Putting the Spring back into the Hare (Pedetes capensis): Meat Chemical Composition of an Underutilized Protein Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Layout and Sample Collection
2.2. Chemical Compositional Analyses
2.2.1. Proximate Analysis
2.2.2. Fatty Acid Analysis
2.3. Statistical Analysis
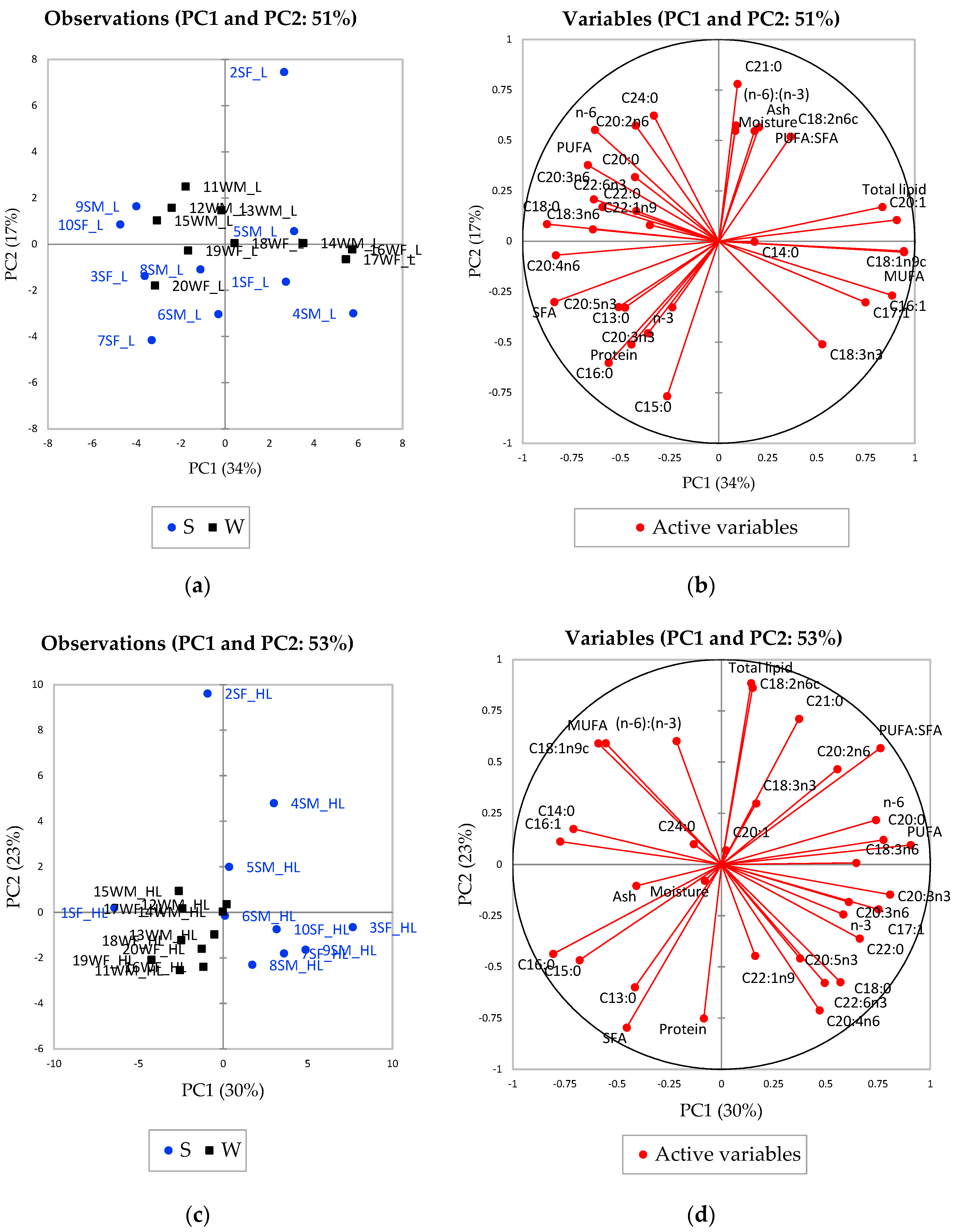
3. Results and Discussion
3.1. Carcass Characteristics
3.2. Chemical Composition
3.2.1. Proximate Composition
3.2.2. Fatty Acid Composition of the Springhare Meat
3.2.3. Effect of Season on the Fatty Acid Composition
3.2.4. Effect of Sex on the Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, L.C. The yield and nutritional value of meat from African ungulates, camelidae, rodents, ratites and reptiles. Meat Sci. 2008, 80, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A. Fatty-acid ratios in free-living and domestic animals. Lancet 1968, 291, 1329–1333. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Wiklund, E. Game and venison—Meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef]
- Blaška, J.; Gašparík, J.; Šmehýl, P.; Gondeková, M. Comparison of basic nutritive components of venison in selected species of hoofed game. J. Cent. Eur. Agric. 2016, 17, 1233–1240. [Google Scholar] [CrossRef][Green Version]
- Neethling, J.; Hoffman, L.C.; Muller, M. Factors influencing the flavour of game meat: A review. Meat Sci. 2016, 113, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Szendrő, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef]
