Isolation, Evaluation, and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Game Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gastrointestinal Digestions of the Tested Meat In Vitro
2.3. Analysis of the Products of In Vitro Meat Digestion
2.4. Assay of ACE Inhibitory Activity of the Products of In Vitro Meat Digestion
2.5. Determination of Imidazole Dipeptides in the Digested Meat Products
2.6. Purification and Identification of ACE Inhibitory Peptides in the Digested Meat
2.7. Statistical Analyses
3. Results and Discussion
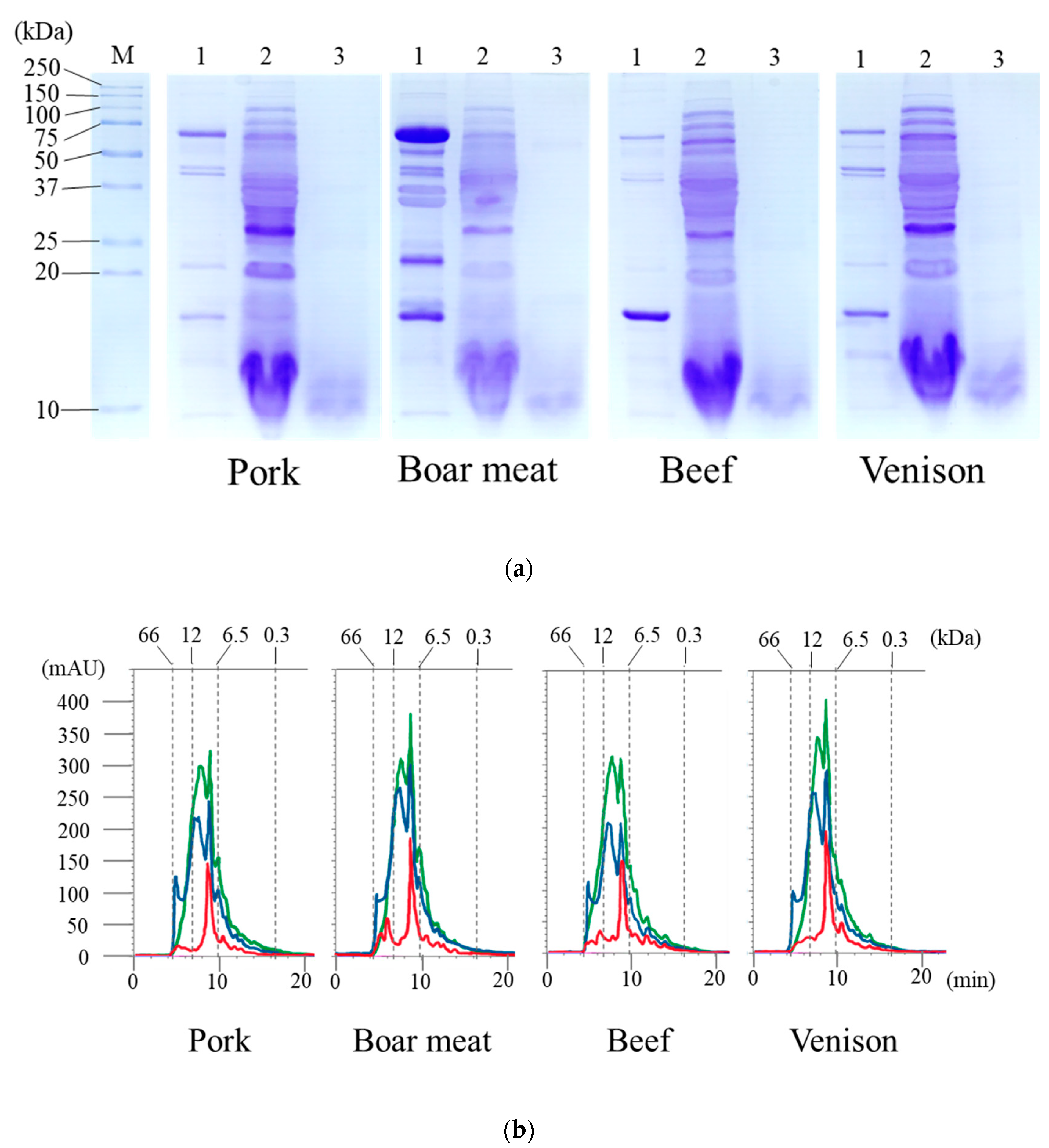
3.1. Products Released from the Digestion of the Tested Meats
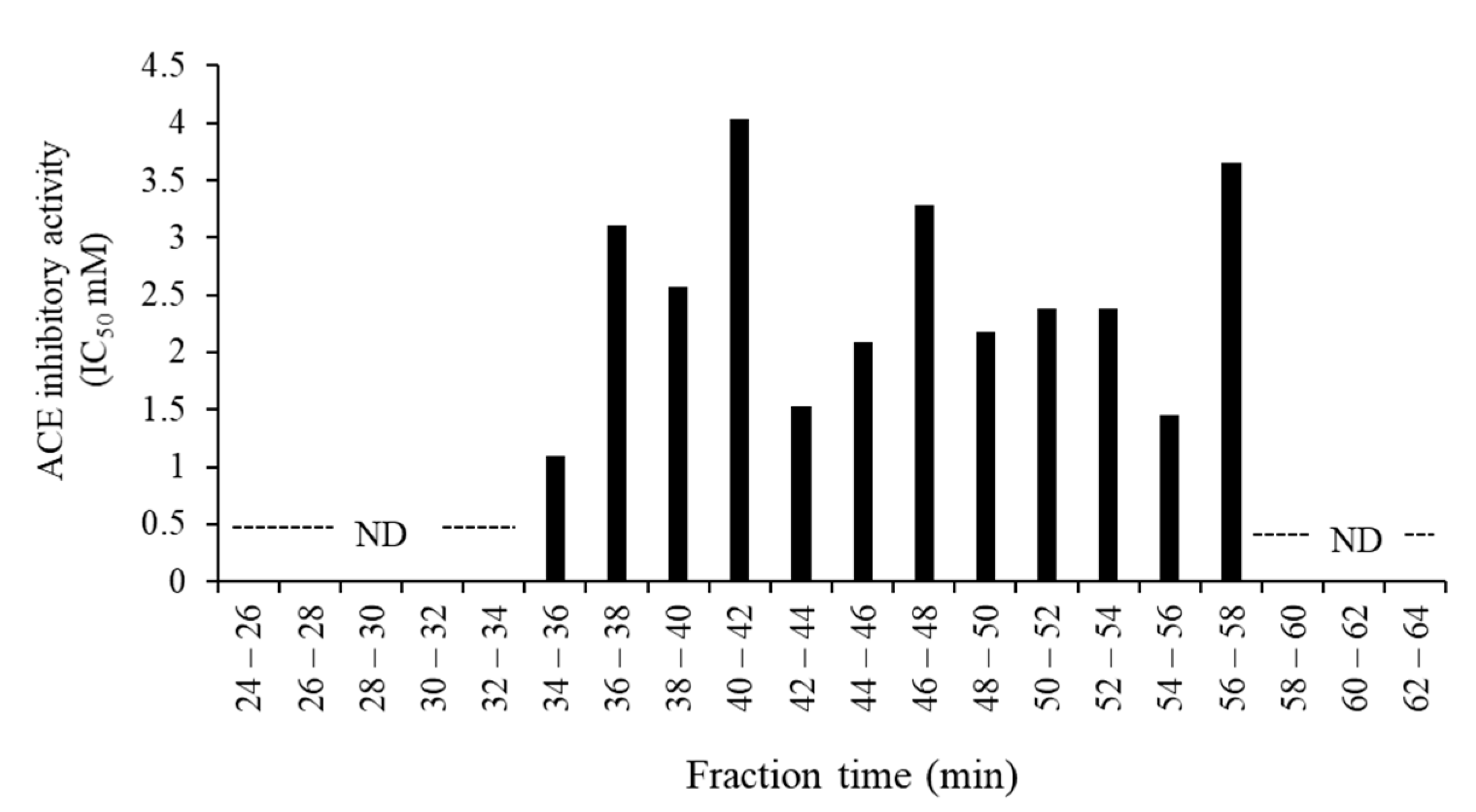
3.2. ACE Inhibitory Activity and Imidazole Dipeptide Content in the Digested Meat Products
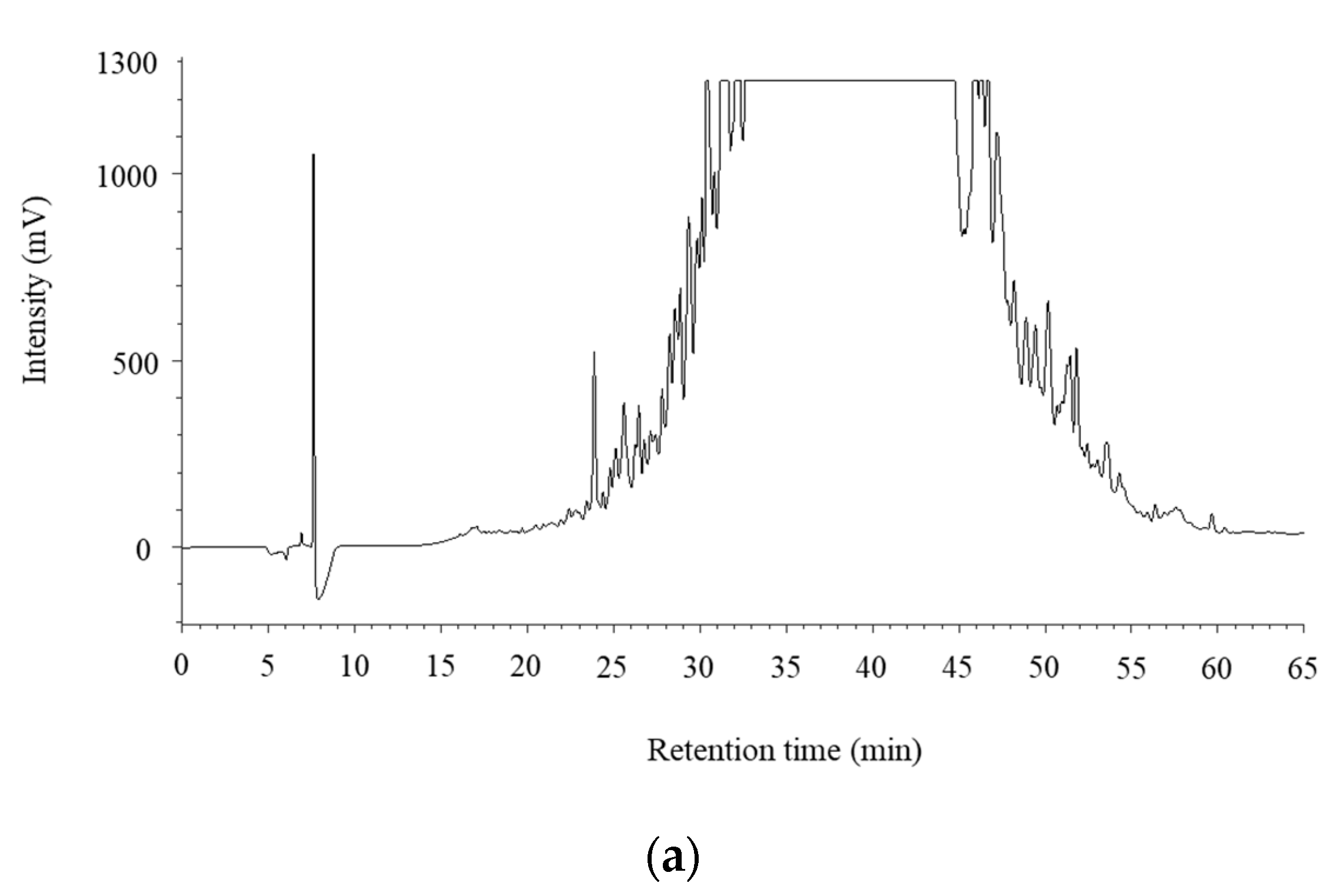
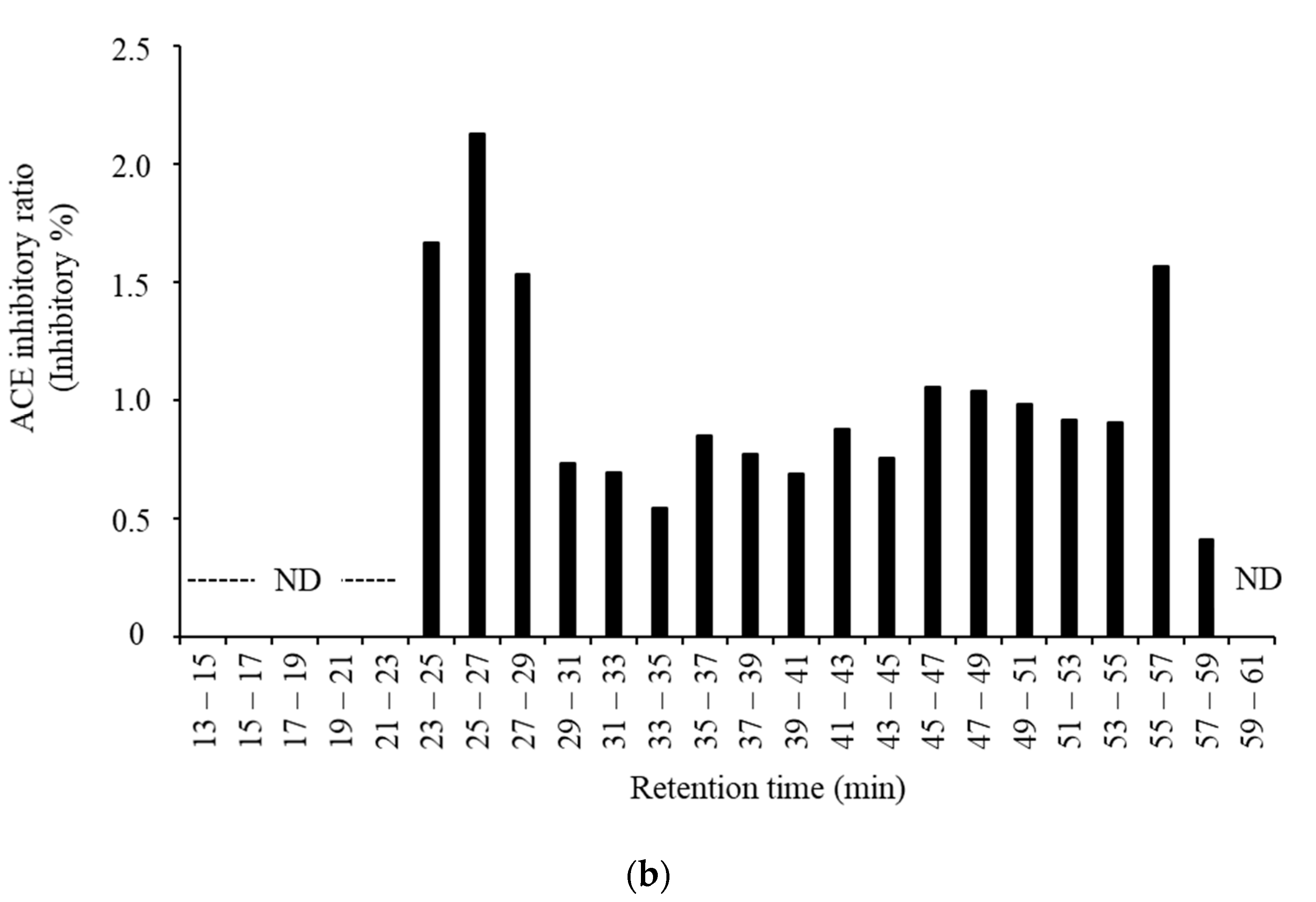
3.3. Purification and Identification of ACE Inhibitory Peptides in the Digested Venison
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, L.C.; Wiklund, E. Game and venison-meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef]
- Hoffman, L.C. The yield and carcass chemical composition of impala (Aepyceros melampus), a Southern African antelope species. J. Sci. Food Agric. 2000, 80, 752–756. [Google Scholar] [CrossRef]
- Viljoen, J.J. A comparison of the lipid components of springbok meat with those of beef and the related importance on aspects of health. S. Afr. J. Sci. Technol. 1999, 18, 51–53. [Google Scholar]
- Razmaitė, V.; Pileckas, V.; Šiukščius, A.; Juškienė, V. Fatty Acid Composition of Meat and Edible Offal from Free-Living Red Deer (Cervus elaphus). Foods 2020, 9, 923. [Google Scholar] [CrossRef]
