Effects of Ultrasound Treatments on Tenderness and In Vitro Protein Digestibility of New Zealand Abalone, Haliotis iris
Abstract
1. Introduction
2. Materials and Methods
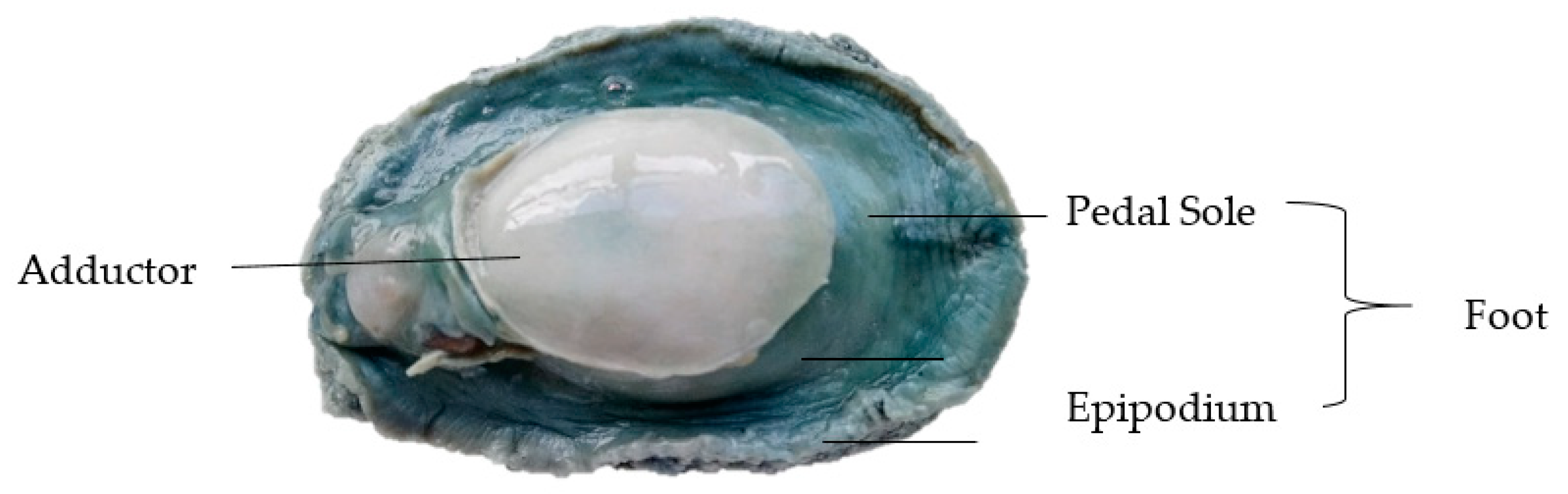
2.1. Materials
2.2. Sample Treatments
2.3. Analyses of Control and Treated Pāua Samples
2.3.1. pH Measurements
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Cook Loss (%)
2.3.4. Microstructure Analysis Using Verhoeff-Van Gieson Staining (VVG)
2.3.5. Ultrastructure Analysis Using Transmission Electron Microscopy (TEM)
2.3.6. Texture Analysis
2.3.7. In Vitro Static Oral-Gastro-Small-Intestinal Digestion
2.4. Statistical Analysis
3. Results
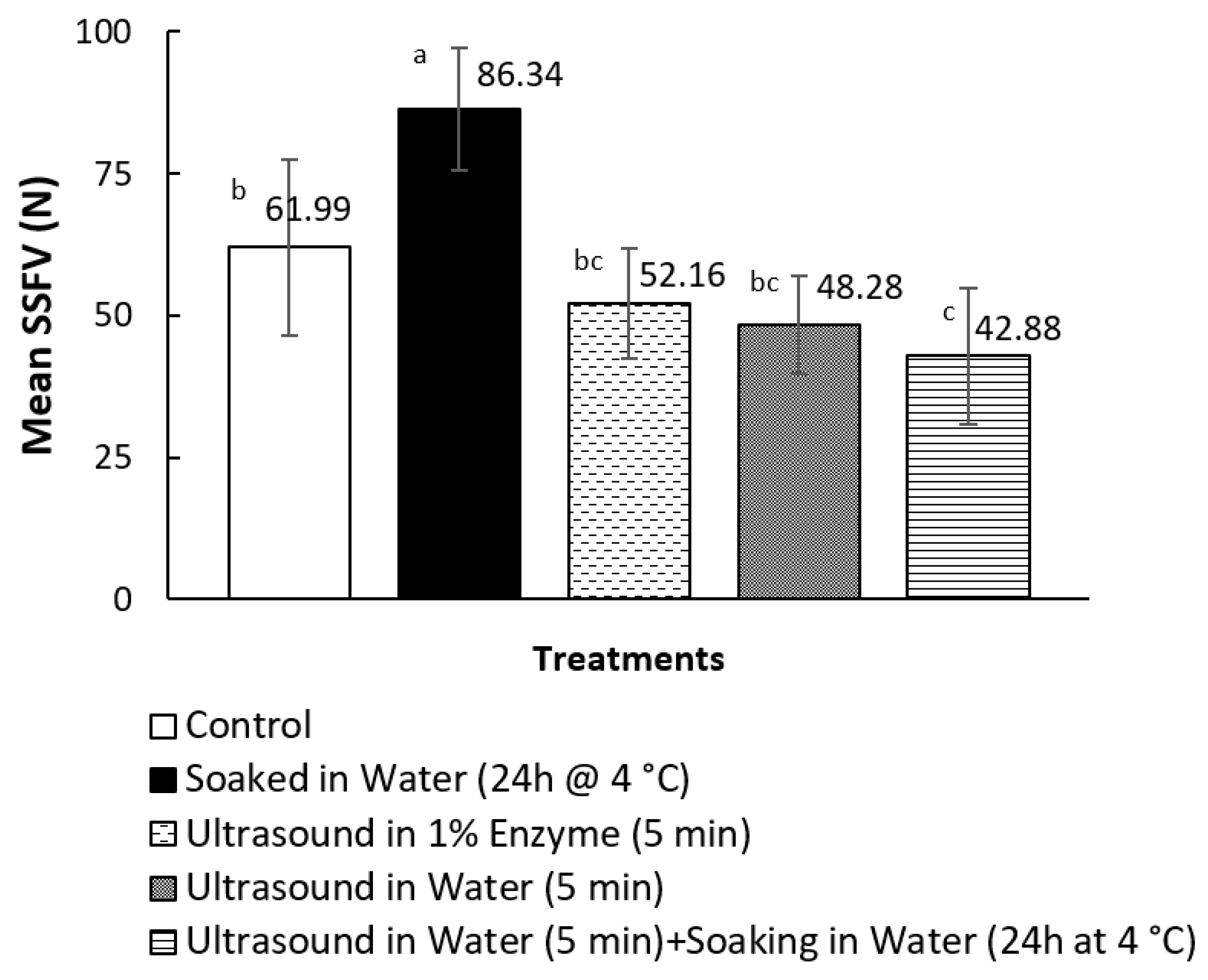
3.1. Texture Measurements on Canned Pāua
3.2. Microscopy
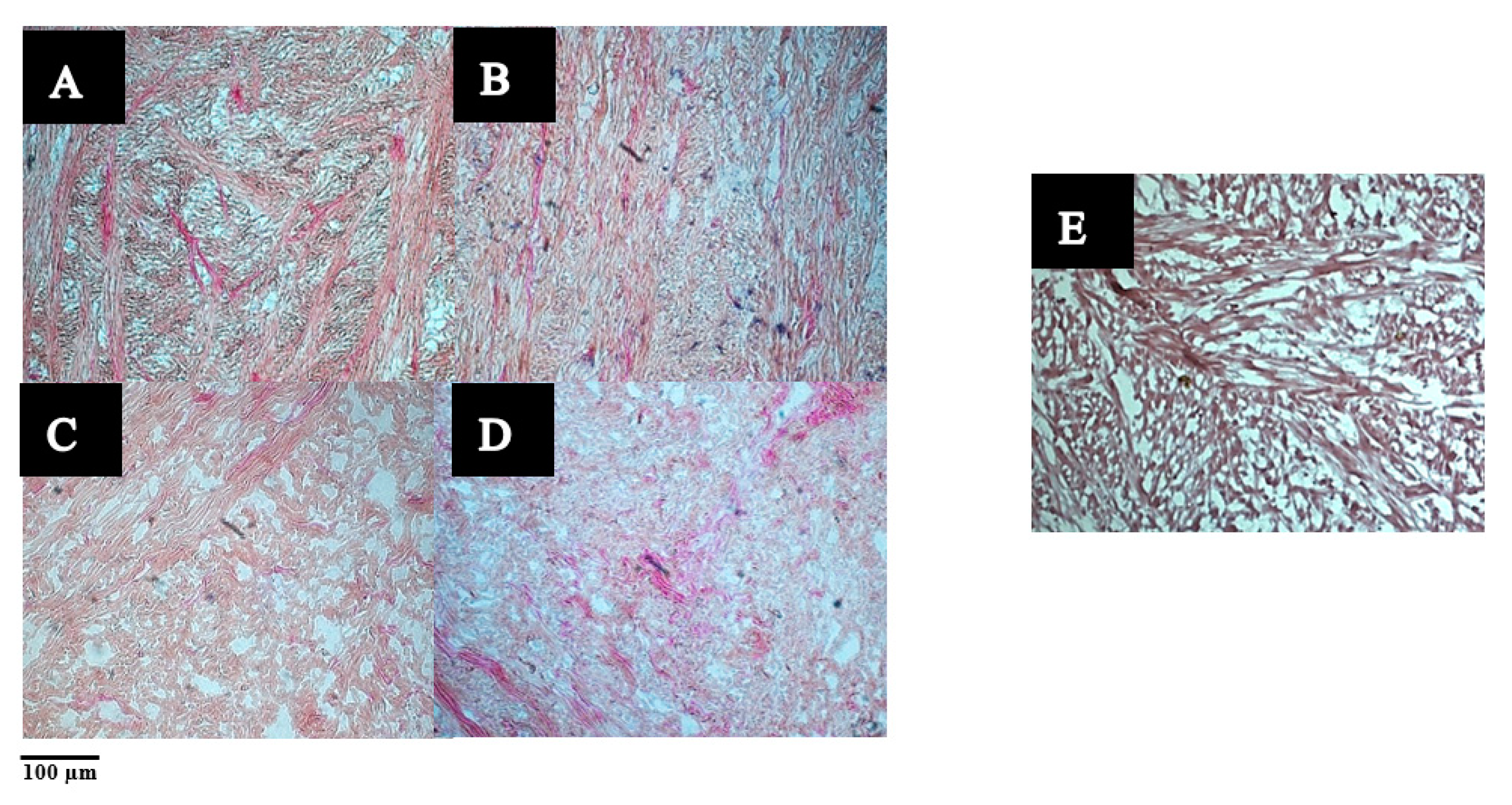
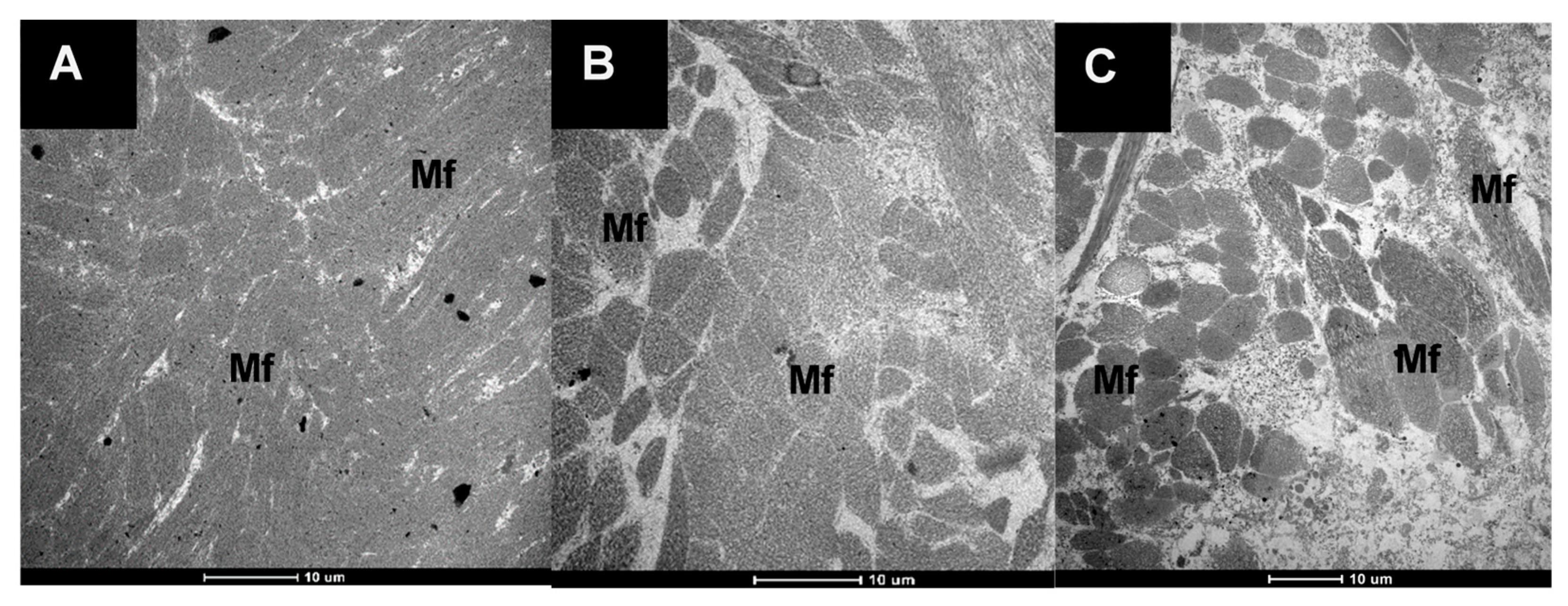
3.2.1. The Microstructure of Raw and Cooked Pāua Muscle
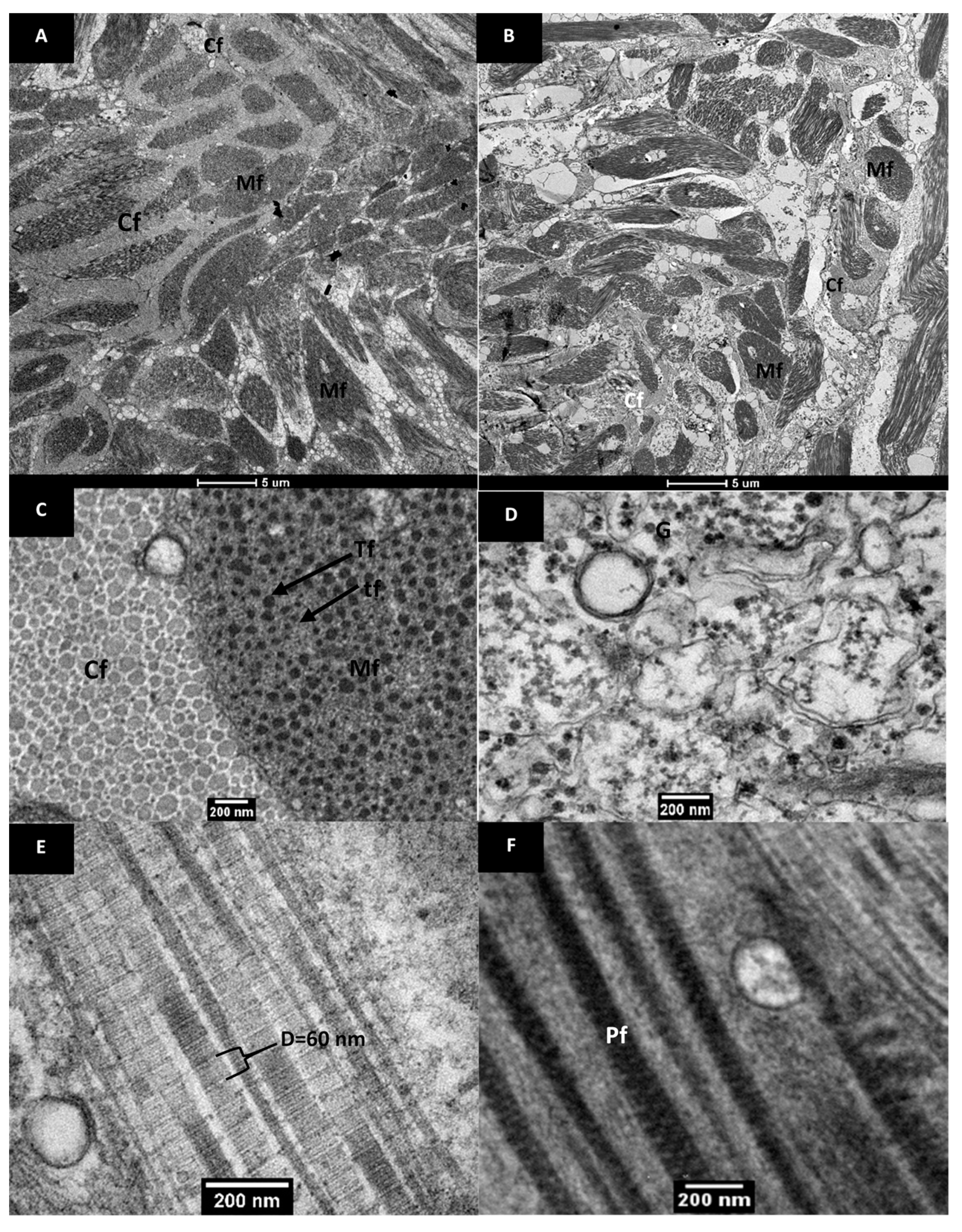
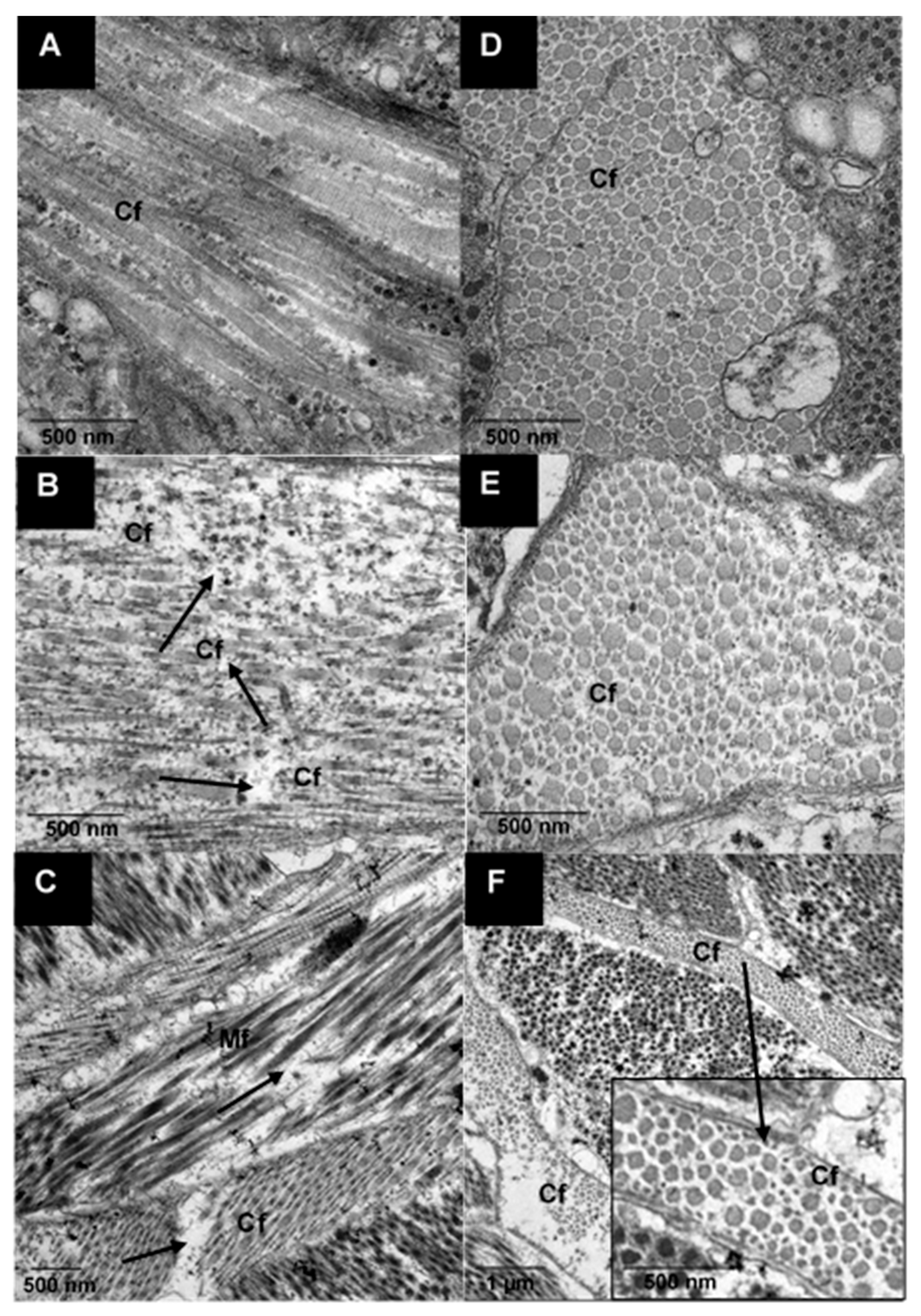
3.2.2. Ultrastructure of Raw and Cooked Pāua Muscle
3.3. pH, Cook Loss, Total Nitrogen Content and Thermal Properties
3.4. In Vitro Gastro-Small Intestinal Digestibility
3.4.1. Ninhydrin Assay
3.4.2. SDS-PAGE
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, J.; Stokes, F.; Dixon, H.; Hurren, K. The Economic Contribution of Commercial Fishing to the New Zealand Economy; BERL: Wellington, New Zealand, 2017; p. 32. [Google Scholar]
- Brown, M.R.; Sikes, A.L.; Elliott, N.G.; Tume, R.K. Physicochemical factors of abalone quality: A review. J. Shellfish Res. 2008, 27, 835–842. [Google Scholar] [CrossRef]
- Chiou, T.K.; Tsai, C.Y.; Lan, H.L. Chemical, physical and sensory changes of small abalone meat during cooking. Fish. Sci. 2004, 70, 867–874. [Google Scholar] [CrossRef]
- Sanchez-Brambila, G.; Lyon, B.; Huang, Y.; Lyon, C.; Gates, K. Sensory characteristics and instrumental texture attributes of abalones, Haliotis fulgens and cracherodii. J. Food Sci. 2002, 67, 1233–1239. [Google Scholar] [CrossRef]
- Zhu, B.; Dong, X.; Sun, L.; Xiao, G.; Chen, X.; Murata, Y.; Yu, C. Effect of thermal treatment on the texture and microstructure of abalone muscle (Haliotis discus). Food Sci. Biotechnol. 2011, 20, 1467–1473. [Google Scholar] [CrossRef]
- Dong, X.; Hou, Y.; Wang, Y.; Xu, X.; Wang, K.; Zhao, M.; Prakash, S.; Yu, C. Effect of temperature–time pretreatments on the texture and microstructure of abalone (Haliotis discus hannai). J. Texture Stud. 2018, 49, 503–511. [Google Scholar] [CrossRef]