- Li, S.; Zeng, W.; Li, R.; Hoffman, L.C.; He, Z.; Sun, Q.; Li, H. Rabbit meat production and processing in China. Meat Sci. 2018, 145, 320–328. [Google Scholar] [CrossRef]
- Tomasevic, N.S.; Solowiej, B.; Zdolec, N.; Skunca, D.; Krocko, M.; Nedomova, S.; Kolaj, R.; Aleksiev, G.; Djekic, I. Consumers’ perceptions, attitudes and perceived quality of game meat in ten European countries. Meat Sci. 2018, 142, 5–13. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Vosloo, C.; Nkhabutlane, P.; De Schutte, W. Associations with rabbits and rabbit meat of three different ethnic groups in Stellenbosch, South Africa. J. Fam. Ecol. Cons. Sci. 2005, 33, 63–72. [Google Scholar] [CrossRef]
- Marescotti, M.E.; Caputo, V.; Demartini, E.; Gaviglio, A. Discovering market segments for hunted wild game meat. Meat Sci. 2019, 149, 163–176. [Google Scholar] [CrossRef]
- Peinke, D.; Wilson, B.; Anderson, C.A. Conservation assessment of Pedetes capensis. In The Red List of Mammals of South Africa, Swaziland and Lesotho; Child, M.F., Roxburgh, L., Do Linh San, E., Raimondo, D., Davies-Mostert, H.T., Eds.; South African National Biodiversity Institute and Endangered Wildlife Trust: Johannesburg, South Africa, 2016; pp. 1–7. [Google Scholar]
- Dupont, B. Original Photo of the Spring Hare (Pedetes capensis). Available online: https://www.flickr.com/photos/berniedup/6042009463 (accessed on 24 June 2020).
- Lee, R.B. Ecology of a contemporary San people. In The Bushmen: San hunters and herders of Southern Africa; Tobias, P., Biesele, M., Eds.; Human & Rousseau: Cape Town, South Africa, 1978; pp. 94–114. Available online: http://hdl.handle.net/1807/18025 (accessed on 24 June 2020).
