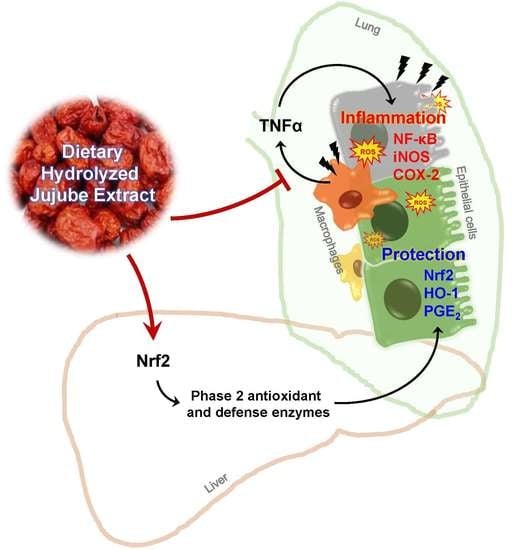
In Vivo Anti-Inflammatory Potential of Viscozyme®-Treated Jujube Fruit
Abstract
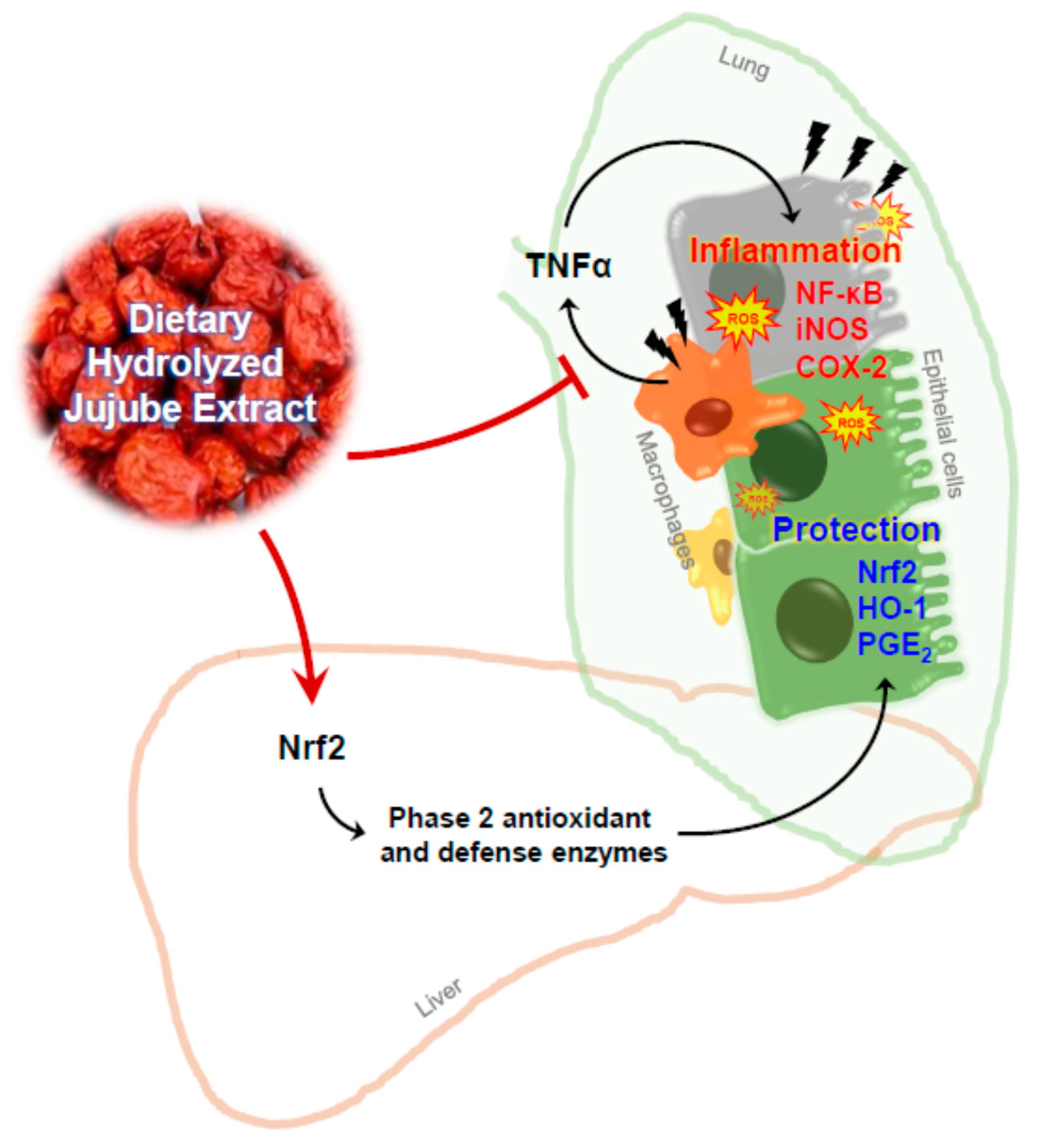
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrolyzed Jujube Extracts
2.2. Quantification of Total Phenolics and Flavonoids
2.3. High-Performance Liquid Chromatography Analysis
2.4. Cell Culture and Treatment
2.5. Antioxidant Response Element–Luciferase Reporter Assay
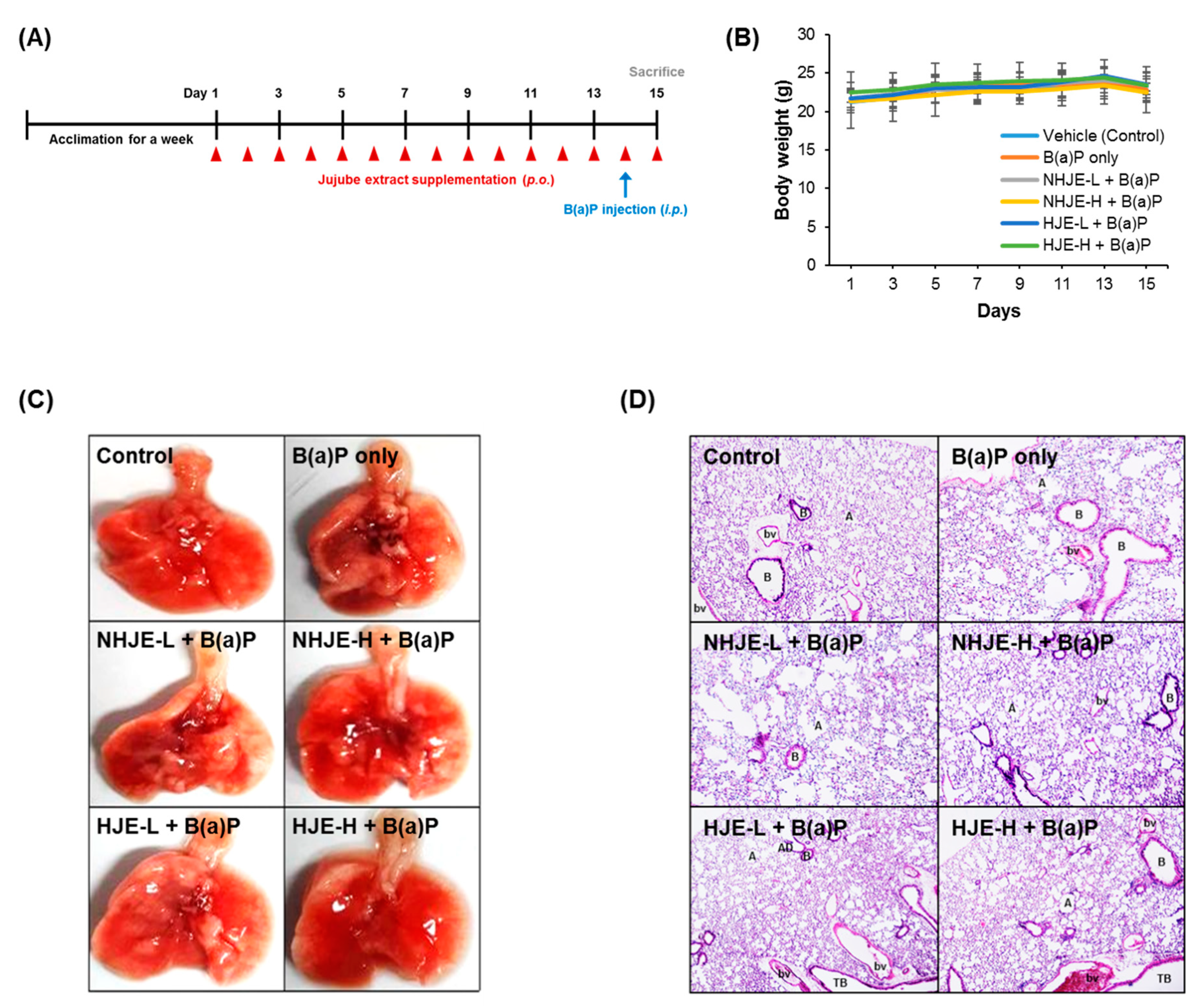
2.6. Animal Treatment
2.7. Histopathological Analysis
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Enzyme Hydrolysis of Jujube Fruit Increased Total Phenolic Content in Ethanolic Extract
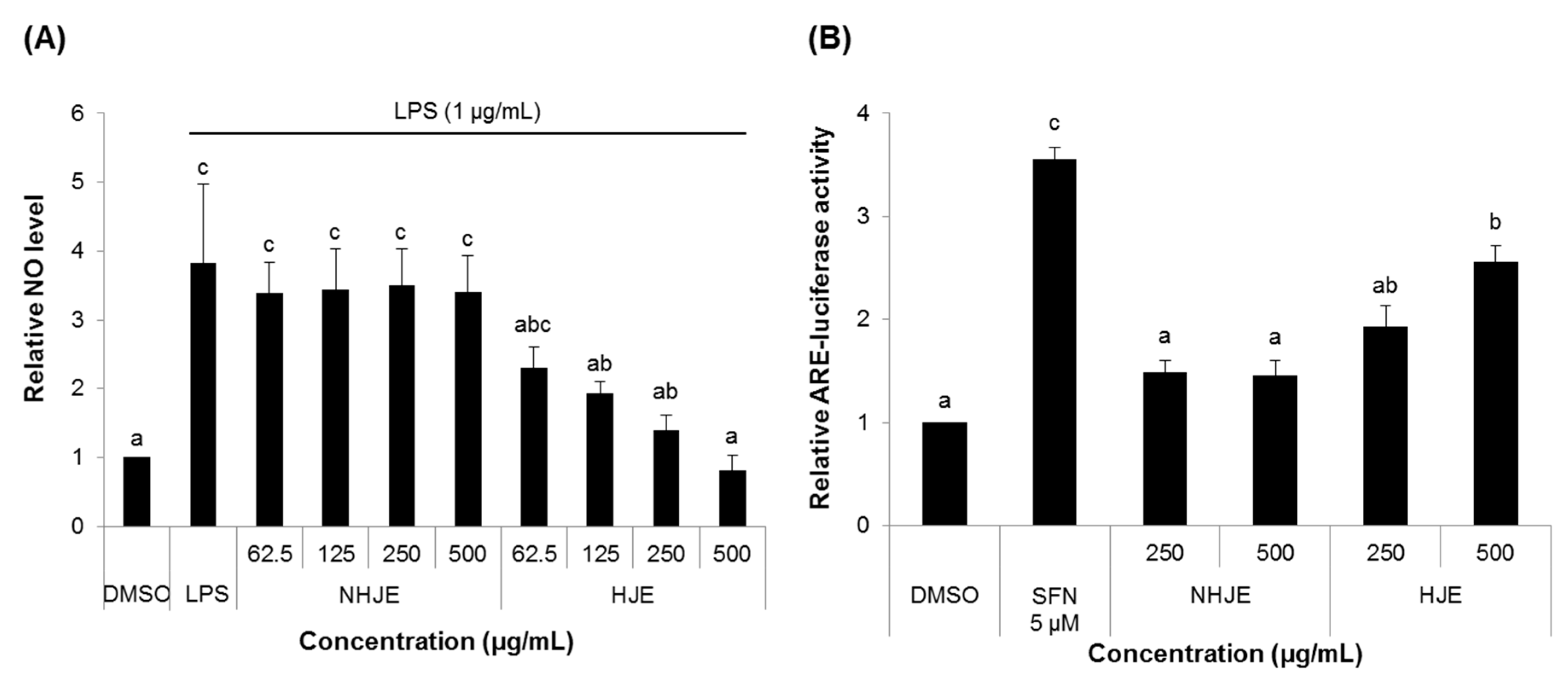
3.2. HJE Decreased NO Production and Increased ARE Transcription Activity in Cultured Cells
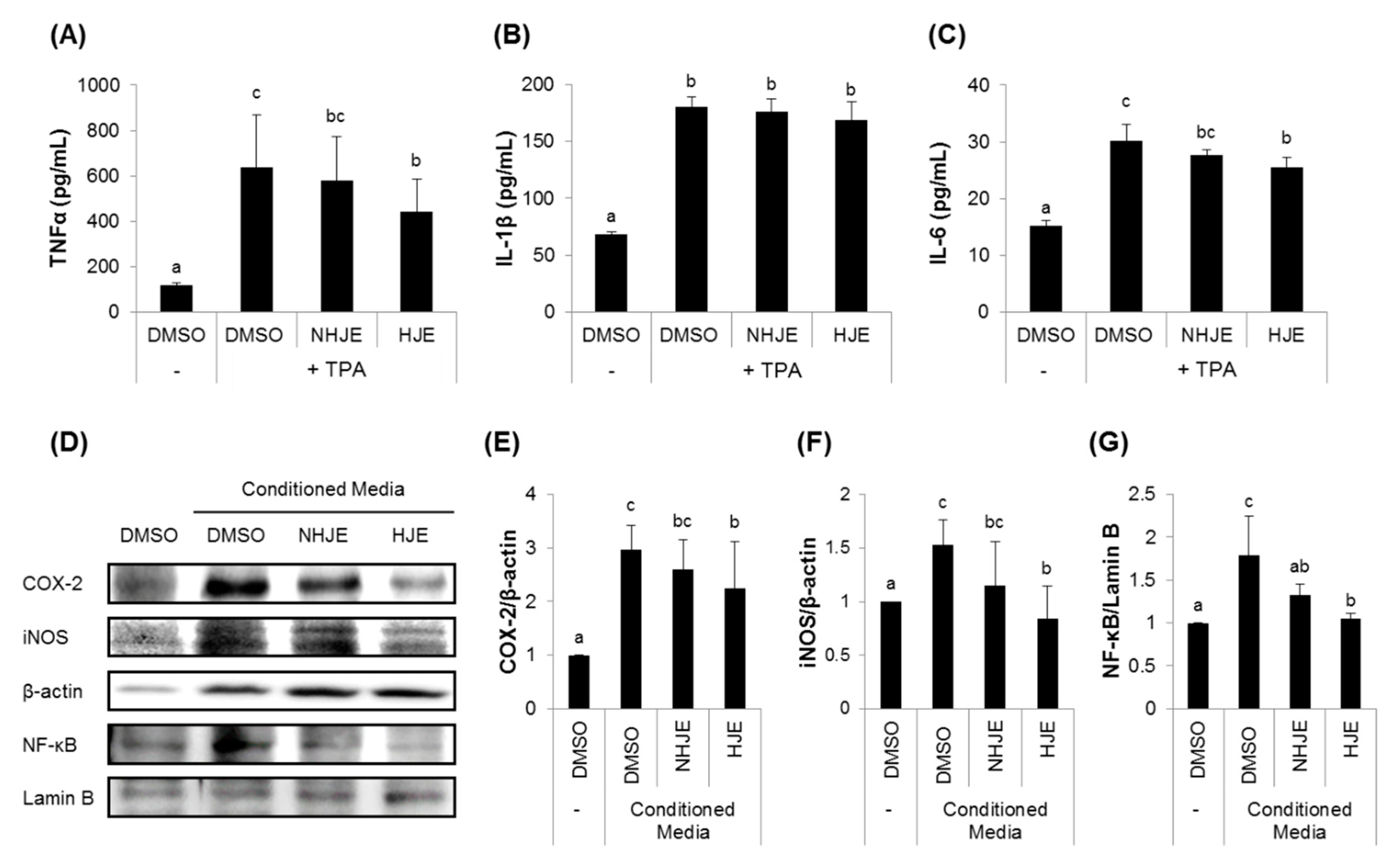
3.3. HJE Inhibited Secretions of Pro-Inflammatory Cytokines from TPA-Challenged THP-1 Cells and Expressions of Inflammation-Related Proteins in A549 Cells
3.4. Oral Supplementation of HJE Alleviated B(a)P-Induced Lung Injury in Mice
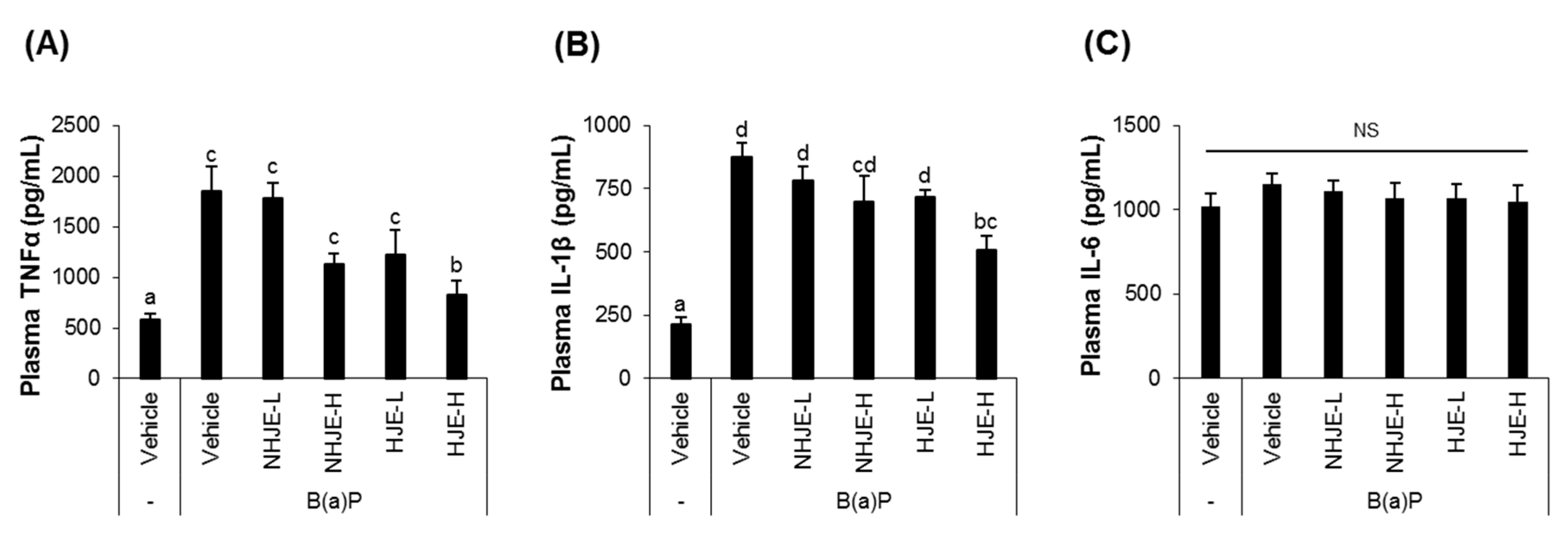
3.5. Oral Supplementation of HJE Lowered Plasma Pro-Inflammatory Cytokine Levels in B(a)P-Injected Mice
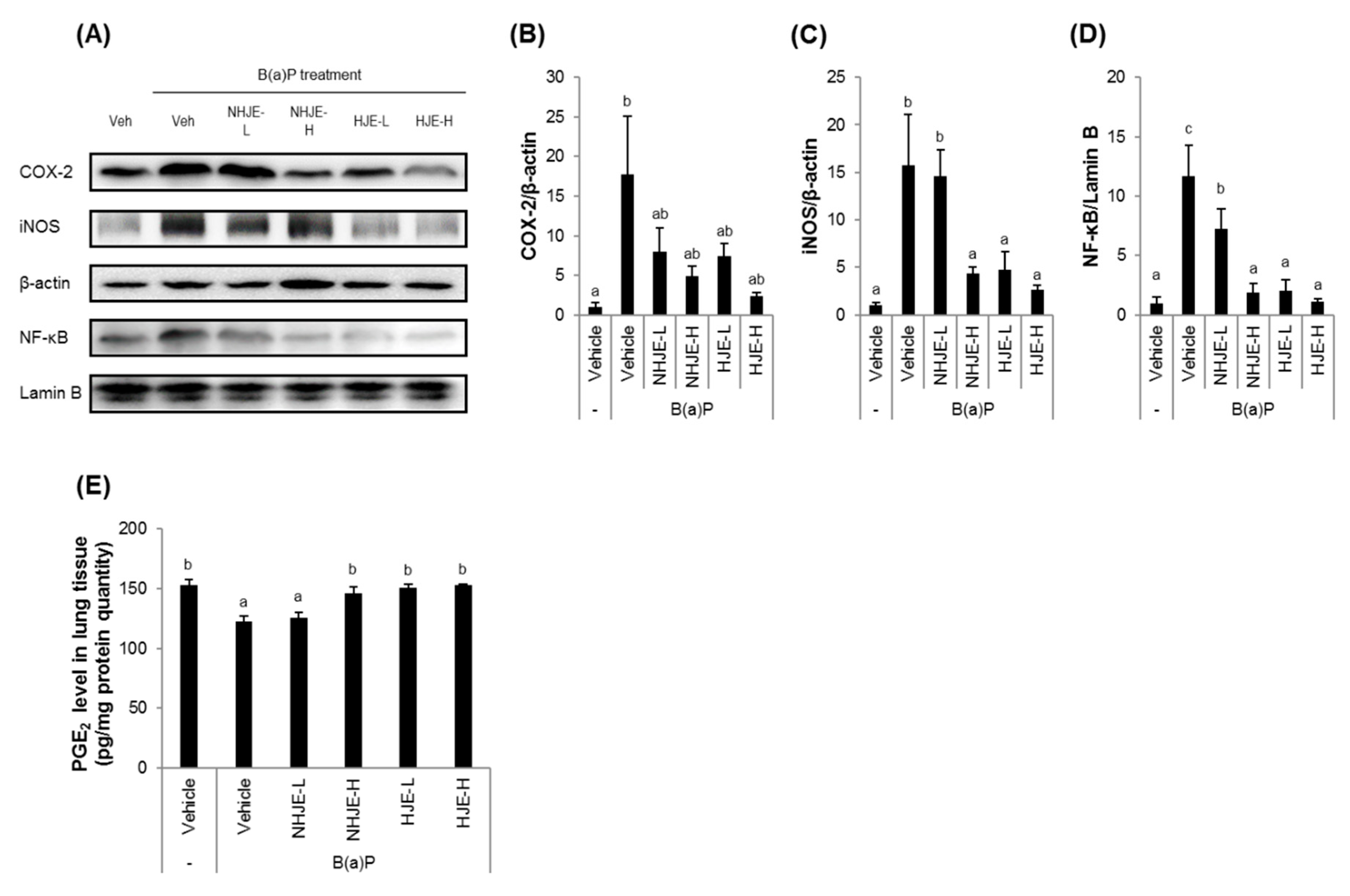
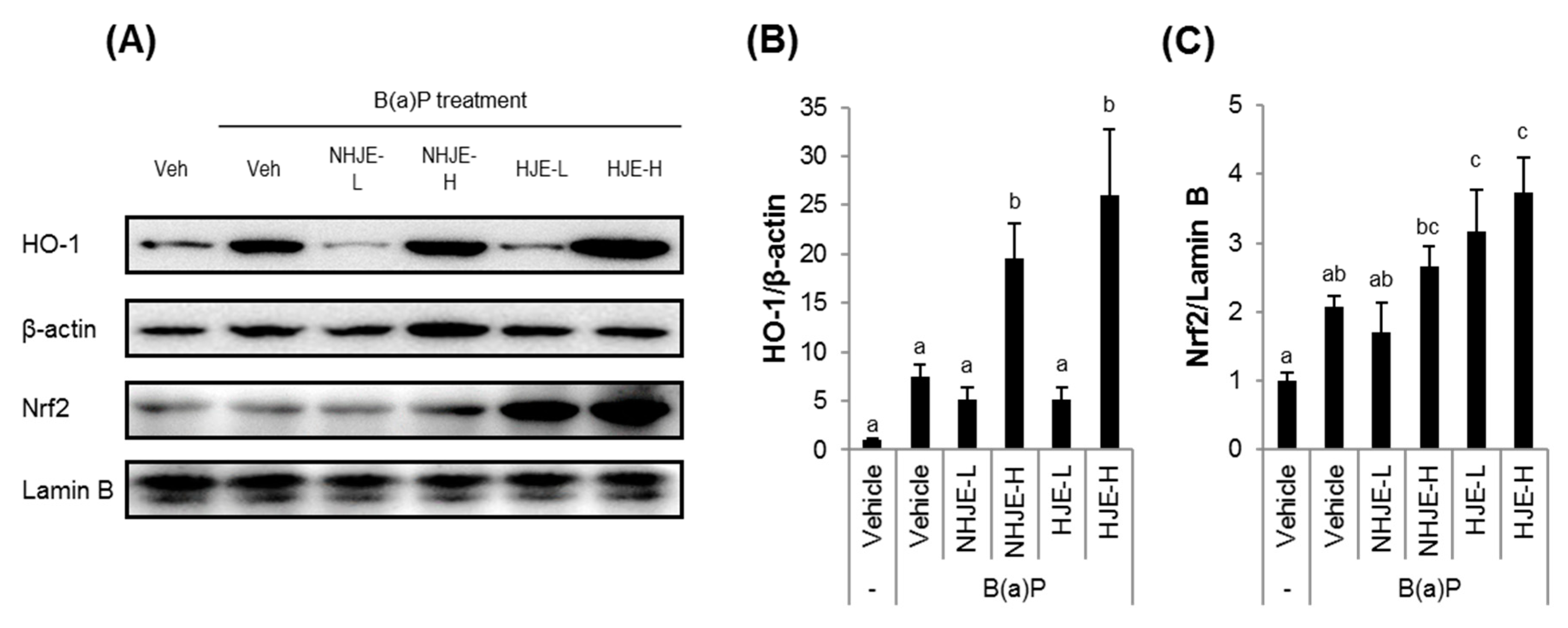
3.6. Oral Supplementation of HJE Suppressed the Expression of Inflammation-Related Proteins and Increased the Expression of Antioxidant Proteins in Lung Tissue
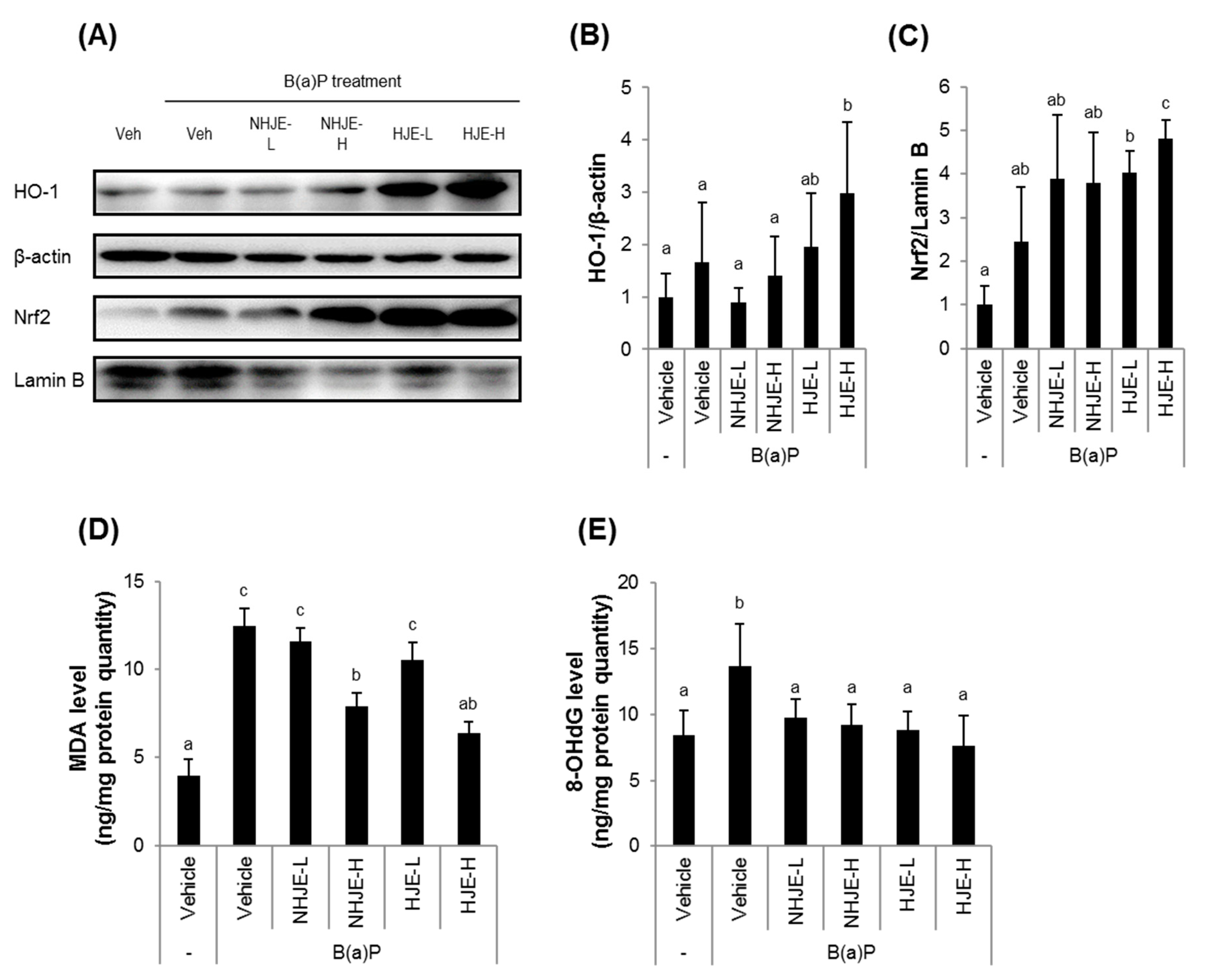