- Gao, X.; Ogawa, H.; Tashiro, Y.; Iso, N. Rheological properties and structural changes in raw and cooked abalone meat. Fish. Sci. 2001, 67, 314–320. [Google Scholar] [CrossRef]
- Hatae, K.; Nakai, H.; Tanaka, C.; Shimada, A.; Watabe, S. Taste and texture of abalone meat after extended cooking. Fish. Sci. 1996, 62, 643–647. [Google Scholar] [CrossRef][Green Version]
- Hughes, B.H.; Greenberg, N.J.; Yang, T.C.; Skonberg, D.I. Effects of rigor status during high-pressure processing on the physical qualities of farm-raised abalone (Haliotis rufescens). J. Food Sci. 2015, 80, C40–C48. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Hong, G.-P. Optimization of hydrostatic pressure processing to extending shelf-life with minimal quality changes of refrigerated abalone. Food Sci. Technol. Res. 2016, 22, 419–428. [Google Scholar] [CrossRef][Green Version]
- Bewick, M.D.; Wells, R.M.G.; Wong, R.J. Free Amino Acid and Nucleotide Concentrations in New Zealand Abalone (Paua), Haliotis iris, Fed Casein-Based, Macroalgal, or Wild Diets. J. Aquat. Food Prod. Technol. 1997, 6, 57–69. [Google Scholar] [CrossRef]
- Preece, M.A. Sensory qualities of the New Zealand abalone, Haliotis iris, reared in offshore structures on artificial diets. N. Z. J. Mar. Freshw. Res. 2006, 40, 223–226. [Google Scholar] [CrossRef]
- Wells, R.M.G.; McShane, P.E.; Ling, N.; Wong, R.J.; Lee, T.O.C.; Baldwin, J. Effect of Wave Action on Muscle Composition, Metabolites and Growth Indices in the New Zealand Abalone, Paua (Haliotis iris), with Implications for Harvesting and Aquaculture. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 1998, 119, 129–136. [Google Scholar] [CrossRef]
- Zhu, X.; Kaur, L.; Boland, M.; Staincliffe, M. Actinidin pretreatment and sous vide cooking of beef brisket: Effects on meat microstructure, texture and in vitro protein digestibility. Meat Sci. 2018, 145, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A Review. Ultrason. Sonochemistry 2018. [Google Scholar] [CrossRef]
- Cepero-Betancourt, Y.; Oliva-Moresco, P.; Pasten-Contreras, A.; Tabilo-Munizaga, G.; Pérez-Won, M.; Moreno-Osorio, L.; Lemus-Mondaca, R. Effect of drying process assisted by high-pressure impregnation on protein quality and digestibility in red abalone (Haliotis rufescens). J. Food Sci. Technol. 2017, 54, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Olley, J.; Thrower, S.J. Abalone-an esoteric food. In Advances in Food Research; Elsevier: Amsterdam, The Netherlands, 1977; Volume 23, pp. 143–186. [Google Scholar]
