High Hydrostatic Pressure Assisted by Celluclast® Releases Oligosaccharides from Apple By-Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Celluclast® 1.5 L Enzyme
2.2. Optimization of Celluclast® Treatment
2.3. HHP Treatment Assisted by Celluclast® Procedure
2.4. High Performance Liquid Chromatography Instrument, Calibration and Validation
2.5. Statistical Analyses
3. Results
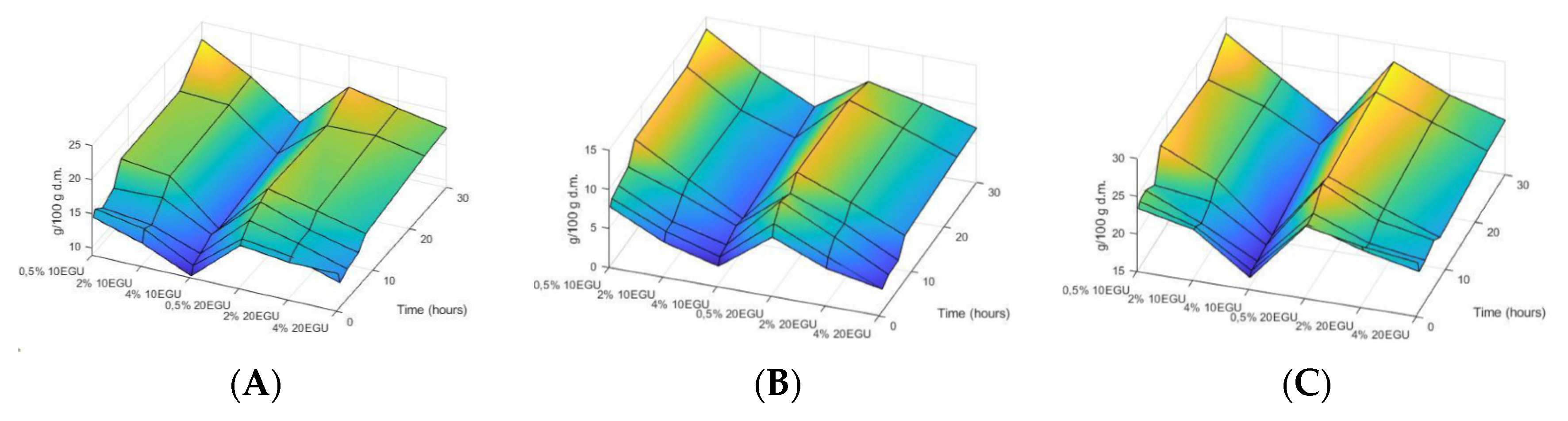
3.1. Optimization of Enzymatic Treatment with Celluclast®
- X1 = Enzyme dose in EGU
- X2 = Time in hours
- X3 = Substrate concentration in percentage (w/v)
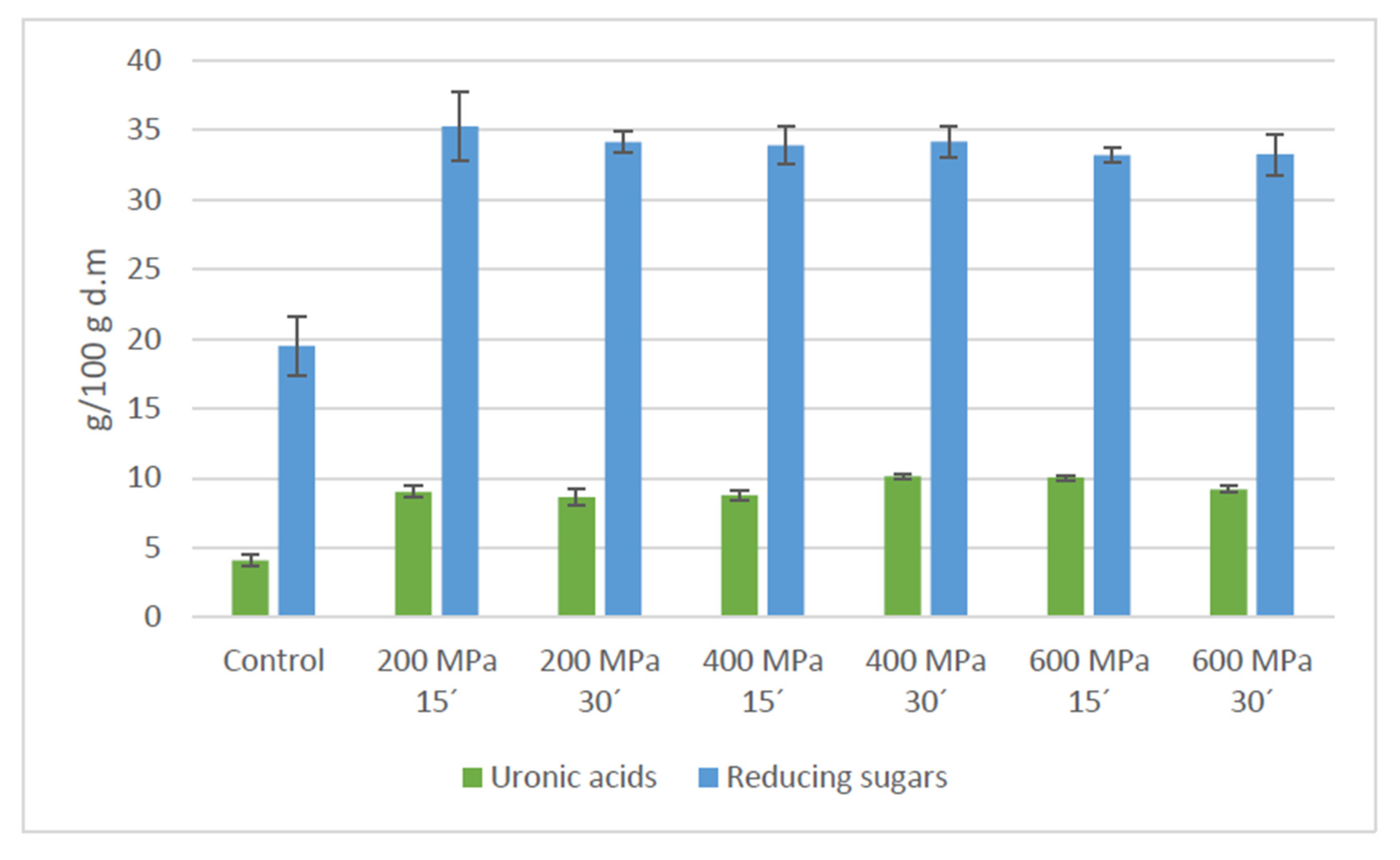
3.2. HHP Assisted by Celluclast® Treatment of Apple By-Product
3.3. HPLC Calibration and Validation Methodology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz, M.G.; Bastos, R.; Pinto, M.; Ferreira, J.M.; Santos, J.F.; Wessel, D.F.; Coelho, E.; Coimbra, M.A. Waste mitigation: From an effluent of apple juice concentrate industry to a valuable ingredient for food and feed applications. J. Clean. Prod. 2018, 193, 652–660. [Google Scholar] [CrossRef]
- Barreira, J.C.; Arraibi, A.A.; Ferreira, I.C. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Le Bourvellec, C.; Renard, C.M.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessela, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Hawken, P. Drawdown: The Most Comprehensive Plan ever Proposed to Reverse Global Warming; Penguin: London, UK, 2018. [Google Scholar]
- Yan, L.; Li, T.; Liu, C.; Zheng, L. Effects of high hydrostatic pressure and superfine grinding treatment on physicochemical/functional properties of pear pomace and chemical composition of its soluble dietary fibre. LWT 2019, 107, 171–177. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; de la Peña, R.J.; Pérez-Cózar, M.L.; Rupérez, P.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J. Apple by-product dietary fibre exhibits potential prebiotic and hypolipidemic effects in high-fat fed Wistar rats. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100219. [Google Scholar] [CrossRef]