3.7. Oral Administration of HJE to Mice Increased Nrf2 and HO-1 Expression and Reduced Oxidative Stress in Liver Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inoue, K.-I.; Takano, H.; Yanagisawa, R.; Hirano, S.; Sakurai, M.; Shimada, A.; Yoshikawa, T. Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ. Health Perspect. 2006, 114, 1325–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qamar, W.; Khan, A.Q.; Khan, R.; Lateef, A.; Tahir, M.; Rehman, M.U.; Ali, F.; Sultana, S. Benzo(a)pyrene-induced pulmonary inflammation, edema, surfactant dysfunction, and injuries in rats: Alleviation by farnesol. Exp. Lung Res. 2012, 38, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Bozinovski, S.; Jones, J.; Powell, J.; Gras, J.; Lilja, A.; Hansen, M.J.; Gualano, R.C.; Irving, L.; Anderson, G. Differential protease, innate immunity, and NF-κB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L931–L945. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, X.T. Cellular and molecular regulation of innate inflammatory responses. Cell. Mol. Immunol. 2016, 13, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1–11. [Google Scholar]
- Benihoud, K.; Esselin, S.; Descamps, D.; Jullienne, B.; Salone, B.; Bobe, P.; Bonardelle, D.; Connault, E.; Opolon, P.; Saggio, I.; et al. Respective roles of TNF-alpha and IL-6 in the immune response-elicited by adenovirus-mediated gene transfer in mice. Gene Ther. 2007, 14, 533–544. [Google Scholar] [CrossRef]
- Brock, T.G.; McNish, R.W.; Peters-Golden, M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E-2. J. Biol. Chem. 1999, 274, 11660–11666. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, N.S.; Chan, M.V.; Zaiss, A.K.; Garcia-Vaz, E.; Jiao, J.; Berglund, L.M.; Verdu, E.F.; Ahmetaj-Shala, B.; Wallace, J.L.; Herschman, H.R.; et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-kappa B and NFAT transcriptional pathways. Proc. Natl. Acad. Sci. USA 2016, 113, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Kensler, T.W.; Wakabayash, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudina, M.; Liu, M.; Veberic, R.; Stampar, F.; Colaric, M. Phenolic compounds in the fruit of different varieties of Chinese jujube (Ziziphus jujuba Mill.). J. Hortic. Sci. Biotechnol. 2008, 83, 305–308. [Google Scholar] [CrossRef]
- Choi, S.-H.; Ahn, J.-B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Khoshkharam, M.; Zandi, P.; Sun, W.; Cheng, Q. Jujube, a super-fruit in traditional Chinese medicine, heading for modern pharmacological science. J. Med. Plants. Stud. 2019, 7, 173–178. [Google Scholar]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Sobhani, Z.; Nikoofal-Sahlabadi, S.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Sahebkar, A. Therapeutic effects of Ziziphus jujuba Mill. fruit in traditional and modern medicine: A review. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Liu, N.; Yang, M.; Huang, W.Z.; Wang, Y.J.; Yang, M.; Wang, Y.; Zhao, Z.X. Composition, antioxidant activities and hepatoprotective effects of the water extract of Ziziphus jujuba cv. Jinsixiaozao. RSC Adv. 2017, 7, 6511–6522. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Kimura, I.; Guo, X.; Luo, B.; Kobayashi, S. Combined Effects of Japanese-Sino Medicine Kakkon-to-Ka-Senkyu-Shin-I and Its Related Combinations and Component Drugs on Adjuvant-Induced Inflammation in Mice. Phytother. Res. 1992, 6, 209–216. [Google Scholar] [CrossRef]