- Cárcel, J.; Benedito, J.; Bon, J.; Mulet, A. High intensity ultrasound effects on meat brining. Meat Sci. 2007, 76, 611–619. [Google Scholar] [CrossRef]
- Chian, F.M.; Kaur, L.; Oey, I.; Hodgkinson, S.; Boland, M.; Astruc, T. Effect of Pulsed Electric Fields (PEF) on the ultrastructure and in vitro protein digestibility of bovine longissimus thoracis. LWT 2019, 103, 253–259. [Google Scholar] [CrossRef]
- Culling, C.F.A. Chapter 19—Tissues requiring special treatment or techniques. In Handbook of Histopathological and Histochemical Techniques, 3rd ed.; Culling, C.F.A., Ed.; Butterworth-Heinemann; Elsevier: Amsterdam, The Netherlands, 1974; pp. 405–466. [Google Scholar] [CrossRef]
- Moore, S. Amino acid analysis: Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem. 1968, 243, 6281–6283. [Google Scholar]
- AOAC. Protein (crude) determination in animal feed: Copper catalyst Kjeldahl method (984.13). In AOAC Official Method; AOAC International: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Kaur, L.; Astruc, T.; Venien, A.; Loison, O.; Cui, J.; Irastorza, M.; Boland, M. High pressure processing of meat: Effects on ultrastructure and protein digestibility. Food Funct. 2016, 7, 2389–2397. [Google Scholar] [CrossRef]
- Olaechea, R.P.; Ushio, H.; Watabe, S.; Takada, K.; Hatae, K. Toughness and Collagen Content of Abalone Muscles. Biosci. Biotechnol. Biochem. 1993, 57, 6–11. [Google Scholar] [CrossRef]
- Hanson, J.; Lowy, J. Structure of smooth muscles. Nature 1957, 180, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Millman, B.M. Mechanisms of Contraction in Molluscan Muscle. Am. Zool. 1967, 7, 583–591. [Google Scholar] [CrossRef]
- Paniagua, R.; Royuela, M.; Garcia-Anchuelo, R.; Fraile, B. Ultrastructure of invertebrate muscle cell types. Histol. Histopathol. 1996, 11, 181–201. [Google Scholar] [PubMed]
- Azuma, N.; Asakura, A.; Yagi, K. Myosin from Molluscan Abalone, Haliotis discus: Isolation and Enzymatic Properties. J. Biochem. 1975, 77, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Oiseth, S.K.; Delahunty, C.; Cochet, M.; Lundin, L. Why is abalone so chewy? Structural characterization and relationship to textural attributes. J. Shellfish Res. 2013, 32, 73–79. [Google Scholar] [CrossRef]
- Ochiai, Y.; Kariya, Y.; Watabe, S.; Hashimoto, K. Heat-induced tendering of turban shell (Batillus cornutus) muscle. J. Food Sci. 1985, 50, 981–984. [Google Scholar] [CrossRef]
