarreche, M.; Blanco Massani, M.; Fontana, C.; Vignolo, G. Strategies for pathogen biocontrol using lactic acid bacteria and their metabolites: A focus on meat ecosystems and industrial environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef]
- Nisiotou, A.; Chorianopoulos, N.G.; Gounadaki, A.; Panagou, E.Z.; Nychas, G.J.E. Effect of wine-based marinades on the behavior of salmonella typhimurium and background flora in beef fillets. Int. J. Food Microbiol. 2013, 164, 119–127. [Google Scholar] [CrossRef]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Theron, M.M.; Lues, J.F.R. Organic acids and meat preservation: A review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Recipes for antimicrobial wine marinades against Bacillus cereus, Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica. J. Food Sci. 2007, 72, M207–M213. [Google Scholar] [CrossRef] [PubMed]

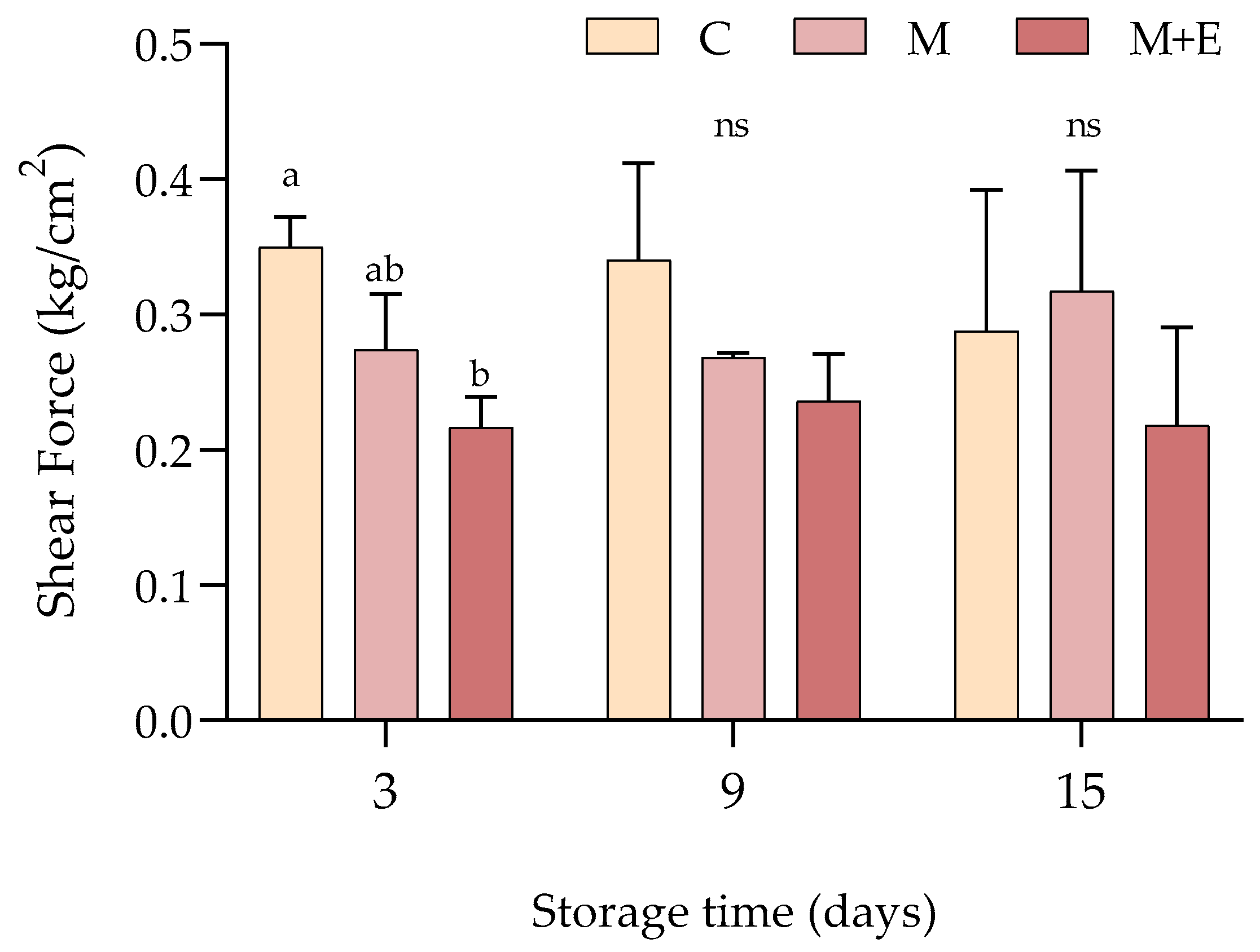

| Marinade Solution | Ingredients Ratio | % of Marinade Solution (w/w) |
|---|---|---|
| Water/lemon juice | 1:1 | 10 |
| Water/lemon juice | 1:1 | 5 |
| Olive oil/lemon juice | 1:2 | 5 |
| Olive oil/lemon juice | 1:2 | 10 |
| Olive oil/balsamic vinegar | 1:1 | 5 |
| Olive oil/balsamic vinegar | 1:1 | 10 |
| Olive oil/red wine | 1:2 | 10 |
| Olive oil/red wine | 1:3 | 10 |
| Olive oil/white wine | 1:2 | 10 |
| Olive oil/white wine | 1:3 | 10 |
| Olive oil/beer | 1:2 | 10 |
| Olive oil/beer | 1:3 | 10 |
| Olive oil/beer/lemon juice | 1:2:1 | 10 |
| Olive oil/mustard/lemon juice | 1:1:1 | 10 |
| Olive oil/mustard/lemon juice | 1:1:1 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siroli, L.; Baldi, G.; Soglia, F.; Bukvicki, D.; Patrignani, F.; Petracci, M.; Lanciotti, R. Use of Essential Oils to Increase the Safety and the Quality of Marinated Pork Loin. Foods 2020, 9, 987. https://doi.org/10.3390/foods9080987
Siroli L, Baldi G, Soglia F, Bukvicki D, Patrignani F, Petracci M, Lanciotti R. Use of Essential Oils to Increase the Safety and the Quality of Marinated Pork Loin. Foods. 2020; 9(8):987. https://doi.org/10.3390/foods9080987
Chicago/Turabian StyleSiroli, Lorenzo, Giulia Baldi, Francesca Soglia, Danka Bukvicki, Francesca Patrignani, Massimiliano Petracci, and Rosalba Lanciotti. 2020. "Use of Essential Oils to Increase the Safety and the Quality of Marinated Pork Loin" Foods 9, no. 8: 987. https://doi.org/10.3390/foods9080987
APA StyleSiroli, L., Baldi, G., Soglia, F., Bukvicki, D., Patrignani, F., Petracci, M., & Lanciotti, R. (2020). Use of Essential Oils to Increase the Safety and the Quality of Marinated Pork Loin. Foods, 9(8), 987. https://doi.org/10.3390/foods9080987