- Chen, P.D.; Zhou, X.; Zhang, L.; Shan, M.Q.; Bao, B.H.; Cao, Y.D.; Kang, A.; Ding, A.W. Anti-inflammatory effects of Huangqin tang extract in mice on ulcerative colitis. J. Ethnopharmacol. 2015, 162, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Peng, Q.; Li, H.Y.; Liu, F.; Wang, M. Chemical Characterization and Anti-infllammatory Activity of Polysaccharides from Zizyphus jujube cv. Muzao. Int. J. Food Eng. 2017, 13, 20160382. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Ninave, P.B.; Patil, S.D. Antiasthmatic potential of Zizyphus jujuba Mill and Jujuboside B.—Possible role in the treatment of asthma. Respir. Physiol. Neurobiol. 2019, 260, 28–36. [Google Scholar] [CrossRef]
- Kwon, J.H.; Won, S.J.; Moon, J.H.; Kim, C.W.; Ahn, Y.S. Control of Fungal Diseases and Increase in Yields of a Cultivated Jujube Fruit (Zizyphus jujuba Miller var. inermis Rehder) Orchard by Employing Lysobacter antibioticus HS124. Forests 2019, 10, 1146. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Wu, Z.; Liu, K.H.; Jang, C.H.; Kim, H.J.; Kim, J.S.; Kim, J.S. Improved extraction of resveratrol and antioxidants from grape peel using heat and enzymatic treatments. J. Sci. Food Agric. 2019, 99, 4043–4053. [Google Scholar] [CrossRef]
- Woo, Y.; Lee, H.; Jeong, Y.S.; Shin, G.Y.; Oh, J.G.; Kim, J.S.; Oh, J. Antioxidant Potential of Selected Korean Edible Plant Extracts. BioMed Res. Int. 2017, 2017, 7695605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.; Oh, J.; Kim, J.S. Suppression of Nrf2 Activity by Chestnut Leaf Extract Increases Chemosensitivity of Breast Cancer Stem Cells to Paclitaxel. Nutrients 