- Butynski, T.M. Life history and economic value of the springhare (Pedetes capensis Forster) in Botswana. Botsw. Notes Rec. 1973, 5, 200–213. [Google Scholar]
- Board on Science and Technology for International Development National Research Council (BOSTID). Microlivestock: Little-Known Small Animals with a Promising Economic Future; National Academy of Sciences, National Academy Press: Washington, DC, USA, 1991. [Google Scholar]
- Blasco, A.; Ouhayoun, J.; Masoero, G. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 1993, 1, 3–10. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Lee, C.M.; Trevino, B.; Chaiyawat, M. A simple and rapid solvent extraction method for determining total lipids in fish tissue. J. AOAC Int. 1996, 79, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- North, M.; Dalle Zotte, A.; Hoffman, L.C. Growth, carcass and meat quality traits of two South African meat rabbit breeds. S. Afr. J. Anim. Sci. 2019, 49, 684–696. [Google Scholar] [CrossRef]
- Butynski, T.M. Body and organ growth of the springhare. Acta Theriol. 1979, 24, 431–448. [Google Scholar] [CrossRef]
- Peinke, D.M.; Brown, C.R. Metabolism and thermoregulation in the springhare (Pedetes capensis). J. Comp. Physiol. B 2003, 173, 347–353. [Google Scholar] [CrossRef]
- Vincenti, A.; Ragni, M.; Di Summa, A.; Marsico, G.; Vonghia, G. Influence of feeds and rearing system on the productive performances and the chemical and fatty acid composition of hare meat. Food Sci. Technol. Int. 2003, 9, 279–284. [Google Scholar] [CrossRef]
- Króliczewska, B.; Miśta, D.; Korzeniowska, M.; Pecka-Kiełb, E.; Zachwieja, A. Comparative evaluation of the quality and fatty acid profile of meat from brown hares and domestic rabbits offered the same diet. Meat Sci. 2018, 145, 292–299. [Google Scholar] [CrossRef]
- North, M.; Dalle Zotte, A.; Hoffman, L.C. The effects of dietary quercetin supplementation and sex on the fatty acid profile of rabbit meat, dissectible fat and caecotrophes. Meat Sci. 2019, 157, 107888. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, L.; JairDiaz, G. Fatty acid profile differences among the muscle tissue of three rodents (Hydroahoerus hidrochaeris, Cuniculus paca and Cavia porcellus) and one lagomorph (Oryctolagus cuniculus). J. Food Nutr. Res. 2014, 2, 744–748. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Brit. J. Nutr. 1997, 78 (Suppl. 1), S49–S60. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Hernandez, P.; Pla, M.; Oliver, M.A.; Blasco, A. Relationships between meat quality measurements in rabbits fed with three diets of different type and content. Meat Sci. 2000, 55, 379–384. [Google Scholar] [CrossRef]
