Phenolic and Aroma Changes of Red and White Wines during Aging Induced by High Hydrostatic Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Experimental Conditions
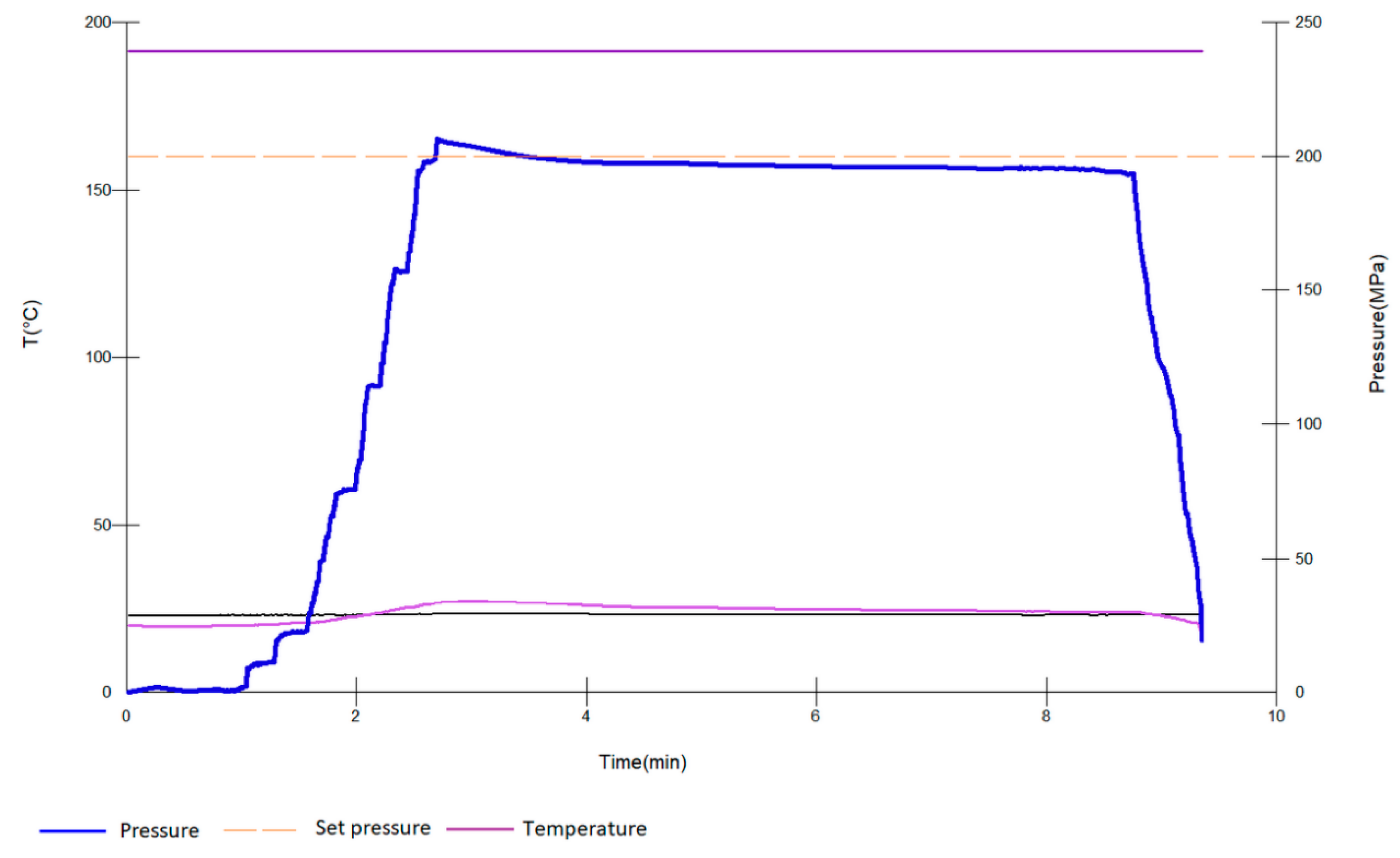
2.2. HHP Treatment and Antioxidants—Wine Bottle Aging
2.3. Chemical Analysis of Wine
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Profile and Color Properties of Red and White Wines after HHP Processing
3.1.1. Red Wine
3.1.2. White Wine
3.2. Phenolic and Aroma Changes of Red Wine during 12 Months of Aging Induced by HHP and Antioxidant Treatments
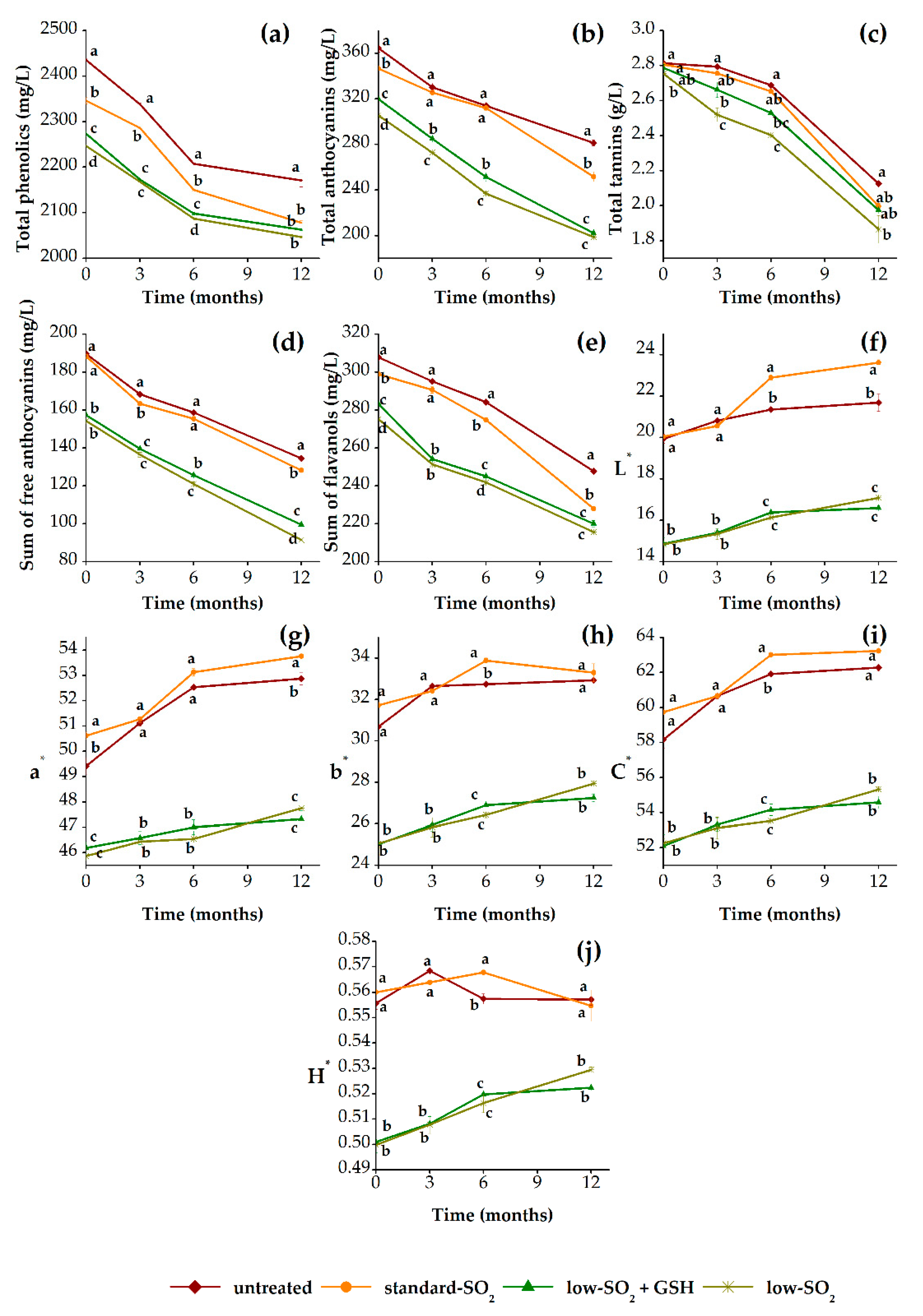
3.2.1. Phenolic Profile and Color Properties
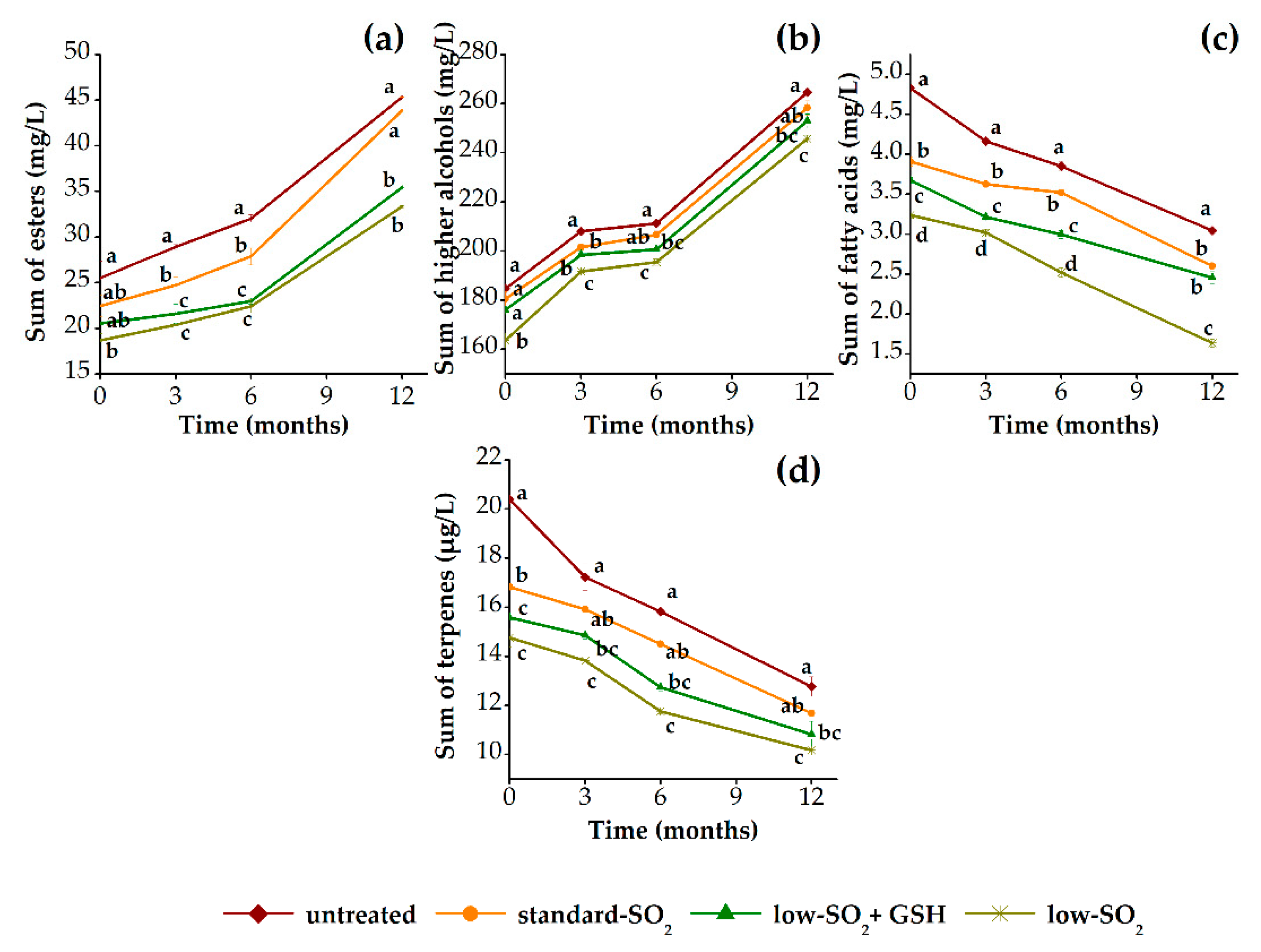
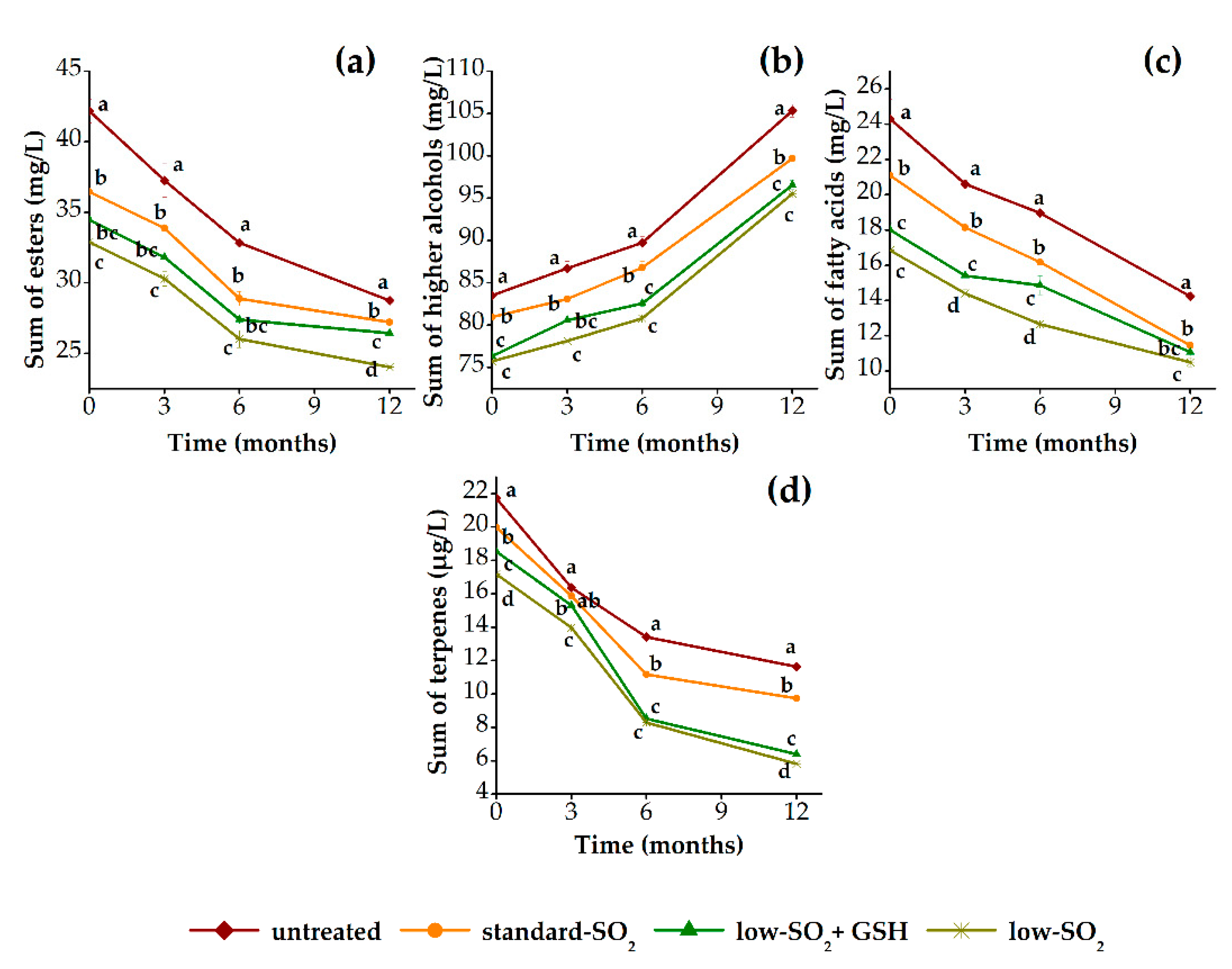
3.2.2. Aroma Profile
3.3. Phenolic and Aroma Changes of White Wine during 12 Months of Aging Induced by HHP and Antioxidant Treatments
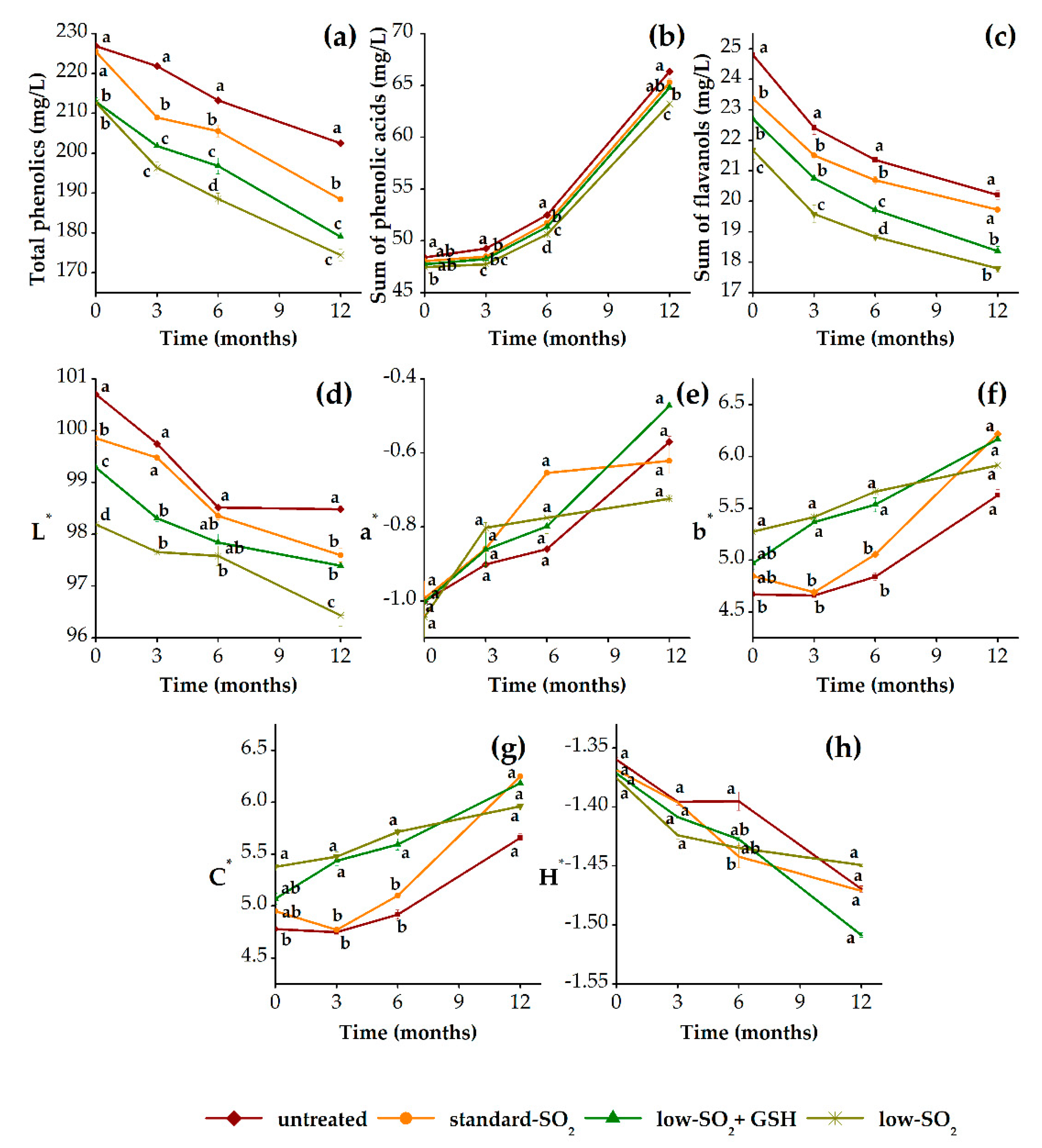
3.3.1. Phenolic Profile and Color Properties
3.3.2. Aroma Profile
3.4. Sensory Changes of Red and White Wines during 12 Months of Aging Induced by HHP and Antioxidant Treatments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Wyk, S.; Silva, F.V. Nonthermal Preservation of Wine. In Preservatives and Preservation Approaches in Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–235. [Google Scholar] [CrossRef]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High pressure processing in food industry–characteristics and applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Puig, A.; Vilavella, M.; Daoudi, L.; Guamis, B.; Minguez, S. Microbiological and biochemical stabilization of wines by application of the high pressure technique. Bull. OIV Fr. 2003, 76, 596–716. [Google Scholar]
- Buzrul, S. High hydrostatic pressure treatment of beer and wine: A review. Innov. Food Sci. Emerg. Technol. 2012, 13, 1–12. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Sevenich, R.; Rauh, C.; López, R.; Knorr, D.; López-Alfaro, I. Inactivation of Brettanomyces bruxellensis by high hydrostatic pressure technology. Food Control 2016, 59, 188–195. [Google Scholar] [CrossRef]
- Van Wyk, S.; Silva, F.V. High pressure processing inactivation of Brettanomyces bruxellensis in seven different table wines. Food Control 2017, 81, 1–8. [Google Scholar] [CrossRef]
- Van Wyk, S.; Silva, F.V. High pressure inactivation of Brettanomyces bruxellensis in red wine. Food Microbiol. 2017, 63, 199–204. [Google Scholar] [CrossRef]