2017, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Oh, J.; Hahn, D.; Kwon, C.S.; Lee, J.S.; Kim, J.S. Protective Effect of Glyceollins in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J. Med. Food 2017, 20, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.; Oh, J.; Lim, J.S.; Lee, J.S.; Kim, J.S. Protective Effects of Dioscorea batatas Flesh and Peel Extracts against Ethanol-Induced Gastric Ulcer in Mice. Nutrients 2018, 10, 1680. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.S.; Oh, J.; Byeon, S.; Lee, J.S.; Kim, J.S. Protective Effect of Dioscorea batatas Peel Extract Against Intestinal Inflammation. J. Med. Food 2018, 21, 1204–1217. [Google Scholar] [CrossRef]
- Barnwal, P.; Vafa, A.; Afzal, S.M.; Shahid, A.; Hasan, S.K.; Alpashree; Sultana, S. Benzo(a)pyrene induces lung toxicity and inflammation in mice: Prevention by carvacrol. Hum. Exp. Toxicol. 2018, 37, 752–761. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alrumaihi, F.; Alsahli, M.A.; Alhommrani, M.F.; Khan, A.; Rahmani, A.H. Curcumin, an Active Constituent of Turmeric Spice: Implication in the Prevention of Lung Injury Induced by Benzo(a) Pyrene (BaP) in Rats. Molecules 2020, 25, 724. [Google Scholar] [CrossRef] [Green Version]
- Guzel, A.; Kanter, M.; Aksu, B.; Basaran, U.N.; Yalcin, O.; Guzel, A.; Uzun, H.; Konukoglu, D.; Karasalihoglu, S. Preventive effects of curcumin on different aspiration material-induced lung injury in rats. Pediatr. Surg. Int. 2009, 25, 83–92. [Google Scholar] [CrossRef]
- Vancheri, C.; Mastruzzo, C.; Sortino, M.A.; Crimi, N. The lung as a privileged site for the beneficial actions of PGE(2). Trends Immunol. 2004, 25, 40–46. [Google Scholar] [CrossRef]