- Macagnan, F.T.; da Silva, L.P.; Hecktheuer, L.H. Dietary fibre: The scientific search for an ideal definition and methodology of analysis, and its physiological importance as a carrier of bioactive compounds. Food Res. Int. 2016, 85, 144–154. [Google Scholar] [CrossRef]
- de la Rosa, O.; Flores-Gallegos, A.C.; Muñíz-Marquez, D.; Nobre, C.; Contreras-Esquivel, J.C.; Aguilar, C.N. Fructooligosaccharides production from agro-wastes as alternative low-cost source. Trends Food Sci. Technol. 2019, 91, 139–146. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Sun, Y.; Zheng, H.; Tang, Y.; Gao, X.; Songa, C.; Liu, J.; Long, Y.; Liu, L.; et al. Apple polysaccharide could promote the growth of Bifidobacterium longum. Int. J. Biol. Macromol. 2020, 152, 1186–1193. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Li, C.; Hu, C.; Su, F.; Liu, R.; Zeng, M.; Zhao, D.; Liu, J.; Guo, J.; et al. A mix of apple pomace polysaccharide improves mitochondrial function and reduces oxidative stress in the liver of high-fat diet-induced obese mice. Mol. Nutr. Food Res. 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Condezo-Hoyos, L.; Pérez-López, E.; Rupérez, P. Improved evaporative light scattering detection for carbohydrate analysis. Food Chem. 2015, 180, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A.; Sowndhararajan, K. Comparison of Acidic and Enzymatic Pectin Extraction from Passion Fruit Peels and Its Gel Properties. J. Food Process. Eng. 2016, 39, 501–511. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef]