- Szent-Györgyi, A.G.; Cohen, C.; Kendrick-Jones, J. Paramyosin and the filaments of molluscan “catch” muscles: II. Native filaments: Isolation and characterization. J. Mol. Biol. 1971, 56, 239–258. [Google Scholar] [CrossRef]
- Chang, H.-J.; Xu, X.-L.; Zhou, G.-H.; Li, C.-B.; Huang, M. Effects of Characteristics Changes of Collagen on Meat Physicochemical Properties of Beef Semitendinosus Muscle during Ultrasonic Processing. Food Bioprocess Technol. 2012, 5, 285–297. [Google Scholar] [CrossRef]
- Christensen, M.; Tørngren, M.A.; Gunvig, A.; Rozlosnik, N.; Lametsch, R.; Karlsson, A.H.; Ertbjerg, P. Injection of marinade with actinidin increases tenderness of porcine M. biceps femoris and affects myofibrils and connective tissue. J. Sci. Food Agric. 2009, 89, 1607–1614. [Google Scholar] [CrossRef]
- Tanikawa, E.; Yamashita, J. Chemical Studies on the meat of abalone (Haliotis discus hannai INO)-I. Bull. Fac. Fish. Hokkaido Univ. 1961, 12, 210–238. [Google Scholar]
- James, D.; Olley, J. Studies on the processing of abalone. III.The effect of processing variables on abalone texture with special reference to brining. Food Technol. Aust. 1971, 23, 444–449. [Google Scholar]
- Kaur, L.; Hui, S.X.; Boland, M. Changes in Cathepsin Activity during Low-Temperature Storage and Sous Vide Processing of Beef Brisket. Food Sci. Anim. Resour. 2020, 40, 415. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, E.; Wellington, G.; Sherbon, J. Lo-temperature, long-time heating of bovine muscle 1. Changes in tenderness, water-binding capacity, pH and amount of water-soluble components. J. Food Sci. 1970, 35, 175–177. [Google Scholar] [CrossRef]
- Hamm, R.; Deatherage, F. Chnages in hydration, solubility and charges of muscle proteins during heating of meat. J. Food Sci. 1960, 25, 587–610. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Boutrou, R.; Dupont, D.; Ménard, O.; Carrière, F.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Suzuki, M.; Kobayashi, Y.; Hiraki, Y.; Nakata, H.; Shiomi, K. Paramyosin of the disc abalone Haliotis discus discus: Identification as a new allergen and cross-reactivity with tropomyosin. Food Chem. 2011, 124, 921–926. [Google Scholar] [CrossRef]
- Oakes, F.R.; Ponte, R.D. The abalone market: Opportunities for cultured abalone. Aquaculture 1996, 140, 187–195. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.C.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef]