- Mok, C.; Song, K.; Park, Y.; Lim, S.; Ruan, R.; Chen, P. High hydrostatic pressure pasteurization of red wine. J. Food Sci. 2006, 71, M265–M269. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Jourdes, M.; Teissedre, P.-L.; Rodrigues, A.; Amado, O.; Saraiva, J.A.; Coimbra, M.A. Evaluation of the potential of high pressure technology as an enological practice for red wines. Innov. Food Sci. Emerg. Technol. 2016, 33, 76–83. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W.; Górecki, A.; Błaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jeliński, T. Effects of high hydrostatic pressure processing on the physicochemical and sensorial properties of a red wine. Innov. Food Sci. Emerg. Technol. 2012, 16, 409–416. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Cappelle, J.; Gonçalves, F.J.; Rodrigues, A.; Saraiva, J.A.; Coimbra, M.A. Effect of high pressure treatments on the physicochemical properties of a sulphur dioxide-free red wine. Food Chem. 2013, 141, 2558–2566. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.M.; Saraiva, J.A.; Coimbra, M.A. Impact of high pressure treatments on the physicochemical properties of a sulphur dioxide-free white wine during bottle storage: Evidence for Maillard reaction acceleration. Innov. Food Sci. Emerg. Technol. 2013, 20, 51–58. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.M.; Saraiva, J.A.; Coimbra, M.A. High pressure treatments accelerate changes in volatile composition of sulphur dioxide-free wine during bottle storage. Food Chem. 2015, 188, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, L.; Ma, T.; Zhao, F.; Yu, D.; Huang, W.; Zhan, J. High hydrostatic pressure treatment: An artificial accelerating aging method which did not change the region and variety non-colored phenolic characteristic of red wine. Innov. Food Sci. Emerg. Technol. 2016, 33, 123–134. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W.; Górecki, A.; Błaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jeliński, T. A preliminary study about the influence of high hydrostatic pressure processing in parallel with oak chip maceration on the physicochemical and sensory properties of a young red wine. Food Chem. 2016, 194, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.; Sun, D.-W. Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technol. 2008, 1, 2–34. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Puxeu, M.; Martín, L.; Nart, E.; Hidalgo, C.; Andorrà, I. Microbiological, Physical, and Chemical Procedures to Elaborate High-Quality SO2-Free Wines. In Grapes and Wines-Advances in Production, Processing, Analysis and Valorization; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Perez-Wom, M.; Habib, G.; Giovagnoli-Vicuña, C.; Cañas-Sarazua, R.; Tabilo-Munizaga, G.; Salazar, F.N. Oenological and quality characteristic on young white wines (sauvignon blanc): Effects of high hydrostatic pressure processing. J. Food Qual. 2017, 2017, 8524073. [Google Scholar] [CrossRef]