- Sabater, C.; Corzo, N.; Olano, A.; Montilla, A. Enzymatic extraction of pectin from artichoke (Cynara scolymus L.) by-products using Celluclast® 1.5 L. Carbohydr. Polym. 2018, 190, 43–49. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Application of Celluclast 1.5L in apple pectin extraction. Carbohydr. Polym. 2015, 134, 251–257. [Google Scholar] [CrossRef]
- Li, P.; Xia, J.; Nie, Z.; Shan, Y. Pectic oligosaccharides hydrolyzed from orange peel by fungal multi- enzyme complexes and their prebiotic and antibacterial potentials. LWT—Food Sci. Technol. 2016, 69, 203–210. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2016, 36, 594–606. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, L.; Zhang, R.; Huang, F.; Deng, Y.; Zhang, M. International Journal of Biological Macromolecules Ultrahigh pressure-assisted enzymatic extraction maximizes the yield of longan pulp polysaccharides and their acetylcholinesterase inhibitory activity in vitro. Int. J. Biol. Macromol. 2017, 96, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Pérez-lópez, E.; Mateos-aparicio, I.; Rupérez, P. Low molecular weight carbohydrates released from Okara by enzymatic treatment under high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2016, 38, 76–82. [Google Scholar] [CrossRef][Green Version]
- Pérez-lópez, E.; Mateos-aparicio, I.; Rupérez, P. Okara treated with high hydrostatic pressure assisted by Ultraflo® L: Effect on solubility of dietary fibre. Innov. Food Sci. Emerg. Technol. 2016, 33, 32–37. [Google Scholar] [CrossRef]
- Pérez-lópez, E.; Mateos-aparicio, I.; Rupérez, P. High hydrostatic pressure aided by food-grade enzymes as a novel approach for Okara valorization. Innov. Food Sci. Emerg. Technol. 2017, 42, 197–203. [Google Scholar] [CrossRef]
