3.1. Small Batch Fermentation
Starter cultures for fermentation of the pea-oat protein blend were selected according to their ability to ferment plant material and possibly degrade phytates. Chosen commercial starters Danisco VEGE 022, 053, and 061 contain lactic acid bacteria
Lactobacillus plantarum,
Lactobacillus delbrueckii, and
Lactobacillus acidophilus, some strains of which were previously shown to have phytase activity [
18]. Additionally, pure cultures of
Weissella sp. and
Lactobacillus plantarum DPPMAB24W were selected for their potential to degrade antinutrients [
18,
27].
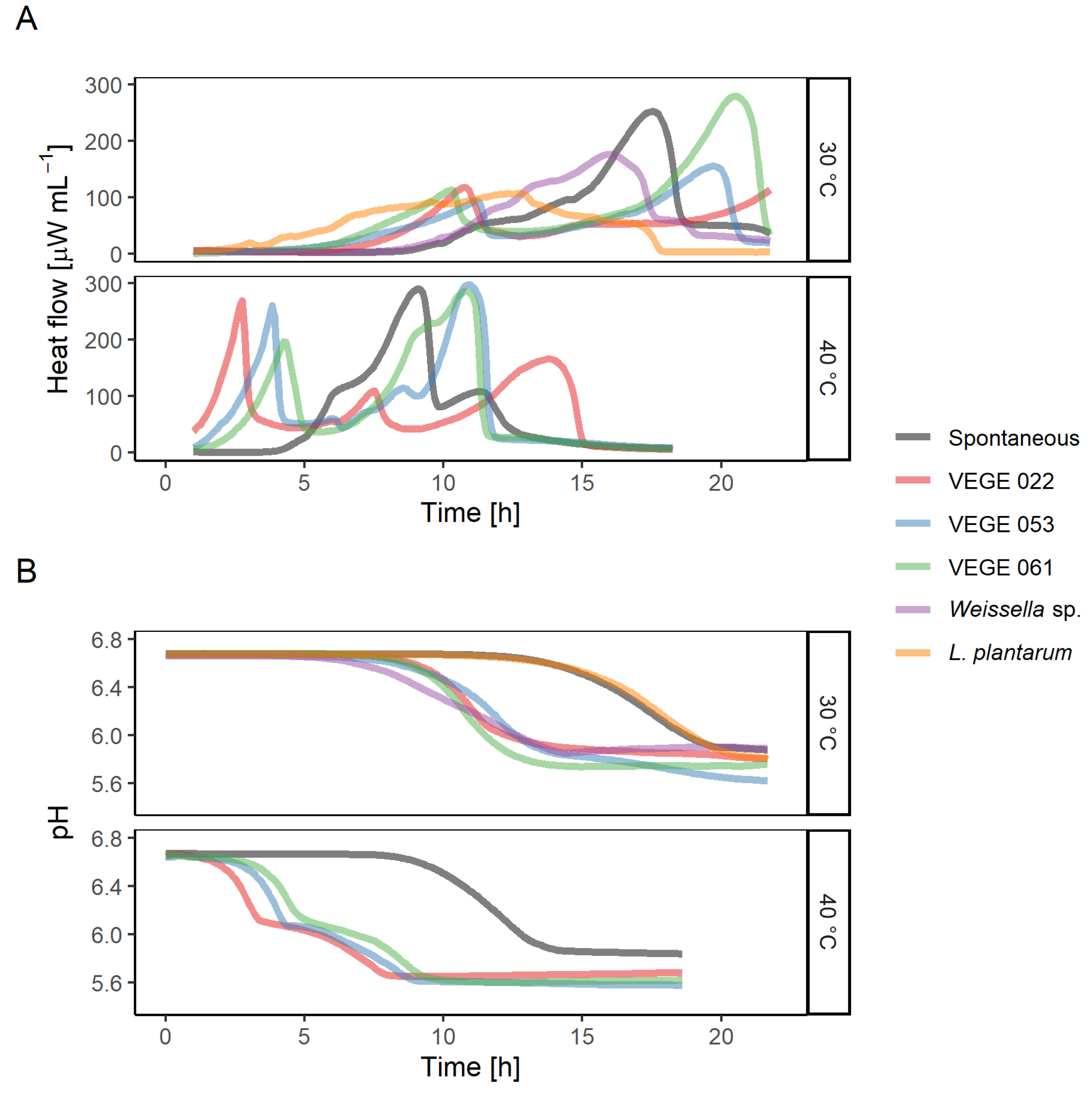
All chosen starters were able to grow in the pea-oat protein suspension supplemented with sucrose, as evidenced by the microcalorimetry heat flow curves (
Figure 2A) and pH drop (
Figure 2B). In all the cases pH has decreased approximately by one unit from 6.7 to 5.7. Spontaneous sample and VEGE starters had multiple heat flow peaks associated with the active growth phases of different bacteria of the starter consortium. Fermentation at 40 °C concluded in 15 h, but at 30 °C, it finished in 22 h, except for VEGE 022, which at the end of the experiment was accelerating its heat production indicating the beginning of an active growth phase.