- Van Wyk, S.; Farid, M.M.; Silva, F.V. SO2, high pressure processing and pulsed electric field treatments of red wine: Effect on sensory, Brettanomyces inactivation and other quality parameters during one year storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 204–211. [Google Scholar] [CrossRef]
- Christofi, S.; Malliaris, D.; Katsaros, G.; Panagou, E.; Kallithraka, S. Limit SO2 content of wines by applying High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2020, 62, 102342. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Bauer, F.F.; Du Toit, W.J. Role of glutathione in winemaking: A review. J. Agric. Food Chem. 2012, 61, 269–277. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Auezova, L.; Sakr, A.; Hajj-Moussa, E. Browning susceptibility of white wine and antioxidant effect of glutathione. Int. J. Food Sci. Technol. 2009, 44, 2459–2463. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of glutathione on wines oxidative stability: A combined sensory and metabolomic study. Front. Chem. 2018, 6, 182. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organization of Vine and Wine: Paris, France, 2009; pp. 154–196. [Google Scholar]
- Lukić, K.; Tomašević, M.; Ćurko, N.; Sivrić, A.; Ružman, E.; Kovačević Ganić, K. Influence of non-thermal processing techniques on sulfur dioxide and oxygen concentrations in young and aged wines. Croat. J. Food Technol. Biotechnol. Nutr. 2019, 14, 65–75. [Google Scholar] [CrossRef][Green Version]
- Nunes, C.; Santos, M.C.; Saraiva, J.A.; Rocha, S.M.; Coimbra, M.A. Influence of high hydrostatic pressure technology on wine chemical and sensorial characteristics: Potentialities and drawbacks. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 82, pp. 205–235. ISBN 1043-4526. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Determination of anthocyanins in red wine. Bull. Soc. Chim. Fr. 1965, 9, 2649. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Concentration of the tannins in red wine and determination of their structure. Chim. Anal. 1966, 48, 188–196. [Google Scholar]
- Lorrain, B.; Chira, K.; Teissedre, P.-L. Phenolic composition of Merlot and Cabernet-Sauvignon grapes from Bordeaux vineyard for the 2009-vintage: Comparison to 2006, 2007 and 2008 vintages. Food Chem. 2011, 126, 1991–1999. [Google Scholar] [CrossRef]