- Shahid, A.; Ali, R.; Ali, N.; Hasan, S.K.; Barnwal, P.; Afzal, S.M.; Vafa, A.; Sultana, S. Methanolic bark extract of Acacia catechu ameliorates benzo(a)pyrene induced lung toxicity by abrogation of oxidative stress, inflammation, and apoptosis in mice. Environ. Toxicol. 2017, 32, 1566–1577. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Sriram, C.S.; Gogoi, R. Benzo (a) pyrene induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacol. Rep. 2015, 67, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Podechard, N.; Lecureur, V.; Le Ferrec, E.; Guenon, I.; Sparfel, L.; Gilot, D.; Gordon, J.R.; Lagente, V.; Fardel, O. Interleukin-8 induction by the environmental contaminant benzo(a)pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol. Lett. 2008, 177, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Isaacs, S.; Trushin, N. Lung-Tumor Induction in a/J Mice by the Tobacco-Smoke Carcinogens 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone and Benzo[a]Pyrene—A Potentially Useful Model for Evaluation of Chemopreventive Agents. Carcinogenesis 1994, 15, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Dang, F.; Gao, J.; Zhao, H.; Qi, S.; Gao, M. Acute benzo[a]pyrene treatment causes different antioxidant response and DNA damage in liver, lung, brain, stomach and kidney. Heliyon 2018, 4, e00898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, S.Y.; Ha, H.J.; Cha, S.H.; Lee, S.K.; Hur, S.J. Rutin attenuates lipopolysaccharide-induced nitric oxide production in macrophage cells. J. Food Nutr. Res. 2015, 3, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharmacol. 2016, 771, 84–92. [Google Scholar] [CrossRef]