- Cloete, S.W.P.; Olivier, J.J. South African industry. In The International Sheep and Wool Handbook; Cottle, D.J., Ed.; Nottingham University Press: Nottingham, UK, 2010; pp. 95–112. [Google Scholar]
- Priolo, A.; Micol, D.; Agabriel, J. Effects of grass feeding systems on ruminant meat colour and flavour: A review. Anim. Res. 2001, 50, 185–200. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Richardson, R.I.; Sheard, P.R. Manipulating meat quality and composition. Proc. Nutr. Soc. 1999, 58, 363–370. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Interim Summary of Conclusions and Dietary Recommendations on Total Fat & Fatty Acids; From The Joint Fao/Who Expert Consultation on Fats and Fatty Acids in Human Nutrition; WHO: Geneva, Switzerland, 10–14 November 2008; Available online: https://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf (accessed on 24 June 2020).
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef]
- Lazzaroni, C.; Biagini, D.; Lussiana, C. Fatty acid composition of meat and perirenal fat in rabbits from two different rearing systems. Meat Sci. 2009, 83, 135–139. [Google Scholar] [CrossRef]

| Season | Sex | |||||
|---|---|---|---|---|---|---|
| Carcass Characteristics | Summer (n = 10) | Winter (n = 10) | p | Male (n = 10) | Female (n = 10) | p |
| Dead weight (kg) | 2.4 ± 0.2 | 2.6 ± 0.1 | 0.453 | 2.6 ± 0.1 | 2.4 ± 0.1 | 0.227 |
| Carcass weight (kg) | 1.6 ± 0.1 | 1.6 ± 0.0 | 0.821 | 1.6 ± 0.1 | 1.5 ± 0.1 | 0.251 |
| Dress out percentage 1 (%) | 64.3 ± 0.7 | 62.1 ± 0.7 | 0.039 | 63.0 ± 0.9 | 63.5 ± 0.6 | 0.691 |
| Total weight 2 (g) | 1450.3 ± 92.3 | 1485.8 ± 46.0 | 0.735 | 1514.3 ± 74.6 | 1421.9 ± 68.6 | 0.374 |
| Forearms (both, g) | 92.6 ± 5.3 | 97.2 ± 3.3 | 0.476 | 98.4 ± 4.1 | 91.4 ± 4.5 | 0.261 |
| Loins 3 (meat, g) | 206.7 ± 18.2 | 215.2 ± 12.5 | 0.703 | 226.8 ± 18.5 | 195.1 ± 10.0 | 0.148 |
| Hind legs (intact, g) | 646.1 ± 32.9 | 617.4 ± 24.2 | 0.492 | 649.8 ± 31.3 | 613.7 ± 25.8 | 0.385 |
| Hind legs (bones, g) | 123.3 ± 7.1 | 113.0 ± 7.2 | 0.319 | 116.7 ± 8.9 | 119.7 ± 5.2 | 0.776 |
| Hind legs (meat, g) | 519.0 ± 29.6 | 475.3 ± 24.9 | 0.272 | 502.9 ± 33.2 | 491.4 ± 22.0 | 0.777 |
| Tail (g) | 70.9 ± 3.6 | 61.1 ± 1.8 | 0.026 | 67.1 ± 3.7 | 65.0 ± 2.7 | 0.653 |
| Rest of carcass 4 (g) | 428.5 ± 43.8 | 492.9 ± 25.3 | 0.219 | 468.1 ± 34.0 | 453.2 ± 40.0 | 0.780 |
| Season | Sex | |||||
|---|---|---|---|---|---|---|
| Parameters | Summer (n = 10) | Winter (n = 10) | p | Male (n = 10) | Female (n = 10) | p |
| Loin muscles 1 | ||||||
| Moisture | 75.1 ± 0.3 | 75.7 ± 0.3 | 0.140 | 75.4 ± 0.3 | 75.4 ± 0.3 | 0.991 |
| Protein | 22.7 ± 0.3 | 22.8 ± 0.3 | 0.855 | 22.7 ± 0.2 | 22.8 ± 0.4 | 0.867 |
| Total lipid | 1.3 ± 0.1 | 1.2 ± 0.1 | 0.611 | 1.3 ± 0.1 | 1.2 ± 0.1 | 0.683 |