- Jayasooriya, S.D.; Torley, P.J.; D’Arcy, B.R.; Bhandari, B.R. Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 2007, 75, 628–639. [Google Scholar] [CrossRef]
- Pohlman, F.; Dikeman, M.; Zayas, J. The effect of low-intensity ultrasound treatment on shear properties, color stability and shelf-life of vacuum-packaged beef semitendinosus and biceps femoris muscles. Meat Sci. 1997, 45, 329–337. [Google Scholar] [CrossRef]
- Aranceta-Garza, F.; Perez-Enriquez, R.; Cruz, P. PCR-SSCP method for genetic differentiation of canned abalone and commercial gastropods in the Mexican retail market. Food Control 2011, 22, 1015–1020. [Google Scholar] [CrossRef]
- Kaur, L.; Maudens, E.; Haisman, D.R.; Boland, M.J.; Singh, H. Microstructure and protein digestibility of beef: The effect of cooking conditions as used in stews and curries. LWT Food Sci. Technol. 2014, 55, 612–620. [Google Scholar] [CrossRef]
- Straadt, I.K.; Rasmussen, M.; Andersen, H.J.; Bertram, H.C. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss–A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007, 75, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.Y.; Marks, B.P. Effect of meat temperature on proteins, texture, and cook loss for ground chicken breast patties1. Poult. Sci. 2000, 79, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.-L.; Aubry, L.; Ferreira, C.; Daudin, J.-D.; Gatellier, P.; Rémond, D.; Santé-Lhoutellier, V. Cooking Temperature Is a Key Determinant of in Vitro Meat Protein Digestion Rate: Investigation of Underlying Mechanisms. J. Agric. Food Chem. 2012, 60, 2569–2576. [Google Scholar] [CrossRef]
- Sweeney, P.J.; Walker, J.M. Proteolytic Enzymes for Peptide Production. In Enzymes of Molecular Biology; Burrell, M.M., Ed.; Humana Press: Totowa, NJ, USA, 1993; pp. 277–303. [Google Scholar] [CrossRef]
- Smith, M.E.; Morton, D.G. 3—The stomach: Basic functions. In The Digestive System, 2nd ed.; Smith, M.E., Morton, D.G., Eds.; Churchill Livingstone; Elsevier: Amsterdam, The Netherlands, 2010; pp. 39–50. [Google Scholar] [CrossRef]
- Santé-Lhoutellier, V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of Meat Cooking on Physicochemical State and in Vitro Digestibility of Myofibrillar Proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. [Google Scholar] [CrossRef]