Evaluation of both heat flow and pH curves provides a deeper understanding of the fermentation process. By looking only at the pH curve (
Figure 2B), one might conclude that
L. plantarum culture did not grow in the medium, as it is not different from the spontaneous curve. But evaluation of the heat flow curves (
Figure 2A) confirms that
L. plantarum did grow and even had the shortest lag-phase of all the bacteria growing at 30 °C.
The total phytic acid content of the fermented samples measured by the Megazyme kit is presented in
Table 1. According to this method, phytic acid content unexpectedly increased after fermentation, particularly in the case of
L. plantarum and spontaneous sample. These peculiar results could be attributed to the non-specificity of the method, but further investigation is needed to explain this inconsistency.
A more targeted LC-MS method was applied to verify the results of the Megazyme kit.
Table 2 shows the content of IP6, IP5, and IP1 that were detected; IP4 was below the quantification limit. In contrast to the Megazyme kit, this method reveals that there is no difference in inositol phosphates content between fermented and non-fermented samples. Multiple studies have demonstrated degradation of phytates by fermentation with lactic acid bacteria [
18], but certain phytase-producing strains are necessary. In this experiment, none of the starters selected for testing had phytase activity.
3.2. Large Batch Fermentation
The recommended incubation temperature for VEGE starters is 40 °C, however, at 30 °C the viscosity of the fermented suspension was visibly higher due to the production of exopolysaccharides (EPS). Lactic acid bacteria can utilize sucrose for the production of a variety of EPS, which can improve the texture of final products [
28]. Viscosity of control, VEGE 022, 053, and 061 at 0.015 s
−1 strain was 0.44 ± 0.02, 0.44 ± 0.01, 0.74 ± 0.08, and 0.52 ± 0.03 Pa s, respectively. Thus, the starter for fermentation in a bigger batch, VEGE 053, was chosen according to its ability to produce EPS as none of the starters degraded phytates.
Bigger batch fermentation with VEGE 053 was carried out in a 7 L bioreactor. The pH curve, in this case, was similar in shape to the small fermentation, but the decrease occurred faster. In small batch pH dropped from 6.7 to 5.7 in approximately 19 h, but in bigger batch in 12 h. This difference can be attributed to the mixing applied in the bigger batch which improved mass transfer and consequently fermentation speed, whereas in small batches powder precipitated and created a thick layer.
3.3. Phytase Treatment
One possibility to reduce phytic acid content is by applying exogenous microbial phytases. Depending on the enzyme source the optimal hydrolysis conditions can vary a lot [
29]. A phytase used in this study, Phyzyme XP, was sourced from an
Escherichia coli and expressed in
Saccharomyces pombe. The highest activity of this enzyme is at low pH values (pH = 4.5) and at 55 °C [
29]. However, for this experiment the incubation temperature (40 °C) was chosen based on the temperature applied also in fermentation experiments. Additionally, the aim was to avoid the effect of high temperature on proteins during incubation. According to
Figure 2A spontaneous fermentation began after 4 h, thus limiting phytase treatment time. To avoid the effect of added acid on the extruded product pH was not adjusted, and as the reaction conditions were not optimal, then the phytase was applied in excess. Used incubation conditions allow a better comparison between control and fermented samples.