- Utrilla, M.C.; García Ruiz, A.; Soriano, A. Effect of partial reduction of pork meat on the physicochemical and sensory quality of dry ripened sausages: Development of a healthy venison salchichon. Meat Sci. 2014, 98, 785–791. [Google Scholar] [CrossRef]
- Cawthorn, D.M.; Fitzhenry, L.B.; Kotrba, R.; Bureš, D.; Hoffman, L.C. Chemical Composition of Wild Fallow Deer (Dama dama) Meat from South Africa: A Preliminary Evaluation. Foods 2020, 9, 598. [Google Scholar] [CrossRef]
- Kudrnáčová, E.; Bartoň, L.; Bureš, D.; Hoffman, L.C. Carcass and meat characteristics from farm-raised and wild fallow deer (Dama dama) and red deer (Cervus elaphus): A review. Meat Sci. 2018, 141, 9–27. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sandanger, T.M.; Brustad, M. Selected vitamins and essential elements in meat from semi-domesticated reindeer (Rangifer tarandus L.) in mid- and northern Norway: Geographical variations and effect of animal population density. Nutrients 2012, 4, 724–739. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sandanger, T.M.; Brustad, M. Level of selected nutrients in meat, liver, tallow and bone marrow from semi-domesticated reindeer (Rangifer t. tarandus L.). Int. J. Circumpolar Health 2012, 71, 17997. [Google Scholar] [CrossRef]
- Nilsson, M.L. Food, Nutrition, and Health in Sápmi. In Nutritional and Health Aspects of Food in Nordic Countries; Andersen, V., Bar, E., Wirtanen, G., Eds.; Academic Press: Cambrige, MA, USA, 2018; pp. 179–195. [Google Scholar]
- Rincker, P.J.; Bechtel, P.J.; Finstadt, G.; Van Buuren, R.G.C.; Killefer, J.; McKeith, F.K. Similarities and differences in composition and selected sensory attributes of reindeer, caribou and beef. J. Muscle Foods 2006, 17, 65–78. [Google Scholar] [CrossRef]