- Shi, L.; Hao, G.; Chen, J.; Ma, S.; Weng, W. Nutritional evaluation of Japanese abalone (Haliotis discus hannai Ino) muscle: Mineral content, amino acid profile and protein digestibility. Food Res. Int. 2020, 129, 108876. [Google Scholar] [CrossRef]
- Quansah, J.K.; Udenigwe, C.C.; Saalia, F.K.; Yada, R.Y. The Effect of Thermal and Ultrasonic Treatment on Amino Acid Composition, Radical Scavenging and Reducing Potential of Hydrolysates Obtained from Simulated Gastrointestinal Digestion of Cowpea Proteins. Plant. Foods Hum. Nutr. 2013, 68, 31–38. [Google Scholar] [CrossRef]
 and cooked (canned) control
and cooked (canned) control  and cooked ultrasound pre-treated
and cooked ultrasound pre-treated  pāua determined based on ninhydrin-reactive free amino N released during gastro-small intestinal digestion (n = 3. Ultrasound treatment was done in water for 5 min followed by soaking in water for 24 h at 4 °C. Digestion times 0, 30 and 60 min represent 2, 30 and 60 min of digestion in the gastric phase following 2 min of oral digestion phase; 70, 120 and 180 represent 10, 60 and 120 min of digestion in the small-intestinal phase following 60 min of gastric and 2 min of oral digestion phases. Different letters in the same column represent a significant difference (p < 0.05).
pāua determined based on ninhydrin-reactive free amino N released during gastro-small intestinal digestion (n = 3. Ultrasound treatment was done in water for 5 min followed by soaking in water for 24 h at 4 °C. Digestion times 0, 30 and 60 min represent 2, 30 and 60 min of digestion in the gastric phase following 2 min of oral digestion phase; 70, 120 and 180 represent 10, 60 and 120 min of digestion in the small-intestinal phase following 60 min of gastric and 2 min of oral digestion phases. Different letters in the same column represent a significant difference (p < 0.05).
 and cooked (canned) control
and cooked (canned) control  and cooked ultrasound pre-treated
and cooked ultrasound pre-treated  pāua determined based on ninhydrin-reactive free amino N released during gastro-small intestinal digestion (n = 3. Ultrasound treatment was done in water for 5 min followed by soaking in water for 24 h at 4 °C. Digestion times 0, 30 and 60 min represent 2, 30 and 60 min of digestion in the gastric phase following 2 min of oral digestion phase; 70, 120 and 180 represent 10, 60 and 120 min of digestion in the small-intestinal phase following 60 min of gastric and 2 min of oral digestion phases. Different letters in the same column represent a significant difference (p < 0.05).