Different studies demonstrate that various exogenous phytases can efficiently reduce phytic acid content from 20% to 100%, depending on reaction conditions [
30,
31]. The results in this study show that under used reaction conditions up to 30% of phytic acid could be degraded. The total inositol phosphates content (IP6, IP5, and IP1) in non-treated and enzymatically treated samples was 1.47% and 1.03% dwb, respectively. However, optimized incubation conditions would allow a higher degree of phytic acid degradation.
3.4. Extrusion of Meat Analogs
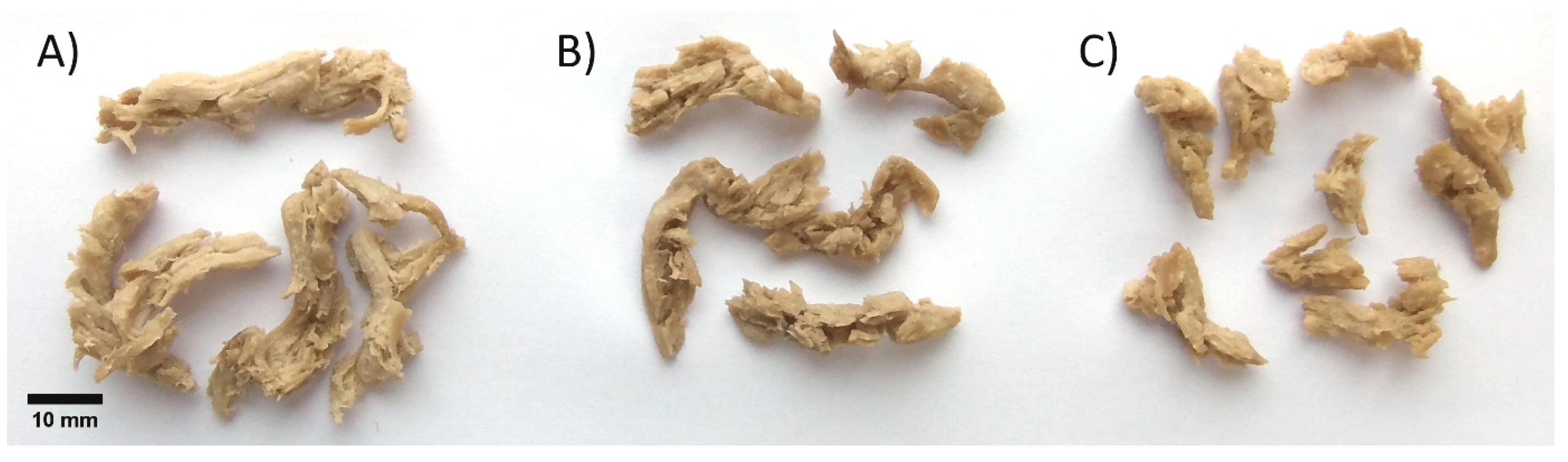
Fermented and enzymatically treated suspensions were lyophilized as accurate dosing into the extruder in hydrated form was not possible for technical reasons. Control and treated powders were extruded and collected in sequence when the extruder reached a steady state. Fibrous meat-like textures were obtained from all powders, as seen in
Figure 3.
Recorded extrusion conditions are presented in
Table 3. The phytase-treated powder was extruding similarly to the control, in contrast, the fermented powder did not initially form fibrous texture at the same extrusion settings, thus treatment intensity was increased by slightly lowering the dough moisture and doubling the screw speed from 600 rpm to 1200 rpm. Fermented powder created significantly lower pressure at the die even at a reduced moisture content as a result of bacterial enzymatic activity that degraded proteins and carbohydrates, which in turn lowered the dough viscosity [
32]. Consequently, higher screw speed was necessary in the case of fermented powder to achieve SME similar to the control powder.
In comparison to expanded snacks produced by do Carmo et al. (2019) [
23] from pea and oat fractions with an analogous extruder setup, meat analogs (
Table 3) extruded at an order of magnitude lower pressure, but achieved higher SME. The values for expanded snacks were in the range 98–213 bar and 88–145 Wh kg
−1. Such divergence in pressure can be attributed to the low viscosity of the dough mainly caused by higher moisture but also by different powder composition, i.e., higher protein content. Previous studies have demonstrated that the addition of proteins in the form of isolates typically reduced SME, however, in this work the screw had multiple kneading and reverse elements that imparted higher mechanical treatment [
33].