| Ash | 1.0± 0.0 | 1.1 ± 0.0 | 0.002 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.933 |
| Hind leg muscles | ||||||
| Moisture | 75.5 ± 0.1 | 76.0 ± 0.2 | 0.038 | 75.6 ± 0.2 | 75.9 ± 0.2 | 0.237 |
| Protein | 22.2 ± 0.3 | 22.5 ± 0.2 | 0.372 | 22.5 ± 0.2 | 22.2 ± 0.3 | 0.277 |
| Total lipid | 1.3 ± 0.1 | 1.1 ± 0.1 | 0.108 | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.665 |
| Ash | 0.9 ± 0.0 | 1.1 ± 0.0 | 0.005 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.183 |
| Fatty Acids | Name | Mean | Standard Error |
|---|---|---|---|
| C13:0 | Tridecylic acid | 0.04 | 0.005 |
| C14:0 | Myristic acid | 0.27 | 0.037 |
| C15:0 | Pentadecylic acid | 0.22 | 0.011 |
| C16:0 | Palmitic acid | 19.45 | 0.454 |
| C18:0 | Stearic acid | 19.53 | 0.348 |
| C20:0 | Arachidic acid | 0.07 | 0.003 |
| C21:0 | Heneicosylic acid | 0.33 | 0.023 |
| C22:0 | Behenic acid | 0.12 | 0.009 |
| C24:0 | Lignoceric acid | 0.21 | 0.014 |
| C16:1 | Palmitoleic acid | 0.30 | 0.025 |
| C17:1 | Heptadecenoic acid | 0.20 | 0.021 |
| C18:1n9c | Oleic acid | 12.69 | 0.765 |
| C20:1 | Gondoic acid | 0.17 | 0.018 |
| C22:1n9 | Erucic acid | 0.14 | 0.013 |
| C18:2n6c | Linoleic acid | 23.72 | 0.650 |
| C18:3n6 | γ-Linolenic acid | 0.25 | 0.021 |
| C18:3n3 | α-Linolenic acid | 1.52 | 0.186 |
| C20:2n6 | Eicosadienoic acid | 0.05 | 0.004 |
| C20:3n6 | Dihomo-γ-linolenic acid | 1.10 | 0.039 |
| C20:3n3 | Eicosatrienoic acid | 0.26 | 0.017 |
| C20:4n6 | Arachidonic acid | 15.14 | 0.632 |
| C20:5n3 | Eicosapentaenoic acid | 0.62 | 0.050 |
| C22:6n3 | Docosahexaenoic acid | 3.47 | 0.178 |
| ΣSFA | Total saturated fatty acids | 40.33 | 0.560 |
| ΣMUFA | Total mono-unsaturated fatty acids | 13.55 | 0.791 |
| ΣPUFA | Total polyunsaturated fatty acids | 46.12 | 0.623 |
| PUFA:SFA | Polyunsaturated fatty acid:Saturated fatty acid | 1.15 | 0.024 |
| Σn-6 | Total n-6 PUFA | 40.25 | 0.561 |
| Σn-3 | Total n-3 PUFA | 5.87 | 0.250 |
| (n-6):(n-3) | Total omega 6:Total omega 3 | 7.46 | 0.429 |
| Loin Muscles (n = 20) 1 | Hind Leg Muscles (n = 20) | ||||
|---|---|---|---|---|---|
| Season | Season | p | |||
| Fatty Acids | Summer | Winter | Summer | Winter | |
| C13:0 | 0.06 a ± 0.008 | 0.03 b ± 0.010 | 0.03 b ± 0.010 | 0.06 a ± 0.007 | 0.012 |
| C14:0 | 0.37 ± 0.096 | 0.23 ± 0.037 | 0.23 ± 0.094 | 0.23 ± 0.053 | 0.158 |
| C15:0 | 0.24 ± 0.017 | 0.23 ± 0.016 | 0.18 ± 0.026 | 0.23 ± 0.024 | 0.195 |
| C16:0 | 20.71 a ± 0.705 | 19.74 ab ± 0.743 | 17.21 b ± 1.072 | 20.14 ab ± 0.760 | 0.043 |
| C18:0 | 19.13 ± 0.759 | 19.15 ± 0.555 | 19.88 ± 0.977 | 19.97 ± 0.440 | 0.712 |
| C20:0 | 0.06 b ± 0.005 | 0.06 b ± 0.004 | 0.09 a ± 0.007 | 0.05 b ± 0.004 | <0.001 |
| C21:0 | 0.38 ± 0.060 | 0.30 ± 0.021 | 0.39 ± 0.058 | 0.28 ± 0.019 | 0.541 |