- Arihara, K. Strategies for designing novel functional meat products. Meat Sci. 2006, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Reig, M. Innovations for healthier processed meats. Trends Food Sci. Technol. 2011, 22, 517–522. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mauri, L.; Concepción Aristoy, M.; Toldrá, F.; Mora, L. Antioxidant peptides profile in dry-cured ham as affected by gastrointestinal digestion. J. Funct. Foods 2020, 69, 103956. [Google Scholar] [CrossRef]
- Simonetti, A.; Gambacorta, E.; Perna, A. Antioxidative and antihypertensive activities of pig meat before and after cooking and in vitro gastrointestinal digestion: Comparison between italian autochthonous pig suino nero lucano and a modern crossbred pig. Food Chem. 2016, 212, 590–595. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Li, Y.; Liu, W.; Jia, X.; Qiao, X.; Qu, C.; Cheng, X.; Wang, S. Antioxidant and angiotensin I-converting enzyme inhibitory activities of Xuanwei ham before and after cooking and in vitro simulated gastrointestinal digestion. R. Soc. Open Sci. 2018, 5, 180276. [Google Scholar] [CrossRef]
- Ahhmed, A.M.; Muguruma, M. A review of meat protein hydrolysates and hypertension. Meat Sci. 2010, 86, 110–118. [Google Scholar] [CrossRef]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef]
- Bah, C.S.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.-D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.J.; Jeon, B.T.; Moon, S.H.; Kim, B.; Park, T.K.; Han, J.-S.; Park, P.J. Purification and characterisation of antioxidative peptides from enzymatic hydrolysates of venison protein. Food Chem. 2009, 114, 1365–1370. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.J.; Moon, S.H.; Jeon, B.T.; Kim, B.; Park, T.K.; Han, J.-S.; Park, P.J. Neuroprotective effects of a novel peptide purified from venison protein. J. Microbiol. Biotechnol. 