- Sun, T.; Tang, J.; Powers, J.R. Effect of pectolytic enzyme preparations on the phenolic composition and antioxidant activity of asparagus juice. J. Agric. Food Chem. 2005, 53, 42–48. [Google Scholar] [CrossRef]
- Sun, T.; Powers, J.R.; Tang, J. Effect of enzymatic macerate treatment on rutin content, antioxidant activity, yield, and physical properties of asparagus juice. J. Food Sci. 2007, 72, S267–S271. [Google Scholar] [CrossRef]
- Xu, C.M.; Yagiz, Y.; Borejsza-Wysocki, W.; Lu, J.; Gu, L.W.; Ramirez-Rodrigues, M.M.; Marshall, M.R. Enzyme release of phenolics from muscadine grape (Vitis rotundifolia Michx.) skins and seeds. Food Chem. 2014, 157, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Hassen, I.; Chaabane, H.; Jemli, M.; Dallali, S.; Sebei, H.; Casabianca, H. Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): Impact on yield, chemical composition and antimicrobial activity. Ind. Crop. Prod. 2013, 47, 291–299. [Google Scholar] [CrossRef]
- Pingili, R.B.; Challa, S.R.; Pawar, A.K.; Toleti, V.; Kodali, T.; Koppula, S. A systematic review on hepatoprotective activity of quercetin against various drugs and toxic agents: Evidence from preclinical studies. Phytother. Res. 2020, 34, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef]
- Araujo, N.P.D.; de Matos, N.A.; Mota, S.L.A.; de Souza, A.B.F.; Cangussu, S.D.; de Menezes, R.C.A.; Bezerra, F.S. Quercetin Attenuates Acute Lung Injury Caused by Cigarette Smoke Both In Vitro and In Vivo. Copd 2020. [Google Scholar] [CrossRef]