3.5. Physicochemical Properties
The physicochemical properties of powders and corresponding extrudates are listed in
Table 4. The WHC, WSI, and OHC characterize interaction with water or oil and are important when extruded materials are processed into final products. Protein constituted 75.6% of the pea-oat blend, thus changes in physicochemical properties can be attributed mostly to it.
Water binding depends on the availability of polar hydrophilic groups and is strongly influenced by changes in the composition and conformation of proteins. Phytase treatment of the control powder did not change WHC as the enzyme was not acting upon proteins and the process was at mild conditions leaving proteins intact. Relatively short time also prevented molecular changes caused by microbiological action. This contrasts with the fermented powder that has 33% lower WHC. In addition to bacterial proteolysis, other factors could have contributed to the decrease of WHC. Protein charge and conformation are influenced by pH, which fell by one unit during fermentation. Degradation and consumption of carbohydrates by bacteria could also have contributed to the WHC, as these molecules bind a lot of water. High temperature and shearing during extrusion denature proteins making hydrophilic groups less available and that explains lower WHC values of extrudates. However, these values were not significantly different across all three samples and also were similar to the WHC of the fermented powder. One explanation is that all these treatments were severe enough to be bound by the lowest possible WHC value [
34].
Oil holding capacity is determined by the nonpolar side chains of proteins but is also dependent on the physical entrapment of oil and can be explained by the material microstructure [
34]. OHC of the control powder and control and phytase-treated extrudates was not different (
Table 4). OHC of the fermented extrudate was statistically significantly lower than the control, though the difference in absolute value was negligible. More than twice higher OHC values were recorded for phytase-treated and fermented powders. In this case, the difference is attributed to the powder drying method and not phytase or fermentation treatments per se, as these lyophilized powders were airy in appearance in contrast to the much denser control powder or ground extrudates. Low-density protein powders with a smaller particle size were shown to adsorb more oil than high-density powders [
34]; for example, spray-dried pea protein isolates had lower OHC than lyophilized [
35].
For reference, studies of pea protein isolates, which is a major constituent in the investigated blend, report WHC values in a wide range of 1.5–4.8 g H
2O g
−1, and OHC 1.1–5.3 g oil g
−1, with differences attributed to pea cultivars and powder production methods [
36]. It should also be noted that both OHC and WHC of extrudates were measured from ground samples. This destroys the macrostructure of meat analogs, including larger pores that could hold additional water or oil. Loosely bound water or oil in large pores can influence sensory characteristics of final products such as juiciness, moistness, and flavor release [
34], thus OHC and WHC might encompass only part of the sensory perception.
Water solubility index estimates the total amount of material that can be extracted by water. Multiple factors can influence WSI, such as composition and particle size of powders, conformational state of proteins, molecular size and cross-linking. Compared to the control powder, phytase-treated powder and control extrudate were not statistically significantly different; phytase-treated extrudate had significantly lower WSI, but at a relatively small absolute difference to the control powder. This contrasts with the WHC of these samples, which was strongly influenced by extrusion. On the other hand, both fermented samples had the highest WSI due to the bacterial breakdown of macromolecules, which increased their solubility. This was also evidenced by the low viscosity of the dough seen as the low pressure at the die during extrusion. Furthermore, WSI was found to be highly negatively correlated with the die pressure (Pearson’s r −0.999).
Color change measurement helps to determine the overall impact of different treatments. As seen in
Table 4, fermentation, phytase treatment, and extrusion, all had a significant effect on color. Extrusion markedly decreased lightness L*, slightly increased redness a*, but the overall effect on yellowness b* was not uniform. These results are consistent with Ilo and Berghofer [
37], who concluded that L* and a* were dependent on extrusion temperature and moisture, and that lightness was the best parameter for tracking Maillard reaction. Phytase treatment darkened the powder, possibly due to the release of minerals bound to phytates. The darker tone of the phytase-treated powder was also clearly observed in extruded samples (
Figure 3B), but according to the colorimetric measurement, the lowest lightness was detected in the fermented extrudate. This discrepancy arises from the fact that color measurements were performed with dried ground extrudates, whilst in
Figure 3 samples are in rehydrated intact form suggesting that other parameters such as WHC or macrostructure of the extrudate influence the color of the product.