2010, 20, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Storcksdieck, S.; Bonsmann, G.; Hurrell, R.F. Iron-Binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J. Food Sci. 2007, 72, S019–S029. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, C.H. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Takeda, S.; Matsufuji, H.; Nakade, K.; Takenoyama, S.; Ahhmed, A.M.; Sakata, R.; Kawahara, S.; Muguruma, M. Investigation of lactic acid bacterial strains for meat fermentation and the product’s antioxidant and angiotensin-I-converting-enzyme inhibitory activities. Anim. Sci. J. 2017, 88, 507–516. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar]
- Terashima, M.; Baba, T.; Ikemoto, N.; Katayama, M.; Morimoto, T.; Matsumura, S. Novel angiotensin-converting enzyme (ACE) inhibitory peptides derived from boneless chicken leg meat. J. Agric. Food Chem. 2010, 58, 7432–7436. [Google Scholar] [CrossRef]
- Maeda, H.; Kobayashi, K.; Watanabe, T.; Satoh, M.; Nomura, F.; Sogawa, K. Urinary carboxylesterase 5A fragment as an early diagnostic marker of cat chronic kidney disease. J. Electrophor. 2019, 63, 39–45. [Google Scholar] [CrossRef]
- Sogawa, K.; Kodera, Y.; Satoh, M.; Kawashima, Y.; Umemura, H.; Maruyama, K.; Takizawa, H.; Yokosuka, O.; Nomura, F. Increased serum levels of pigment epithelium-derived factor by excessive alcohol consumption-detection and identification by a three-step serum proteome analysis. Alcohol. Clin. Exp. Res. 2011, 35, 211–217. [Google Scholar] [CrossRef]
- Maeda, H.; Sogawa, K.; Sakaguchi, K.; Abe, S.; Sagizaka, W.; Mochizuki, S.; Horie, W.; Watanabe, T.; Shibata, Y.; Satoh, M.; et al. Urinary albumin and transferrin as early diagnostic markers of chronic kidney disease. J. Vet. Med. Sci. 2015, 77, 937–943. [Google Scholar] [CrossRef]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Ahhmed, M.A.; Özer, N.; Özcan, C.; Çam, M.; Sağdic, O.; Arici, M.; Yilmaz, T.M.; Yetim, H.; Kim, J.; Muguruma, M.; et al. Solubility, stability and blood pressure lowering-properties of fresh and cured beef proteins. Acta Sci. Nutr. Health 2019, 3, 16–26. [Google Scholar]