- Zhang, X.C.; Cai, Y.L.; Zhang, W.; Chen, X.H. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem. Cell Biol. 2018, 96, 742–751. [Google Scholar] [CrossRef]
- Farazuddin, M.; Mishra, R.; Jing, Y.X.; Srivastava, V.; Comstock, A.T.; Sajjan, U.S. Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PLoS ONE 2018, 13, e0199612. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Jan, A.T.; Kamli, M.R.; Murtaza, I.; Singh, J.B.; Ali, A.; Haq, Q. Dietary flavonoid quercetin and associated health benefits—An overview. Food Rev. Int. 2010, 26, 302–317. [Google Scholar] [CrossRef]
| NHJE | HJE | |
|---|---|---|
| Total phenolics (mg GAE 2/g dry weight) | 6.73 ± 0.83 a | 12.50 ± 1.69 b |
| Rutin (mg/g dry weight) | 12.68 ± 6.06 a | 11.94 ± 4.99 a |
| Quercetin (mg/g dry weight) | 0.34 ± 0.59 a | 3.72 ± 2.54 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Oh, J.; Jang, C.H.; Lim, J.S.; Lee, J.S.; Kim, J.-S. In Vivo Anti-Inflammatory Potential of Viscozyme®-Treated Jujube Fruit. Foods 2020, 9, 1033. https://doi.org/10.3390/foods9081033
Kim Y, Oh J, Jang CH, Lim JS, Lee JS, Kim J-S. In Vivo Anti-Inflammatory Potential of Viscozyme®-Treated Jujube Fruit. Foods. 2020; 9(8):1033. https://doi.org/10.3390/foods9081033
Chicago/Turabian StyleKim, Yoonsu, Jisun Oh, Chan Ho Jang, Ji Sun Lim, Jeong Soon Lee, and Jong-Sang Kim. 2020. "In Vivo Anti-Inflammatory Potential of Viscozyme®-Treated Jujube Fruit" Foods 9, no. 8: 1033. https://doi.org/10.3390/foods9081033
APA StyleKim, Y., Oh, J., Jang, C. H., Lim, J. S., Lee, J. S., & Kim, J.-S. (2020). In Vivo Anti-Inflammatory Potential of Viscozyme®-Treated Jujube Fruit. Foods, 9(8), 1033. https://doi.org/10.3390/foods9081033