Table 5 summarizes the results of the texture profile analysis of rehydrated extrudates. The hardness relates to the maximum force required to compress the sample, chewiness is a measure of the energy required to masticate the sample, cohesiveness indicates the strength of internal bonds, springiness is how much the sample recovers after deformation, and resilience measures how fast and how strong the recovery is [
38]. According to these attributes, the control and the phytase-treated meat analogs were not significantly different; in contrast, the fermented extrudate was 40% harder and 25% chewier, but at the same time slightly less cohesive. Fermented meat analog pieces were noticeably smaller in size and this lower expansion could explain their higher hardness and chewiness. Studies of expanded snacks confirm that texture properties, such as hardness, are directly related to expansion [
24]. Higher dough viscosity was shown to favor higher expansion [
23], whereas in this work fermented sample dough was less viscous due to macromolecular degradation and accordingly the extrudate was less expanded. Macromolecular degradation is also reflected in the lower cohesiveness of the fermented extrudate as smaller molecules formed weaker internal structures. A study of cooked chicken meat showed cohesiveness 0.37 and springiness 0.57, which are much lower than the values found in this work [
39]. Interestingly, the fermented sample was the closest. Therefore further optimization of extrusion conditions is necessary for the texture of meat analogs to resemble meat more.
3.6. Nutritional Aspects
The nutritional quality of a protein source depends upon bioavailability, digestibility, amino acid profile, antinutrients, and other factors [
40].
Phytates have a strong affinity to minerals and proteins and can hinder the absorption of nutrients. Stability of
myo-inositol phosphate-mineral complexes has been shown to decrease with dephosphorylation of phytate, therefore improving the bioavailability of minerals [
29].
The content of inositol phosphates of control powder, treated powders, and corresponding extrudates is shown in
Table 6. Only IP6, IP5, and IP1 were quantified in these samples and IP4 was below the quantification limit. The most abundant form was IP6.
The lowest IP6 and IP5 content was detected in the phytase-treated and the corresponding extruded sample. IP6 was reduced by 32% and 35% from the control, respectively. Fermentation did not reduce IP6, however, IP1 concentration was 4x higher compared to the control. The same trend was also seen in small-batch spontaneous fermentation, but not in VEGE 022 (
Table 2). One possible explanation for this is an enzyme with phosphatase activity that produced IP1 but could not hydrolyze IP6. Assuming that IP4, IP3, and IP2 were not detected due to some limitation of the analytical method, these inositol phosphate forms could be the substrate for the enzyme. Indeed, phosphatases with different substrate specificities and end products are well documented [
41].
Extrusion degraded IP6 in the control and the fermented sample (by 18% and 8%, respectively), whereas IP5 content was significantly higher only in the fermented sample. This supports the results of Alonso et al. (2001) and Pontoppidan et al. (2007) [
14,
42]. In these experiments high temperature and pressure during extrusion cooking degraded IP6 to lower phosphate forms and this reduction depended on the extruded material.
The content of essential amino acids defines the nutritional quality of a protein. The Food and Agriculture Organization of the United Nations (FAO) has established a standard for protein quality evaluation that sets a minimum amount of essential amino acids per gram of total protein for different age groups [
43]. Plant proteins are regarded as nutritionally incomplete: cereals are typically deficient in lysine and legumes are low in methionine and cysteine [
40]. This is seen in
Table 7 that shows the amino acid composition of pea and oat proteins and their blend. According to the FAO recommendation for infants, both protein concentrates are nutritionally incomplete. Yet, the content of essential amino acids of pea isolate is above the recommended minimum for adults, while oat concentrate is deficient only in lysine. Therefore, powder blending improved nutritional quality, and the control blend fulfilled FAO recommendations for adults.
The free amino acid content is summarized by sparse principal component analysis in
Figure 4. The largest difference is seen between the fermented and other samples, as captured by the first principal component. Fermentation is known to increase free amino acids due to proteolytic activity [
44,
45]. In this work, the total free amino acid content of the fermented sample was twice higher compared to the control (1.77 ± 0.12 and 0.85 ± 0.08 mg g
−1 dwb). Fermentation increased content of free Ala, Cys, His, Ile, Leu, Lys, Phe, Pro, Ser, Tyr, and Val, but decreased Arg, Asn, and Asp. The second principal component represents the effect of extrusion, which was much smaller than fermentation. Extrusion cooking mostly had reduced the availability of free Gln, Met, and Trp; the effect on Gly was not statistically significant. Small soluble molecules such as free amino acids contribute to WSI (
Table 4). This is corroborated by a high Pearson’s correlation coefficient of 0.929.