- Wen, S.; Zhou, G.; Song, S.; Xu, X.; Voglmeir, J.; Liu, L.; Zhao, F.; Li, M.; Li, L.; Yu, X.; et al. Discrimination of in vitro and in vivo digestion products of meat proteins from pork, beef, chicken, and fish. Proteomics 2015, 15, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Montowska, M.; Rao, W.; Alexander, M.R.; Tucker, G.A.; Barrett, D.A. Tryptic digestion coupled with ambient desorption electrospray ionization and liquid extraction surface analysis mass spectrometry enabling identification of skeletal muscle proteins in mixtures and distinguishing between beef, pork, horse, chicken, and turkey meat. Anal. Chem. 2014, 86, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.J.; Dort, J.; Eilertsen, K.E. Proximate composition, antihypertensive and antioxidative properties of the semimembranosus muscle from pork and beef after cooking and in vitro digestion. Meat Sci. 2014, 96, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Paleari, M.A.; Moretti, V.M.; Beretta, G.; Mentasti, T.; Bersani, C. Cured products from different animal species. Meat Sci. 2003, 63, 485–489. [Google Scholar] [CrossRef]
- Hou, W.C.; Chen, H.J.; Lin, Y.H. Antioxidant peptides with angiotensin converting enzyme inhibitory activities and applications for angiotensin converting enzyme purification. J. Agric. Food Chem. 2003, 51, 1706–1709. [Google Scholar] [CrossRef]
- Marcolini, E.; Babini, E.; Bordoni, A.; Di Nunzio, M.; Laghi, L.; Maczó, A.; Picone, G.; Szerdahelyi, E.; Valli, V.; Capozzi, F. Bioaccessibility of the bioactive peptide carnosine during in vitro digestion of cured beef meat. J. Agric. Food Chem. 2015, 63, 4973–4978. [Google Scholar] [CrossRef]