pāua determined based on ninhydrin-reactive free amino N released during gastro-small intestinal digestion (n = 3. Ultrasound treatment was done in water for 5 min followed by soaking in water for 24 h at 4 °C. Digestion times 0, 30 and 60 min represent 2, 30 and 60 min of digestion in the gastric phase following 2 min of oral digestion phase; 70, 120 and 180 represent 10, 60 and 120 min of digestion in the small-intestinal phase following 60 min of gastric and 2 min of oral digestion phases. Different letters in the same column represent a significant difference (p < 0.05).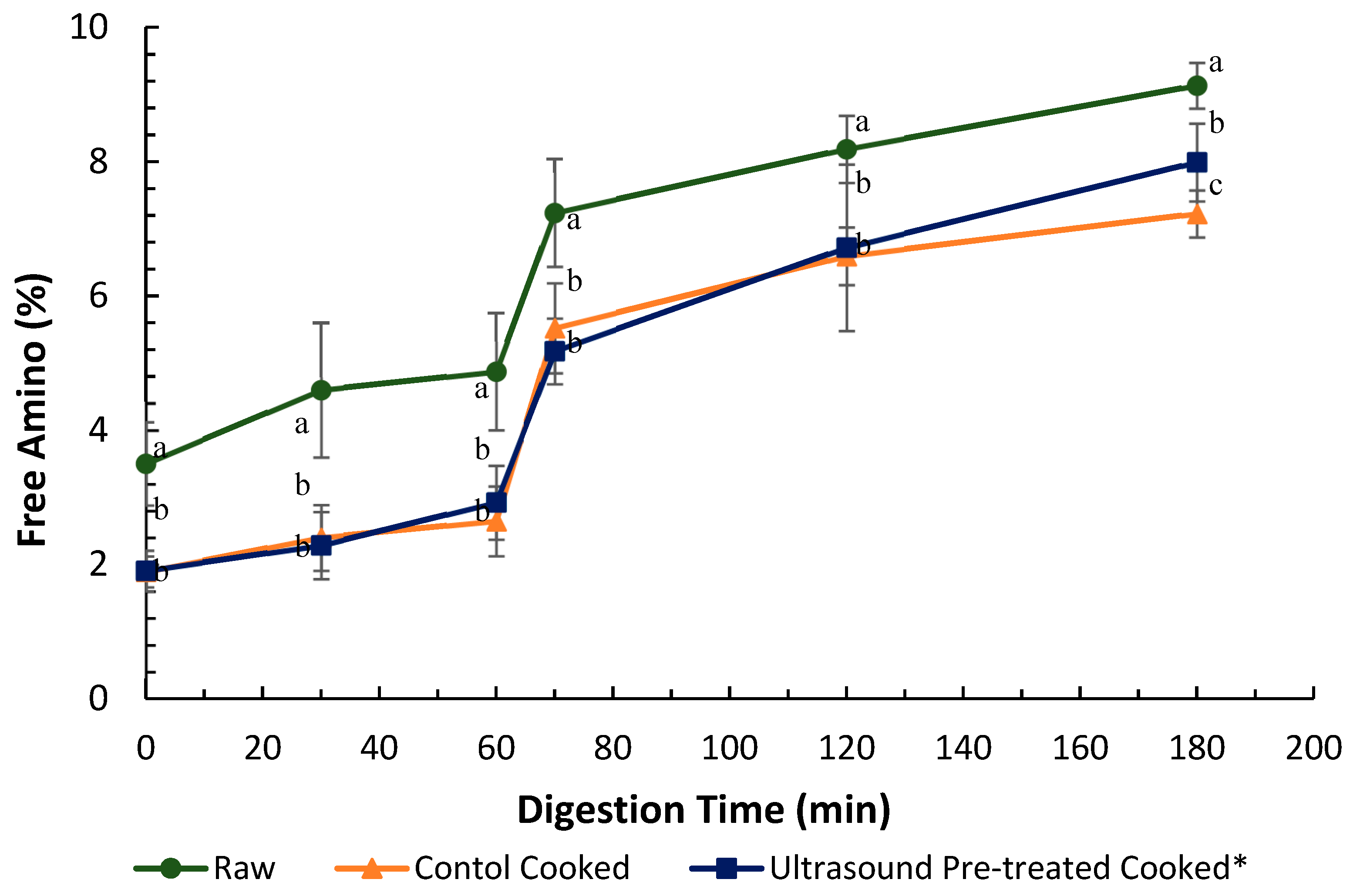
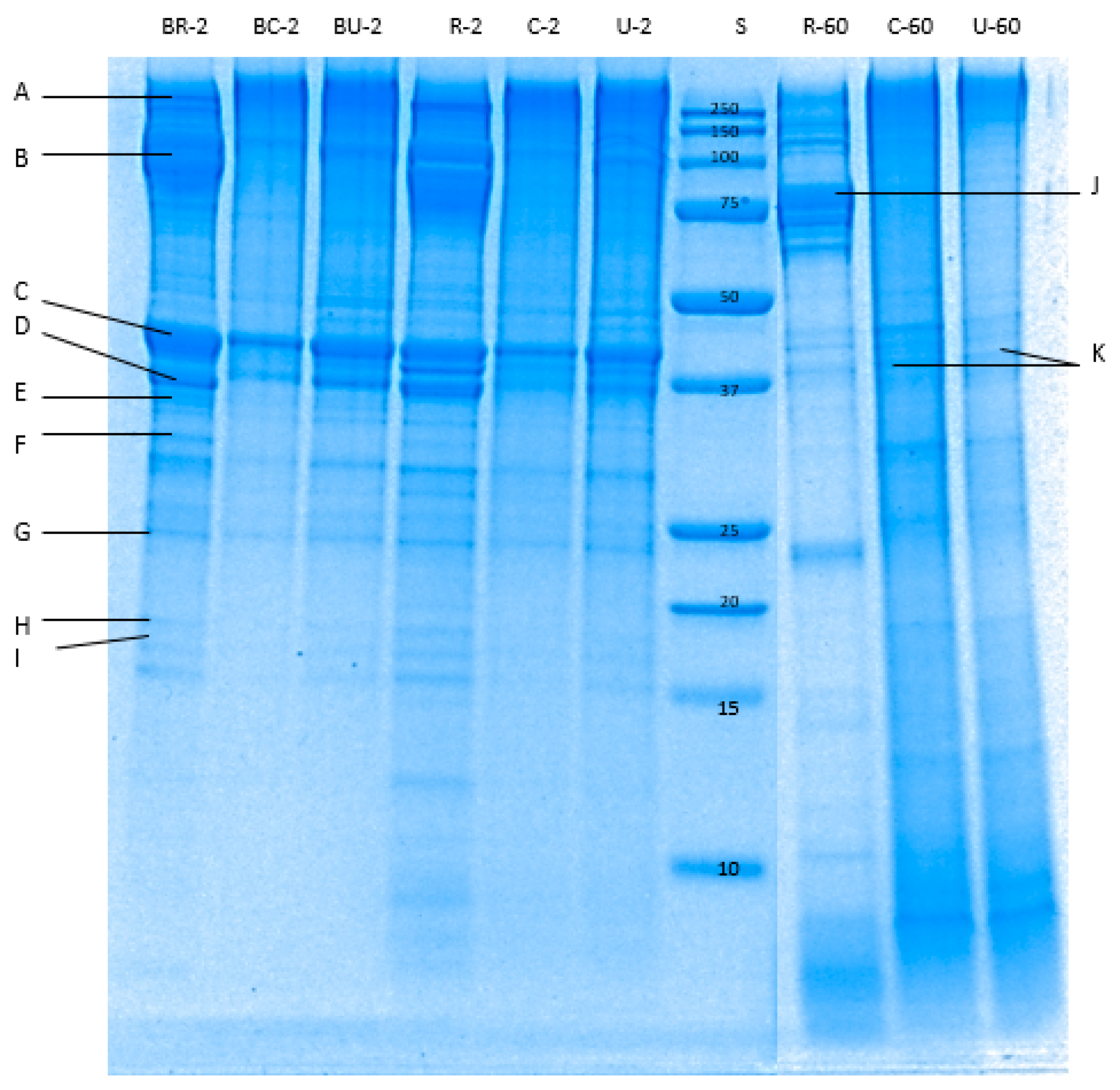
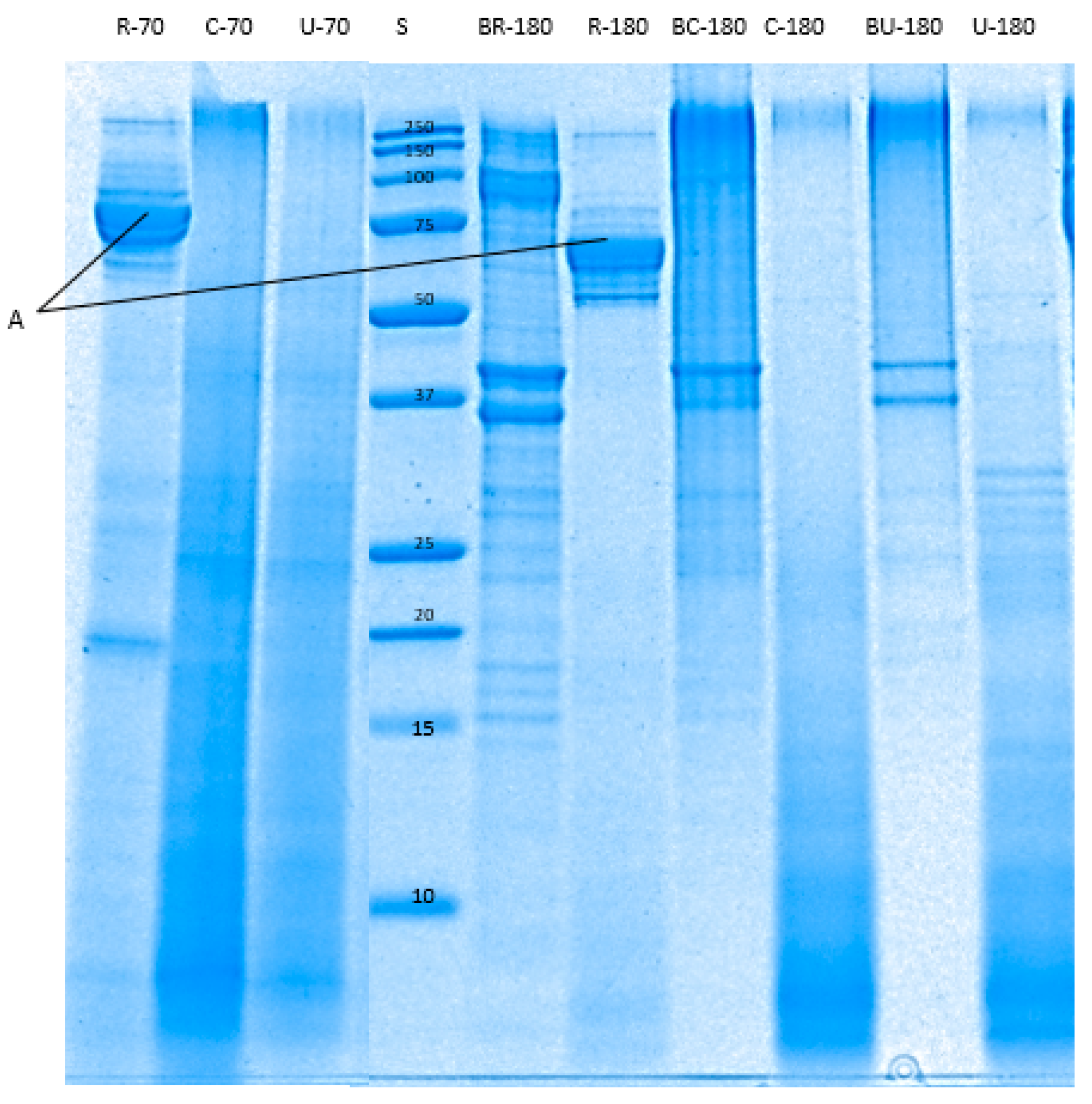
| Samples | pH of Abalone | Total Nitrogen (%) of Abalone | Cook Loss (%) |
|---|---|---|---|
| Control raw | 6.05 ± 0.13 b | 3.06 ± 0.14 a | - |
| Control cooked | 6.63 ± 0.02 a | 2.49 ± 0.24 c | 6.55 ± 0.23 a |
| Ultrasound pre-treated cooked | 6.68 ± 0.10 a | 2.72 ± 0.08 b | 9.14 ± 2.89 a |
| p value | <0.001 | <0.001 | 0.196 |
| Digestion Time (min) | Soluble Nitrogen (%) | ||
|---|---|---|---|
| Control Raw | Control Cooked | Ultrasound Cooked | |
| 2 | 34.09 ± 3.4 a | 18.06 ± 5.10 b | 15.23 ± 1.07 b |
| 30 | 46.84 ±5.35 a | 33.90 ± 3.73 ab | 25.49 ± 9.83 b |