| C22:0 | 0.09 b ± 0.010 | 0.12 ab ± 0.015 | 0.15 a ± 0.027 | 0.13 a ± 0.012 | 0.049 |
| C24:0 | 0.20 ab ± 0.029 | 0.17 b ± 0.012 | 0.19 ab ± 0.026 | 0.29 a ± 0.025 | 0.009 |
| C16:1 | 0.26 ± 0.055 | 0.30 ± 0.039 | 0.30 ± 0.064 | 0.33 ± 0.044 | 0.685 |
| C17:1 | 0.12 b ± 0.024 | 0.14 b ± 0.022 | 0.36 a ± 0.048 | 0.17 ab ± 0.012 | <0.001 |
| C18:1n9c | 11.40 ± 1.593 | 14.61 ± 1.981 | 12.07 ± 1.424 | 12.68 ± 1.001 | 0.656 |
| C20:1 | 0.15 ± 0.024 | 0.19 ± 0.030 | 0.22 ± 0.057 | 0.14 ± 0.016 | 0.557 |
| C22:1n9 | 0.14 ± 0.016 | 0.17 ± 0.038 | 0.11 ± 0.027 | 0.13 ± 0.016 | 0.426 |
| C18:2n6c | 25.16 ± 1.495 | 21.71 ± 0.833 | 25.41 ± 1.672 | 22.59 ± 0.671 | 0.121 |
| C18:3n6 | 0.19 b ± 0.025 | 0.26 ab ± 0.031 | 0.38 a ± 0.053 | 0.17 b ± 0.015 | 0.005 |
| C18:3n3 | 1.82 ab ± 0.397 | 0.95 b ± 0.157 | 2.30 a ± 0.512 | 1.01 b ± 0.114 | 0.026 |
| C20:2n6 | 0.04 ab ± 0.004 | 0.05 ab ± 0.004 | 0.07 a ± 0.010 | 0.03 b ± 0.005 | 0.018 |
| C20:3n6 | 1.14 ± 0.092 | 1.12 ± 0.068 | 1.09 ± 0.095 | 1.03 ± 0.055 | 0.780 |
| C20:3n3 | 0.29 ab ± 0.030 | 0.17 b ± 0.019 | 0.34 a ± 0.042 | 0.23 ab ± 0.021 | 0.007 |
| C20:4n6 | 14.12 ± 1.354 | 16.15 ± 1.280 | 14.36 ± 1.603 | 15.93 ± 0.713 | 0.665 |
| C20:5n3 | 0.67 ± 0.132 | 0.58 ± 0.073 | 0.66 ± 0.120 | 0.56 ± 0.072 | 0.853 |
| C22:6n3 | 3.23 ± 0.380 | 3.50 ± 0.366 | 3.63 ± 0.450 | 3.52 ± 0.246 | 0.876 |
| ΣSFA | 41.27 ± 1.267 | 40.10 ± 1.127 | 38.48 ± 1.106 | 41.47 ± 0.842 | 0.339 |
| ΣMUFA | 12.07 ± 1.674 | 15.40 ± 2.051 | 13.27 ± 1.446 | 13.45 ± 1.058 | 0.670 |
| ΣPUFA | 46.66 ± 0.868 | 44.49 ± 1.430 | 48.25 ± 1.488 | 45.07 ± 0.872 | 0.084 |
| PUFA:SFA | 1.14 ab ± 0.035 | 1.11 ab ± 0.040 | 1.27 a ± 0.062 | 1.09 b ± 0.035 | 0.044 |
| Σn-6 | 40.65 ± 0.940 | 39.29 ± 1.251 | 41.32 ± 1.405 | 39.75 ± 0.869 | 0.408 |
| Σn-3 | 6.02 ab ± 0.549 | 5.20 b ± 0.341 | 6.93 a ± 0.629 | 5.32 b ± 0.241 | 0.049 |
| (n-6):(n-3) | 7.60 ± 1.089 | 7.76 ± 0.423 | 6.87 ± 1.268 | 7.62 ± 0.393 | 0.077 |
| Loin Muscles (n = 20) 1 | Hind Leg Muscles (n = 20) | ||||
|---|---|---|---|---|---|
| Sex | Sex | p | |||
| Fatty Acids | Male | Female | Male | Female | |
| C13:0 | 0.04 ± 0.008 | 0.06 ± 0.011 | 0.04 ± 0.010 | 0.04 ± 0.010 | 0.737 |
| C14:0 | 0.32 ± 0.100 | 0.29 ± 0.039 | 0.17 ± 0.062 | 0.28 ± 0.084 | 0.129 |
| C15:0 | 0.23 ± 0.013 | 0.24 ± 0.019 | 0.19 ± 0.025 | 0.21 ± 0.028 | 0.386 |
| C16:0 | 19.79 ± 0.471 | 20.66 ± 0.914 | 17.98 ± 0.863 | 19.37 ± 1.162 | 0.243 |
| C18:0 | 18.97 ± 0.646 | 19.32 ± 0.677 | 19.50 ± 0.660 | 20.35 ± 0.820 | 0.551 |
| C20:0 | 0.06 ± 0.004 | 0.06 ± 0.004 | 0.07 ± 0.006 | 0.07 ± 0.010 | 0.495 |
| C21:0 | 0.33 ± 0.034 | 0.34 ± 0.057 | 0.34 ± 0.035 | 0.32 ± 0.057 | 0.717 |
| C22:0 | 0.11 ± 0.015 | 0.10 ± 0.012 | 0.13 ± 0.009 | 0.15 ± 0.028 | 0.108 |