- Han, Y.; Gao, B.; Zhao, S.; Wang, M.; Jian, L.; Han, L.; Liu, X. Simultaneous detection of carnosine and anserine by UHPLC-MS/MS and its application on biomarker analysis for differentiation of meat and bone meal. Molecules 2019, 24, 217. [Google Scholar] [CrossRef]
- Gil-Agustí, M.; Esteve-Romero, J.; Carda-Broch, S. Anserine and carnosine determination in meat samples by pure micellar liquid chromatography. J. Chromatogr. A 2008, 1189, 444–450. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vazquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Jang, A.; Jo, C.; Kang, K.S.; Lee, M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008, 107, 327–336. [Google Scholar] [CrossRef]
- Katayama, K.; Fuchu, H.; Sugiyama, M.; Kawahara, S.; Yamauchi, K.; Kawamura, Y.; Muguruma, M. Peptic hydrolysate of porcine crude myosin has many active fractions inhibiting angiotensin I-converting enzyme. Asian-australas. J. Anim. Sci. 2003, 16, 1384–1389. [Google Scholar] [CrossRef]
| Peptide Concentration (mM) | |||
|---|---|---|---|
| Untreated | Pep | Pep/Try/Pan | |
| Pork | 22.28 ± 0.99 a | 52.80 ± 6.65 b | 80.10 ± 5.52 c |
| Boar meat | 20.32 ± 1.36 a | 58.03 ± 6.50 b | 78.45 ± 8.09 c |
| Beef | 23.56 ± 1.83 a | 51.62 ± 1.89 b | 78.95 ± 3.28 c |
| Venison | 22.20 ± 2.00 a | 50.56 ± 2.37 b | 75.70 ± 4.87 c |
| Meat Type | ACE Inhibitory Percentage (%) | ||
|---|---|---|---|
| Untreated | Pep | Pep/Try/Pan | |
| Pork | 43.71 ± 5.42 a, A | 64.36 ± 1.00 b, A | 65.00 ± 6.43 b, A |
| Boar meat | 44.67 ± 5.03 a, A | 79.44 ± 1.70 b, B | 78.04 ± 2.47 b, B |
| Beef | 47.18 ± 5.90 a, A | 64.15 ± 2.81 b, A | 63.20 ± 3.26 b, A |
| Venison | 52.60 ± 2.89 a, A | 77.29 ± 5.96 b, AB | 89.38 ± 5.57 c, C |