3.7. Sensory Assessment
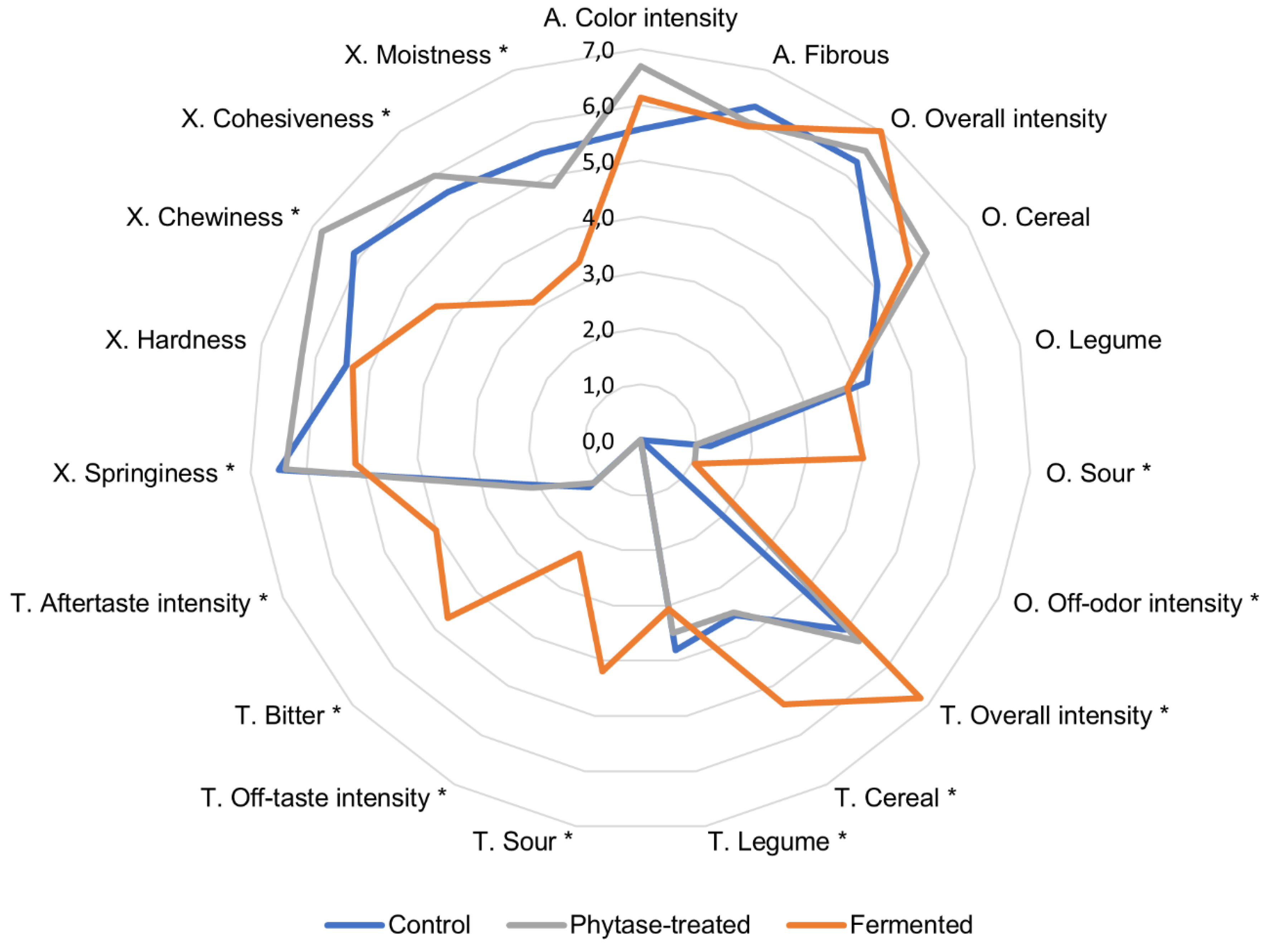
The sensory assessment of extrudates is presented as a radar diagram in
Figure 5. The difference between the control and the phytase-treated sample was small, as evidenced by the overlap of the lines. The phytase-treated extrudate tended to be significantly harder, more intense in color, and had stronger off-odor in comparison to the control. Interestingly, the assessors commented on this sample as having sweet, bready, roasty, and ashy odors. The fermented sample was more clearly distinguished from the other two extrudates. The following attributes were statistically significantly higher in the fermented sample compared to the control: overall taste intensity, sour odor and taste, cereal and legume taste, bitter taste, off-odor and off-taste intensity, and aftertaste intensity. On the other hand, springiness, chewiness, cohesiveness, and moistness were graded lower. Some assessors described fermented samples as being dry and easily disintegrating and noted medicinal, fermented, soapy, and citrus off-flavors.