- Lukić, K.; Brnčić, M.; Ćurko, N.; Tomašević, M.; Valinger, D.; Denoya, G.I.; Barba, F.J.; Kovačević Ganić, K. Effects of high power ultrasound treatments on the phenolic, chromatic and aroma composition of young and aged red wine. Ultrason. Sonochem. 2019, 59, 104725. [Google Scholar] [CrossRef] [PubMed]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.-L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Suárez, R.; Gómez-Cordovés, C.; Bartolomé, B. Simultaneous determination of nonanthocyanin phenolic compounds in red wines by HPLC-DAD/ESI-MS. Am. J. Enol. Vitic. 2005, 56, 139–147. [Google Scholar]
- Lukić, K.; Brnčić, M.; Ćurko, N.; Tomašević, M.; Tušek, A.J.; Kovačević Ganić, K. Quality characteristics of white wine: The short-and long-term impact of high power ultrasound processing. Ultrason. Sonochem. 2020, 68, 105194. [Google Scholar] [CrossRef] [PubMed]
- Tomašević, M.; Gracin, L.; Ćurko, N.; Kovačević Ganić, K. Impact of pre-fermentative maceration and yeast strain along with glutathione and SO2 additions on the aroma of Vitis vinifera L. Pošip wine and its evaluation during bottle aging. LWT 2017, 81, 67–76. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices, 2nd ed.; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Tao, Y.; Wu, D.; Sun, D.-W.; Górecki, A.; Błaszczak, W.; Fornal, J.; Jeliński, T. Quantitative and predictive study of the evolution of wine quality parameters during high hydrostatic pressure processing. Innov. Food Sci. Emerg. Technol. 2013, 20, 81–90. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; You, Y.; Mao, B.; Zhao, W.; Zhan, J. The effects of ultra-high pressure treatment on the phenolic composition of red wine. S. Afr. J. Enol. Vitic. 2012, 33, 203–213. [Google Scholar] [CrossRef][Green Version]
- Sun, X.; Chen, X.; Li, L.; Ma, T.; Zhao, F.; Huang, W.; Zhan, J. Effect of ultra-high pressure treatment on the chemical properties, colour and sensory quality of young red wine. S. Afr. J. Enol. Vitic. 2015, 36, 393–401. [Google Scholar] [CrossRef][Green Version]
- Martínez, J.; Melgosa, M.; Pérez, M.; Hita, E.; Negueruela, A. Note. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Tomašević, M.; Lukić, K.; Bosiljkov, T.; Kelšin, K.; Ćurko, N.; Kovačević Ganić, K. Effect of high hydrostatic pressure on the volatile compounds in wine. Rad. Poljopr. Fak. Univ. U Sarajev. Fac. Agric. Univ. Sarajevo 2017, 62, 505–516. [Google Scholar]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Evolution of polyphenols in red wines from Vitis vinifera L. during aging in the bottle. Eur. Food Res. Technol. 