| Meat Type | Anserine (μM) | Carnosine (μM) |
|---|---|---|
| Pork | 66.67 ± 1.15 a | 2750 ± 100 a |
| Beef | 323.33 ± 7.02 b | 2190 ± 40 a |
| Boar meat | 401.33 ± 9.45 c | 1730 ± 30 b |
| Venison | 668.67 ± 1.15 d | 1040 ± 10 c |
| Peptide Sequence | Origin | Ace Inhibitory Activity (IC50: μg/mL) |
|---|---|---|
| VcNYVNWIQQTIAAN | Tropomyosin alpha-3 chain | 358.64 ± 64.71 a |
| mQGTLEDQIISANPLLEAFGNAK | Myosin-1 | 292.83 ± 38.27 a,b |
| IKEVTER | Myosin-1 | 86.52 ± 18.84 c |
| TEAGATVTVK | Myosin-1 | 208.64 ± 5.57 b |
| SEIQAALEEAEASLEHEEGK | Myosin-1 | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, S.; Kaneko, S.; Sogawa, K.; Ahhmed, A.M.; Enomoto, H.; Kawarai, S.; Taira, K.; Mizunoya, W.; Minami, M.; Sakata, R. Isolation, Evaluation, and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Game Meat. Foods 2020, 9, 1168. https://doi.org/10.3390/foods9091168
Takeda S, Kaneko S, Sogawa K, Ahhmed AM, Enomoto H, Kawarai S, Taira K, Mizunoya W, Minami M, Sakata R. Isolation, Evaluation, and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Game Meat. Foods. 2020; 9(9):1168. https://doi.org/10.3390/foods9091168
Chicago/Turabian StyleTakeda, Shiro, Sakurako Kaneko, Kazuyuki Sogawa, Abdulatef M Ahhmed, Hirofumi Enomoto, Shinpei Kawarai, Kensuke Taira, Wataru Mizunoya, Masato Minami, and Ryoichi Sakata. 2020. "Isolation, Evaluation, and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Game Meat" Foods 9, no. 9: 1168. https://doi.org/10.3390/foods9091168
APA StyleTakeda, S., Kaneko, S., Sogawa, K., Ahhmed, A. M., Enomoto, H., Kawarai, S., Taira, K., Mizunoya, W., Minami, M., & Sakata, R. (2020). Isolation, Evaluation, and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Game Meat. Foods, 9(9), 1168. https://doi.org/10.3390/foods9091168