The low sensorial moistness of the fermented extrudate was reflected in its low water holding capacity (
Table 4). Yet, other sensory texture attributes had a very low correlation with the texture profile analysis results underlining the difficulty of evaluating this type of material.
Fermentation developed flavor compounds that enhanced the overall taste profile, but some attributes, such as cereal or bitter taste, can be considered negative or unwanted in a meat analog product. In contrast to these results, Rani et al. (2018) [
44] demonstrated that puffed snacks prepared from fermented rice-black gram mixed flour had superior acceptability as fermentation improved sensory attributes of texture, mouthfeel, taste, and aftertaste. Thus it can be understood, that fermentation has to be optimized to avoid the formation of unwanted compounds.
3.8. Volatile Compounds
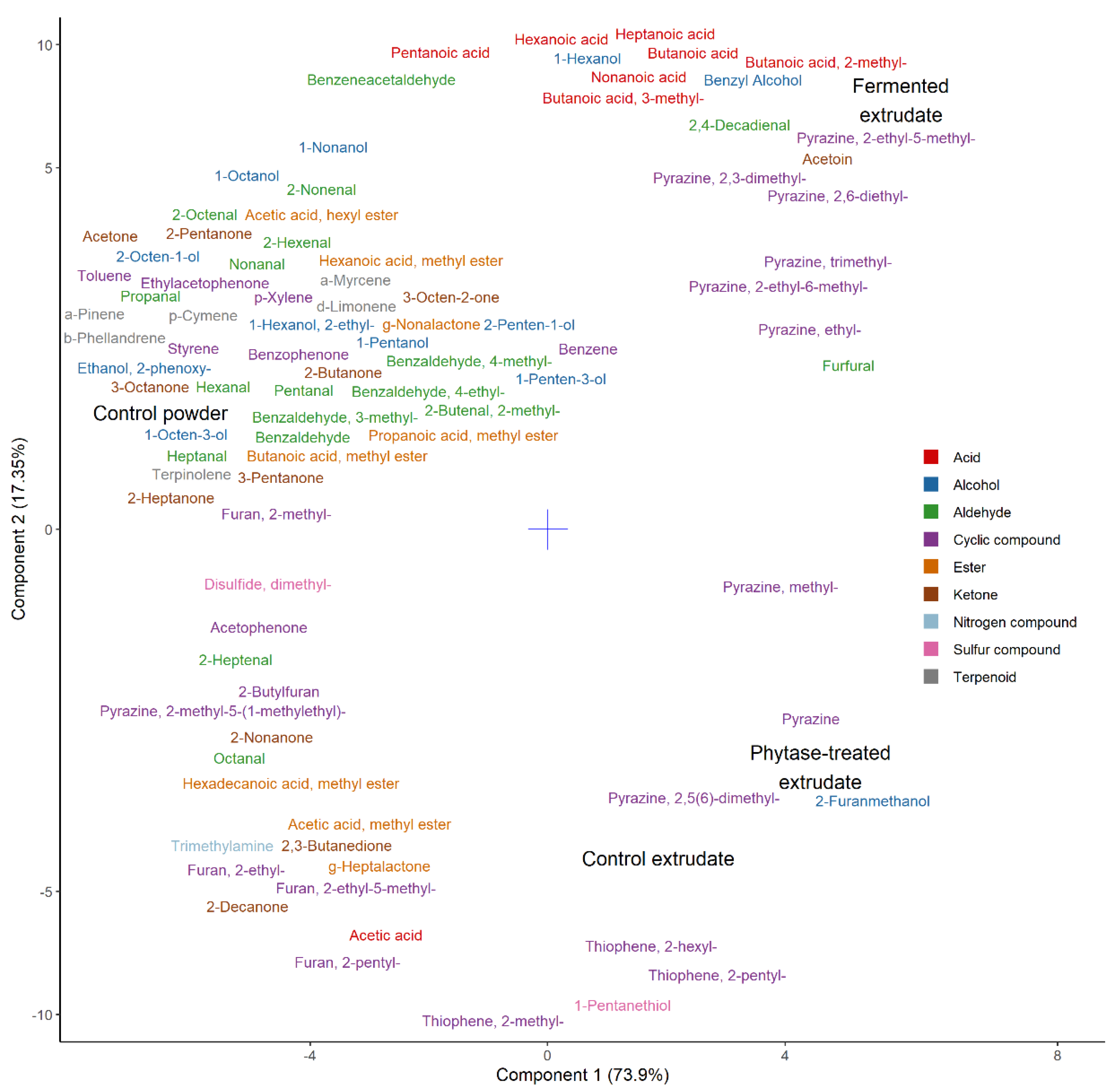
Volatiles are represented by a partial least squares discriminant analysis in
Figure 6. As evident from the plot, the control powder used in this trial correlated with most of the identified volatile compounds. These compounds can all be traced back to the raw materials—pea and oat—and can be considered as remnants of protein powder production [
46,
47]. The amount of these compounds, however, noticeably decreased as a result of the extrusion process which can be primarily attributed to the use of heat (up to 150 °C). This compares to some of the earlier studies where the application of heat during extrusion has been tied to compositional losses [
48,
49].
The extruded samples were different from the control powder due to an increase in various pyrazines, thiophenes (thiophene, 2-methyl-; thiophene, 2-pentyl-; thiophene, 2-hexyl-), furans (furfural, 2-furanmethanol), and 1-pentanethiol. These compounds could all be viewed as products of the Maillard reaction. The latter could also have formed as a result of interaction between the by-products of Maillard reaction and lipid oxidation [
50].
In the Maillard reaction, reductive sugars react with amino acids to produce brown pigments (melanoidins) and characteristic volatile compounds [