2005, 220, 331–340. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Evolution of the phenolic content of red wines from Vitis vinifera L. during ageing in bottle. Food Chem. 2006, 95, 405–412. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Ferreira, A.S.; Jourdes, M.; Teissedre, P.-L.; Rodrigues, A.; Amado, O.; Saraiva, J.A.; Coimbra, M.A. Comparison of high pressure treatment with conventional red wine aging processes: Impact on phenolic composition. Food Res. Int. 2019, 116, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.G.; García-Carpintero, E.G.; Sánchez-Palomo, E.; Viñas, M.G.; Hermosín-Gutiérrez, I. Evolution of the phenolic content, chromatic characteristics and sensory properties during bottle storage of red single-cultivar wines from Castilla La Mancha region. Food Res. Int. 2013, 51, 554–563. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine-Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 2, ISBN 0-470-01038-X. [Google Scholar]
- Coetzee, C.; Du Toit, W. Sauvignon blanc wine: Contribution of ageing and oxygen on aromatic and non-aromatic compounds and sensory composition-A review. S. Afr. J. Enol. Vitic. 2015, 36, 347–365. [Google Scholar] [CrossRef][Green Version]
- Pérez-Prieto, L.; López-Roca, J.; Gómez-Plaza, E. Differences in major volatile compounds of red wines according to storage length and storage conditions. J. Food Compos. Anal. 2003, 16, 697–705. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of SO2 on the formation and evolution of volatile compounds in wines. Food Control 2007, 18, 1501–1506. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Waterhouse, A.L. A method to quantify quinone reaction rates with wine relevant nucleophiles: A key to the understanding of oxidative loss of varietal thiols. J. Agric. Food Chem. 2012, 60, 8484–8491. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Hernanz, D.; Gallo, V.; Recamales, Á.F.; Meléndez-Martínez, A.J.; González-Miret, M.L.; Heredia, F.J. Effect of storage on the phenolic content, volatile composition and colour of white wines from the varieties Zalema and Colombard. Food Chem. 2009, 113, 530–537. [Google Scholar] [CrossRef]
- Ferreira-Lima, N.E.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Impact of pressing conditions on the phenolic composition, radical scavenging activity and glutathione content of Brazilian Vitis vinifera white wines and evolution during bottle ageing. Food Bioprocess Technol. 2016, 9, 944–957. [Google Scholar] [CrossRef]