- Pérez-lópez, E.; Mateos-aparicio, I.; Rupérez, P. Determination of soluble dietary fibre content of Okara treated with high hydrostatic pressure and enzymes: A comparative evaluation of two methods (AOAC and HPLC-ELSD). J. Food Sci. Technol. 2017, 54, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Scott, R.W. Colorimetric determination of hexuronic acids in plant materials. Anal. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Molecular weight distribution of polysaccharides from edible seaweeds by high-performance size-exclusion chromatography (HPSEC). Talanta 2012, 93, 153–159. [Google Scholar] [CrossRef]
- Boccia, F.; Di, P.; Covino, D.; Poli, A. Food waste and bio-economy: A scenario for the Italian tomato market. J. Clean. Prod. 2019, 227, 424–433. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Food and Bioproducts Processing Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2014, 94, 668–674. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.; Yu, W.; Imm, J.; Joo, H. Enzyme-assisted extraction of cactus bioactive molecules under high hydrostatic pressure. J. Sci. Food Agric. 2013, 94, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Encalada, A.M.I.; Pérez, C.D.; Flores, S.K.; Rossetti, L.; Fissore, E.N.; Rojas, A.M. Antioxidant pectin enriched fractions obtained from discarded carrots (Daucus carota L.) by ultrasound-enzyme assisted extraction. Food Chem. 2019, 289, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willfo, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Lan, C.; Ding, H.; Yan, C.; Dou, Y. Protective effect of polysaccharide from Sophora japonica L. flower buds against UVB radiation in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B Biol. 2019, 191, 135–142. [Google Scholar] [CrossRef]

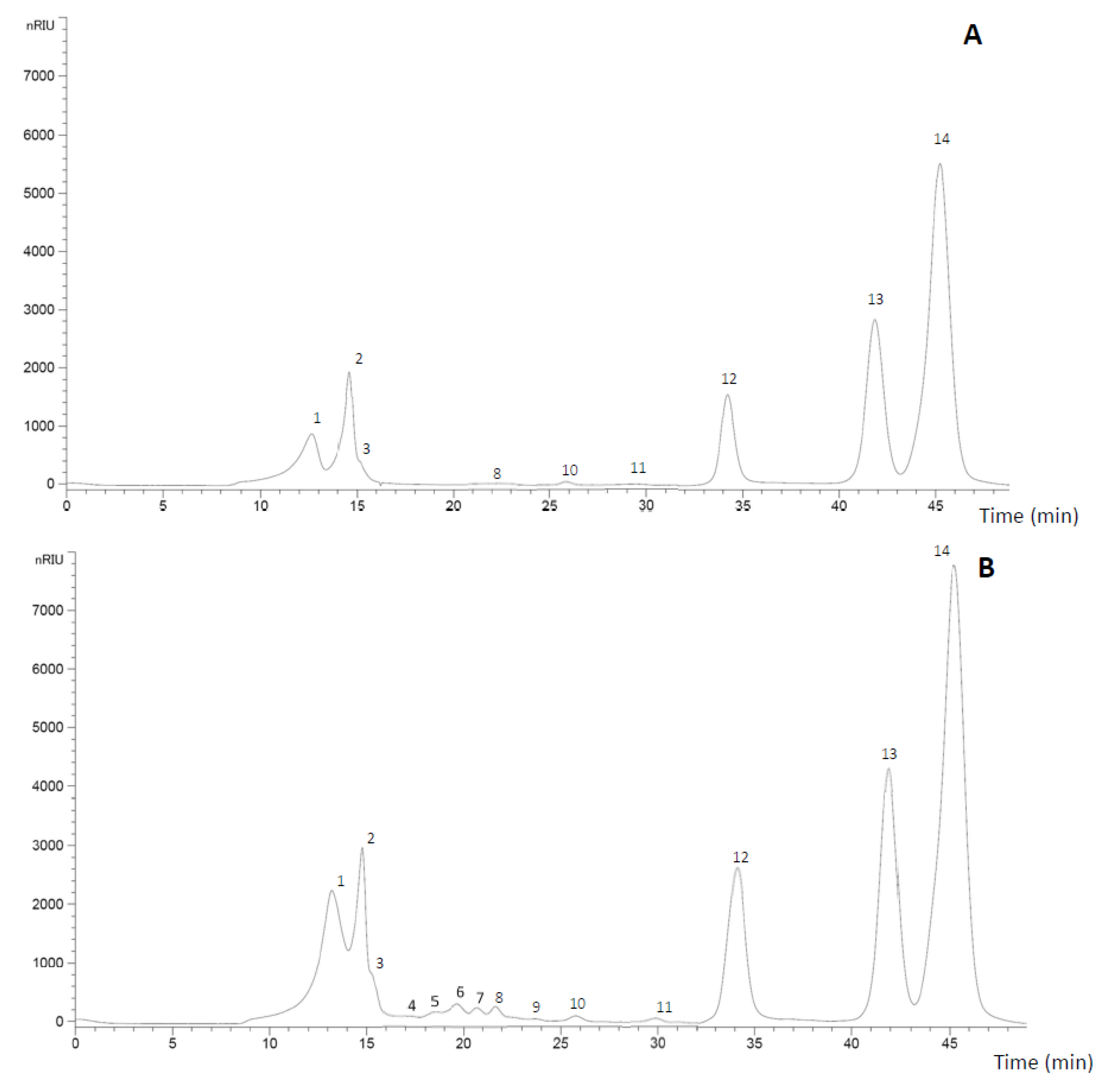
| RT (min) | Sample Time (min) | Control | 200 MPa | 400 MPa | 600 MPa | ||||
|---|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 15 | 30 | 15 | 30 | ||||