51]. Volatile compounds produced as a result include but not limited to pyrazines, pyridines, pyrroles, furans, furanones, pyranones, oxazoles, thiophenes [
52]. The Maillard reaction compounds associated with extrusion were previously found to depend on the initial amino acid composition of the extrudate. For example, increased production of sulfur compounds was noted in extrudates supplemented with cysteine [
53]. The formation of thiophenes and furans could also be tied to specific amino acids contained in the samples: the formation of thiophenes could be attributed to methionine and its inclusion in the Maillard reaction pathways; the formation of furans—to alanine [
54,
55]. The increase in pyrazines was also previously connected to individual amino acid supplementation [
56]. Hence the fermented extrudate correlated with most of the identified pyrazines due to the increase in free amino acid content. Additionally, higher production of pyrazines in the fermented sample could be attributed to its lower pH, corroborating other studies [
57].
The phytase-treated sample can be considered similar to the extruded control which means that phytase action did not add to the release of volatile compounds or their precursors. The fermented extruded sample, on the other hand, additionally contained elevated amounts of carboxylic acids (butanoic acid; butanoic acid, 2-methyl-; butanoic acid, 3-methyl-; pentanoic acid; hexanoic acid; heptanoic acid; nonanoic acid), alcohols (1-hexanol, benzyl alcohol), acetoin, and 2,4-decadienal. These can be considered as products of metabolic activity of the chosen starter culture (VEGE 053) that consists primarily of lactic acid bacteria (
Streptococcus,
Lactobacillus,
Lactococcus) and bifidobacteria [
58,
59]. The higher amount of carboxylic acids in this sample correlated with the sourness in both perceived odor and taste. This could have also contributed to the “fermented” off-flavor perceived by the panel. The off-flavor described as ”medicinal”, “soapy”, and “citrus-like” could be attributed to the presence of 2,4-decadienal in this sample (based on
www.thegoodscentscompany.com database description for this compound).