- Di Lecce, G.; Boselli, E.; D’Ignazi, G.; Frega, N.G. Evolution of phenolics and glutathione in Verdicchio wine obtained with maceration under reductive conditions. LWT 2013, 53, 54–60. [Google Scholar] [CrossRef]
- Recamales, Á.F.; Sayago, A.; González-Miret, M.L.; Hernanz, D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006, 39, 220–229. [Google Scholar] [CrossRef]
- Sonni, F.; Clark, A.C.; Prenzler, P.D.; Riponi, C.; Scollary, G.R. Antioxidant action of glutathione and the ascorbic acid/glutathione pair in a model white wine. J. Agric. Food Chem. 2011, 59, 3940–3949. [Google Scholar] [CrossRef] [PubMed]
- Antoce, A.O.; Badea, G.A.; Cojocaru, G.A. Effects of glutathione and ascorbic acid addition on the CIELab chromatic characteristics of Muscat Ottonel wines. Agric. Agric. Sci. Procedia 2016, 10, 206–214. [Google Scholar] [CrossRef][Green Version]
- Roussis, I.G.; Sergianitis, S. Protection of some aroma volatiles in a model wine medium by sulphur dioxide and mixtures of glutathione with caffeic acid or gallic acid. Flavour Fragr. J. 2008, 23, 35–39. [Google Scholar] [CrossRef]
- Kilmartin, P.A. The oxidation of red and white wines and its impact on wine aroma. Chem. N. Z. 2009, 73, 79–83. [Google Scholar]
- Panero, L.; Motta, S.; Petrozziello, M.; Guaita, M.; Bosso, A. Effect of SO 2, reduced glutathione and ellagitannins on the shelf life of bottled white wines. Eur. Food Res. Technol. 2015, 240, 345–356. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Frost, S.; Ugliano, M.; Cantu, A.R.; Currie, B.L.; Anderson, M.; Chassy, A.W.; Vidal, S.; Diéval, J.-B.; Aagaard, O. Sulfur dioxide–oxygen consumption ratio reveals differences in bottled wine oxidation. Am. J. Enol. Vitic. 2016, 67, 449–459. [Google Scholar] [CrossRef]
- Roussis, I.; Patrianakou, M.; Drossiadis, A. Protection of aroma volatiles in a red wine with low sulphur dioxide by a mixture of glutathione, caffeic acid and gallic acid. S. Afr. J. Enol. Vitic. 2013, 34, 262–265. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Savastano, M.L.; Benucci, I.; Esti, M. Evolution of phenolic and volatile compounds during bottle storage of a white wine without added sulfite. J. Sci. Food Agric. 2020, 100, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Carnieli, G.J.; Cardozo, A.; Vanderlinde, R. Effect of glutathione during bottle storage of sparkling wine. Food Chem. 2017, 216, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Volkova, N.; Suraski, A.; Aviram, M. White wine with red wine-like properties: Increased extraction of grape skin polyphenols improves the antioxidant capacity of the derived white wine. J. Agric. Food Chem. 2001, 49, 3164–3168. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; Cox, A.; Williams, E.R.; Boss, P.K. Associations between the Sensory Attributes and Volatile Composition of Cabernet Sauvignon Wines and the Volatile Composition of the Grapes Used for Their Production. J. Agric. Food Chem. 2011, 59, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