| Peak 1 | 13.20 | 3.63 ± 0.10 a | 8.51 ± 0.33 cd | 7.73 ± 0.38 b | 8.02 ± 0.22 bc | 8.97 ± 0.33 d | 8.62 ± 0.24 d | 8.49 ± 0.32 cd | |
| Peak 2 | 14.77 | 3.13 ± 0.05 a | 3.95 ± 0.51 e | 3.51 ± 0.10 b | 3.71 ± 0.10 c | 4.05 ± 0.05 d | 3.77 ± 0.14 c | 3.96 ± 0.14 d | |
| Peak 3 | 15.04 | 0.46 ± 0.07 a | 0.87 ± 0.08 d | 0.73 ± 0.07 c | 0.82 ± 0.02 cd | 0.89 ± 0.05 d | 0.58 ± 0.11 b | 0.56 ± 0.04 b | |
| Peak 4 | 17.21 | Nd | 1.01 ± 0.15 b | 0.84 ± 0.20 b | 0.74 ± 0.13 b | 0.77 ± 0.07 b | 2.16 ± 0.32 c | 2.13 ± 0.29 c | |
| Peak 5 | 18.57 | Nd | 1.32 ± 0.03 d | 1.08 ± 0.15 c | 1.01 ± 0.09 bc | 1.14 ± 0.08 cd | 1.15 ± 0.17 cd | 0.82 ± 0.14 b | |
| Peak 6 | 19.68 | Nd | 1.09 ± 0.15 e | 0.88 ± 0.07 de | 0.82 ± 0.06 cd | 0.94 ± 0.03 de | 0.70 ± 0.04 c | 0.52 ± 0.14 b | |
| Peak 7 | 20.74 | Nd | 0.73 ± 0.09 d | 0.59 ± 0.15 c | 0.50 ± 0.05 bc | 0.55 ± 0.03 c | 0.49 ± 0.02 bc | 0.41 ± 0.07 b | |
| Peak 8 | 21.69 | 0.27 ± 0.05 a | 0.72 ± 0.07 e | 0.68 ± 0.03 de | 0.59 ± 0.03 c | 0.64 ± 0.04 cd | 0.75 ± 0.06 e | 0.49 ± 0.18 b | |
| Peak 9 | 22.33 | Nd | 0.77 ± 0.10 d | 0.60 ± 0.07 bc | 0.56 ± 0.07 bc | 0.65 ± 0.07 cd | 0.52 ± 0.08 b | 0.49 ± 0.02 b | |
| Peak 10 | 25.81 | 0.27 ± 0.06 a | 0.89 ± 0.30 b | 0.68 ± 0.15 b | 0.60 ± 0.10 b | 0.63 ± 0.08 b | 0.70 ± 0.15 b | 0.64 ± 0.39 b | |
| Peak 11 | 29.92 | 0.06 ± 0.01 a | 0.81 ± 0.13 b | 0.58 ± 0.12 b | 0.50 ± 0.16 b | 0.67 ± 0.07 b | 0.66 ± 0.18 b | 0.66 ± 0.39 b | |
| Peak 12 | 34.13 | 2.64 ± 0.08 a | 5.24 ± 0.60 bc | 5.06 ± 0.21 bc | 5.30 ± 0.11 bc | 5.55 ± 0.63 c | 4.92 ± 0.01 bc | 4.44 ± 0.05 b | |
| Peak 13 | 41.91 | 5.19 ± 0.34 a | 7.13 ± 0.46 c | 6.25 ± 0.10 b | 6.77 ± 0.39 bc | 6.84 ± 0.23 bc | 6.37 ± 0.04 b | 6.53 ± 0.31 b | |
| Peak 14 | 45.29 | 13.03 ± 0.28 a | 16.73 ± 1.57 bc | 15.85 ± 0.15 b | 16.77 ± 0.03 bc | 17.38 ± 0.36 c | 16.19 ± 0.02 bc | 16.55 ± 0.36 bc | |
| Total | 29.00 ± 0.73 a | 49.62 ± 2.11 c | 49.76 ± 1.59 c | 46.68 ± 1.82 b | 49.55 ± 1.52 c | 47.58 ± 0.49 bc | 46.69 ± 2.56 c | ||