| C24:0 | 0.17 ± 0.017 | 0.20 ± 0.026 | 0.23 ± 0.028 | 0.25 ± 0.032 | 0.109 |
| C16:1 | 0.30 ± 0.046 | 0.27 ± 0.049 | 0.30 ± 0.034 | 0.33 ± 0.070 | 0.813 |
| C17:1 | 0.13 b ± 0.023 | 0.13 b ± 0.023 | 0.26 a ± 0.039 | 0.26 ab ± 0.054 | 0.004 |
| C18:1n9c | 12.76 ± 1.570 | 13.26 ± 2.135 | 12.92 ± 1.244 | 11.84 ± 1.199 | 0.969 |
| C20:1 | 0.16 ± 0.022 | 0.17 ± 0.032 | 0.21 ± 0.057 | 0.15 ± 0.020 | 0.900 |
| C22:1n9 | 0.17 ± 0.037 | 0.14 ± 0.018 | 0.14 ± 0.026 | 0.10 ± 0.015 | 0.345 |
| C18:2n6c | 22.96 ± 1.063 | 23.92 ± 1.552 | 23.93 ± 0.816 | 24.07 ± 1.738 | 0.897 |
| C18:3n6 | 0.24 ± 0.035 | 0.21 ± 0.025 | 0.29 ± 0.052 | 0.26 ± 0.054 | 0.891 |
| C18:3n3 | 1.47 ± 0.399 | 1.31 ± 0.252 | 1.84 ± 0.529 | 1.47 ± 0.284 | 0.872 |
| C20:2n6 | 0.05 ± 0.004 | 0.04 ± 0.004 | 0.05 ± 0.010 | 0.05 ± 0.010 | 0.912 |
| C20:3n6 | 1.12 ± 0.061 | 1.14 ± 0.096 | 1.07 ± 0.062 | 1.05 ± 0.092 | 0.765 |
| C20:3n3 | 0.23 ± 0.018 | 0.23 ± 0.041 | 0.30 ± 0.031 | 0.27 ± 0.043 | 0.344 |
| C20:4n6 | 16.01 ± 1.428 | 14.26 ± 1.220 | 15.28 ± 0.970 | 15.02 ± 1.507 | 0.772 |
| C20:5n3 | 0.56 ± 0.063 | 0.70 ± 0.135 | 0.50 ± 0.050 | 0.72 ± 0.122 | 0.497 |
| C22:6n3 | 3.84 ab ± 0.440 | 2.89 b ± 0.198 | 4.09 a ± 0.373 | 3.06 b ± 0.258 | 0.039 |
| ΣSFA | 40.02 ± 0.880 | 41.35 ± 1.441 | 38.78 ± 0.938 | 41.17 ± 1.110 | 0.375 |
| ΣMUFA | 13.51 ± 1.634 | 13.96 ± 2.224 | 13.88 ± 1.274 | 12.84 ± 1.237 | 0.946 |
| ΣPUFA | 46.47 ± 1.112 | 44.69 ± 1.284 | 47.34 ± 1.089 | 45.98 ± 1.498 | 0.474 |
| PUFA:SFA | 1.16 ± 0.031 | 1.09 ± 0.040 | 1.23 ± 0.049 | 1.13 ± 0.062 | 0.259 |
| Σn-6 | 40.38 ± 0.975 | 39.56 ± 1.249 | 40.61 ± 0.957 | 40.46 ± 1.396 | 0.887 |
| Σn-3 | 6.09 ± 0.451 | 5.12 ± 0.446 | 6.73 ± 0.489 | 5.52 ± 0.526 | 0.142 |
| (n-6):(n-3) | 6.99 ± 0.567 | 8.37 ± 0.969 | 6.31 ± 0.452 | 8.18 ± 1.182 | 0.283 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erasmus, S.W.; Hoffman, L.C. Putting the Spring back into the Hare (Pedetes capensis): Meat Chemical Composition of an Underutilized Protein Source. Foods 2020, 9, 1096. https://doi.org/10.3390/foods9081096
Erasmus SW, Hoffman LC. Putting the Spring back into the Hare (Pedetes capensis): Meat Chemical Composition of an Underutilized Protein Source. Foods. 2020; 9(8):1096. https://doi.org/10.3390/foods9081096
Chicago/Turabian StyleErasmus, Sara Wilhelmina, and Louwrens Christiaan Hoffman. 2020. "Putting the Spring back into the Hare (Pedetes capensis): Meat Chemical Composition of an Underutilized Protein Source" Foods 9, no. 8: 1096. https://doi.org/10.3390/foods9081096
APA StyleErasmus, S. W., & Hoffman, L. C. (2020). Putting the Spring back into the Hare (Pedetes capensis): Meat Chemical Composition of an Underutilized Protein Source. Foods, 9(8), 1096. https://doi.org/10.3390/foods9081096