| Analysis | RW | High Hydrostatic Pressure Processing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | 200 MPa/5 min | 200 MPa/15 min | 200 MPa/25 min | 400 MPa/5 min | 400 MPa/15 min | 400 MPa/25 min | 600 MPa/5 min | 600 MPa/15 min | 600 MPa/25 min | |
| TP (mg/L) | 2455.0 ± 3.2 a | 2440.5 ± 4.5 ab | 2436.4 ± 6.4 bc | 2424.6 ± 5.1 cd | 2417.7 ± 0.6 d | 2396.4 ± 1.3 e | 2358.2 ± 3.9 f | 2366.8 ± 3.2 f | 2364.6 ± 3.9 f | 2337.3 ± 3.9 g |
| TA (mg/L) | 333.1 ± 0.4 a | 331.9 ± 2.9 ab | 328.6 ± 0.7 abc | 326.0 ± 2.1 bc | 330.4 ± 0.4 abc | 329.2 ± 0.4 abc | 326.4 ± 0.9 bc | 324.6 ± 2.2 c | 315.7 ± 2.5 d | 315.6 ± 1.6 d |
| TT (g/L) | 2.94 ± 0.06 a | 2.94 ± 0.06 a | 2.92 ± 0.07 a | 2.92 ± 0.06 a | 2.94 ± 0.02 a | 2.91 ± 0.11 a | 2.91 ± 0.00 a | 2.88 ± 0.05 a | 2.86 ± 0.03 a | 2.85 ± 0.05 a |
| FA (mg/L) | ||||||||||
| Dph | 18.42 ± 0.34 a | 17.92 ± 0.09 ab | 18.20 ± 0.15 a | 17.31 ± 0.02 c | 17.57 ± 0.15 bc | 17.21 ± 0.06 c | 17.62 ± 0.12 bc | 15.82 ± 0.01 d | 14.28 ± 0.17 e | 14.44 ± 0.03 e |
| Cy | 2.66 ± 0.09 bc | 2.56 ± 0.03 cd | 2.49 ± 0.00 cde | 2.96 ± 0.03 b | 2.68 ± 0.17 bc | 2.31 ± 0.02 def | 3.47 ± 0.05 a | 2.25 ± 0.12 ef | 2.13 ± 0.08 f | 2.23 ± 0.04 ef |
| Pt | 17.64 ± 0.39 a | 17.65 ± 0.16 a | 17.09 ± 0.02 abc | 17.35 ± 0.06 ab | 16.68 ± 0.15 bc | 17.20 ± 0.12 abc | 16.47 ± 0.17 c | 14.35 ± 0.29 d | 13.64 ± 0.17 d | 13.85 ± 0.04 d |
| Pn | 14.33 ± 0.34 a | 13.39 ± 0.31 b | 13.42 ± 0.11 b | 12.15 ± 0.10 c | 13.34 ± 0.37 b | 12.40 ± 0.02 c | 13.70 ± 0.31 ab | 12.29 ± 0.10 c | 11.90 ± 0.04 c | 12.37 ± 0.02 c |
| Mv | 92.36 ± 0.77 a | 93.87 ± 0.38 a | 85.15 ± 0.51 b | 85.93 ± 0.13 b | 85.88 ± 0.60 b | 85.71 ± 1.01 b | 84.15 ± 0.14 bc | 82.20 ± 0.56 c | 82.27 ± 0.12 c | 82.19 ± 0.18 c |
| PnAc | 4.76 ± 0.16 a | 2.68 ± 0.22 c | 4.55 ± 0.07 a | 2.83 ± 0.07 c | 4.42 ± 0.14 a | 2.73 ± 0.13 c | 4.66 ± 0.31 a | 3.73 ± 0.12 b | 3.26 ± 0.03 b | 3.08 ± 0.06 c |
| MvAc | 25.35 ± 0.74 a | 24.73 ± 0.15 ab | 23.75 ± 0.10 bc | 22.67 ± 0.08 c | 23.50 ± 0.74 bc | 23.58 ± 0.31 bc | 22.31 ± 0.08 c | 22.34 ± 0.33 c | 18.44 ± 0.21 c | 15.11 ± 0.07 d |
| PnCm | 2.26 ± 0.06 a | 2.27 ± 0.15 a | 2.18 ± 0.01 a | 2.27 ± 0.00 a | 2.09 ± 0.09 ab | 2.29 ± 0.01 a | 2.27 ± 0.14 a | 2.15 ± 0.02 a | 1.49 ± 0.05 c | 1.85 ± 0.03 b |