| Sample | 200 MPa | 400 MPa | 600 MPa | |||
|---|---|---|---|---|---|---|
| Time (min) | 15 | 30 | 15 | 30 | 15 | 30 |
| Peak 1 | 7.88 ± 0.16 b | 8.93 ± 1.27 b | 8.69 ± 0.74 b | 8.71 ± 0.99 b | 8.02 ± 0.68 b | 9.52 ± 0.61 b |
| Peak 2 | 3.29 ± 0.12 a | 3.53 ± 0.40 a | 3.24 ± 0.22 a | 3.51 ± 0.21 a | 3.10 ± 0.20 a | 3.24 ± 0.06 a |
| Peak 3 | 0.70 ± 0.06 b | 0.82 ± 0.11 b | 0.78 ± 0.07 b | 0.86 ± 0.06 b | 0.70 ± 0.11 b | 0.76 ± 0.07 b |
| Peak 8 | 0.33 ± 0.02 a | 0.33 ±0.02 a | 0.28 ± 0.01 a | 0.35 ± 0.05 a | 0.33 ± 0.03 a | 0.35 ± 0.03 a |
| Peak 10 | 0.21 ± 0.01 a | 0.21 ± 0.01 a | 0.20 ± 0.02 a | 0.21 ± 0.02 a | 0.21 ± 0.01 a | 0.22 ± 0.02 a |
| Peak 11 | 0.10 ± 0.01 a | 0.11 ± 0.02 a | 0.11 ± 0.02 a | 0.13 ± 0.06 a | 0.07 ± 0.01 a | 0.13 ± 0.05 a |
| Peak 12 | 2.77 ±0.17 a | 2.91 ± 0.12 a | 2.60 ± 0.23 a | 2.69 ± 0.45 a | 2.37 ± 0.36 a | 2.29 ± 1.30 a |
| Peak 13 | 6.09 ± 0.18 b | 6.12 ± 0.06 b | 6.51 ± 0.20 b | 6.39 ± 0.43 b | 6.53 ± 0.23 b | 6.32 ± 0.28 b |
| Peak 14 | 15.07 ± 0.27 b | 15.27 ± 0.09 b | 15.58 ± 0.11 b | 15.39 ± 0.64 b | 15.53 ± 0.39 b | 15.21 ± 0.37 b |
| Total | 36.19 ± 1.05 b | 37.35 ± 2.27 b | 37.96 ± 0.52 b | 37.50 ± 0.74 b | 36.04 ± 1.42 b | 37.90 ± 0.80 b |
| Standard | MW (kDa) | RT (min) | Linearity (R2) | Sensitivity | Estimated MW a (kDa) | LOD b (µg/mL) | LOQ c (µg/mL) | Repeatability RSD (%) | Inter-Day Precision RSD (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Slope (m) | Intercept (b) | |||||||||
| Pullulan 100 | 100 | 14.99 | 0.999 | 1.032 | 1.957 | 104.06 | 1.81 | 6.02 | 0.01 | 0.01 |
| Pullulan 50 | 50 | 15.18 | 0.999 | 1.033 | 2.166 | 40.09 | 1.23 | 4.09 | 0.01 | <0.01 |
| Pullulan 20 | 20 | 15.31 | 0.998 | 1.054 | 1.963 | 20.88 | 1.68 | 5.59 | 0.01 | 0.01 |
| Pullulan 10 | 10 | 15.40 | 0.999 | 0.991 | 2.130 | 13.29 | 1.82 | 6.08 | 0.01 | 0.04 |