| 60 | 52.79 ± 5.82 a | 42.03 ± 7.98 a | 36.61 ± 10.57 a |
| 70 | 66.65 ± 4.73 a | 62.10 ± 4.89 ab | 50.97 ± 6.96 b |
| 120 | 72.49 ± 6.83 a | 75.82 ± 8.38 a | 68.15 ± 3.46 a |
| 180 | 84.72 ± 11.82 a | 76.21 ± 6.52 a | 74.73 ± 4.13 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagarinao, N.C.; Kaur, L.; Boland, M. Effects of Ultrasound Treatments on Tenderness and In Vitro Protein Digestibility of New Zealand Abalone, Haliotis iris. Foods 2020, 9, 1122. https://doi.org/10.3390/foods9081122
Bagarinao NC, Kaur L, Boland M. Effects of Ultrasound Treatments on Tenderness and In Vitro Protein Digestibility of New Zealand Abalone, Haliotis iris. Foods. 2020; 9(8):1122. https://doi.org/10.3390/foods9081122
Chicago/Turabian StyleBagarinao, Norma Cecille, Lovedeep Kaur, and Mike Boland. 2020. "Effects of Ultrasound Treatments on Tenderness and In Vitro Protein Digestibility of New Zealand Abalone, Haliotis iris" Foods 9, no. 8: 1122. https://doi.org/10.3390/foods9081122
APA StyleBagarinao, N. C., Kaur, L., & Boland, M. (2020). Effects of Ultrasound Treatments on Tenderness and In Vitro Protein Digestibility of New Zealand Abalone, Haliotis iris. Foods, 9(8), 1122. https://doi.org/10.3390/foods9081122