| Inulin | 5.94 | 15.59 | 0.999 | 1.068 | 1.519 | 5.12 | 1.22 | 4.07 | 0.01 | 0.06 |
| Verbascose | 0.83 | 23.40 | 0.999 | 0.948 | 2.207 | 0.77 | 3.25 | 10.83 | <0.01 | 0.01 |
| Stachyose | 0.67 | 25.93 | 0.999 | 1.044 | 1.947 | 0.64 | 3.32 | 11.08 | 0.01 | 0.02 |
| Cellotriose | 0.50 | 28.10 | 0.999 | 1.041 | 1.943 | 0.55 | 3.33 | 11.11 | 0.01 | 0.03 |
| Raffinose | 0.50 | 29.58 | 0.999 | 1.017 | 1.966 | 0.49 | 3.29 | 10.96 | 0.01 | 0.02 |
| Cellobiose | 0.34 | 33.80 | 0.999 | 0.982 | 2.165 | 0.36 | 3.34 | 11.15 | 0.03 | 0.03 |
| Sucrose | 0.34 | 34.28 | 0.999 | 0.968 | 2.213 | 0.35 | 3.49 | 11.63 | <0.01 | <0.01 |
| Glucose | 0.18 | 41.93 | 0.999 | 0.911 | 2.461 | 0.20 | 3.51 | 11.70 | <0.01 | <0.01 |
| Fructose | 0.18 | 45.34 | 0.999 | 0.936 | 2.364 | 0.16 | 3.52 | 11.72 | 0.03 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Rupérez, P.; Mateos-Aparicio, I. High Hydrostatic Pressure Assisted by Celluclast® Releases Oligosaccharides from Apple By-Product. Foods 2020, 9, 1058. https://doi.org/10.3390/foods9081058
De la Peña-Armada R, Villanueva-Suárez MJ, Rupérez P, Mateos-Aparicio I. High Hydrostatic Pressure Assisted by Celluclast® Releases Oligosaccharides from Apple By-Product. Foods. 2020; 9(8):1058. https://doi.org/10.3390/foods9081058
Chicago/Turabian StyleDe la Peña-Armada, Rocío, María José Villanueva-Suárez, Pilar Rupérez, and Inmaculada Mateos-Aparicio. 2020. "High Hydrostatic Pressure Assisted by Celluclast® Releases Oligosaccharides from Apple By-Product" Foods 9, no. 8: 1058. https://doi.org/10.3390/foods9081058
APA StyleDe la Peña-Armada, R., Villanueva-Suárez, M. J., Rupérez, P., & Mateos-Aparicio, I. (2020). High Hydrostatic Pressure Assisted by Celluclast® Releases Oligosaccharides from Apple By-Product. Foods, 9(8), 1058. https://doi.org/10.3390/foods9081058