| MvCm | 8.81 ± 0.28 ab | 8.96 ± 0.16 a | 8.46 ± 0.05 abc | 7.96 ± 0.05 cd | 8.12 ± 0.25 cd | 7.78 ± 0.30 d | 8.86 ± 0.01 ab | 8.23 ± 0.13 bcd | 6.74 ± 0.04 e | 6.74 ± 0.13 e |
| ∑ FA | 186.6 ± 1.6 a | 184.0 ± 0.1 a | 175.3 ± 1.0 b | 171.4 ± 0.4 c | 174.3 ± 1.1 bc | 171.2 ± 0.6 c | 173.5 ± 0.5 bc | 163.4 ± 1.0 d | 154.2 ± 0.3 e | 151.9 ± 0.1 e |
| Fl (mg/L) | ||||||||||
| Pro B1 | 33.65 ± 0.14 a | 33.78 ± 0.56 a | 33.35 ± 0.01 a | 32.02 ± 0.08 ab | 32.96 ± 0.22 a | 32.60 ± 0.62 ab | 31.98 ± 1.11 ab | 32.74 ± 0.75 ab | 32.06 ± 0.52 ab | 30.77 ± 0.47 b |
| Cat | 52.89 ± 0.55 a | 52.57 ± 0.30 a | 51.47 ± 0.73 ab | 51.16 ± 0.05 ab | 51.52 ± 0.43 ab | 51.02 ± 0.65 ab | 50.45 ± 0.14 ab | 50.81 ± 1.75 ab | 50.34 ± 0.59 ab | 49.51 ± 0.92 b |
| Pro B2 | 35.84 ± 0.39 a | 35.78 ± 0.51 a | 35.59 ± 2.49 a | 33.60 ± 1.37 ab | 35.30 ± 2.09 a | 33.79 ± 2.23 ab | 32.47 ± 1.23 ab | 32.27 ± 0.92 ab | 28.95 ± 0.04 b | 28.91 ± 0.55 b |
| Epicat | 51.43 ± 1.30 a | 47.98 ± 2.14 ab | 46.17 ± 2.30 b | 45.65 ± 1.18 b | 46.04 ± 0.62 b | 45.90 ± 0.53 b | 43.59 ± 0.12 b | 45.79 ± 0.29 b | 45.69 ± 0.97 b | 43.46 ± 1.12 b |
| Pro B3 | 4.41 ± 0.18 a | 4.37 ± 0.06 a | 4.28 ± 0.00 ab | 4.18 ± 0.15 ab | 4.22 ± 0.07 ab | 4.11 ± 0.06 ab | 4.07 ± 0.08 ab | 4.07 ± 0.02 ab | 4.06 ± 0.05 ab | 3.95 ± 0.02 b |
| Pro B4 | 10.30 ± 0.49 a | 10.06 ± 0.07 a | 9.85 ± 0.51 a | 9.49 ± 0.42 ab | 9.30 ± 0.22 ab | 8.50 ± 0.26 ab | 7.62 ± 0.56 ab | 8.45 ± 0.83 ab | 7.63 ± 1.78 ab | 7.02 ± 0.34 b |
| Pro C1 | 12.47 ± 0.31 a | 11.55 ± 0.85 ab | 10.51 ± 0.49 bc | 10.03 ± 0.06 bc | 10.47 ± 0.03 bc | 9.81 ± 0.18 bc | 9.70 ± 0.75 bc | 9.62 ± 0.56 c | 9.46 ± 0.46 c | 8.94 ± 0.31 c |
| ∑ Fl | 201.0 ± 1.1 a | 196.1 ± 0.5 ab | 191.2 ± 6.5 abc | 186.1 ± 0.2 bcd | 189.8 ± 2.3 bc | 185.7 ± 4.0 bcd | 179.9 ± 0.4 cde | 183.8 ± 3.4 cd | 178.2 ± 2.3 de | 172.6 ± 1.0 e |
| Color | ||||||||||
| L* | 14.6 ± 0.2 e | 14.6 ± 0.1 de | 15.0 ± 0.1 bc | 14.8 ± 0.1 cde | 15.0 ± 0.0 bc | 15.1 ± 0.1 bc | 15.2 ± 0.0 b | 16.4 ± 0.1 a | 16.5 ± 0.2 a | 16.3 ± 0.1 a |
| a* | 45.8 ± 0.1 e | 46.0 ± 0.1 cde | 46.2 ± 0.1 cde | 45.9 ± 0.1 de | 46.3 ± 0.0 bcd | 46.4 ± 0.1 bc | 46.1 ± 0.3 b | 47.9 ± 0.0 a | 47.9 ± 0.2 a | 47.7 ± 0.2 a |
| b* | 24.8 ± 0.1 d | 25.4 ± 0.2 bc | 25.3 ± 0.1 bcd | 25.0 ± 0.1 cd | 25.5 ± 0.0 bc | 25.6 ± 0.1 b | 25.3 ± 0.1 bcd | 27.7 ± 0.1 a | 27.7 ± 0.2 a | 27.4 ± 0.2 a |
| C* | 52.2 ± 0.1 e | 52.4 ± 0.2 cde | 52.7 ± 0.1 cde | 52.3 ± 0.1 de | 52.8 ± 0.0 bcd | 53.0 ± 0.1 bc | 53.4 ± 0.0 b | 55.3 ± 0.1 a | 55.3 ± 0.3 a | 55.0 ± 0.3 a |
| H* | 0.5 ± 0.0 d | 0.5 ± 0.0 cd | 0.5 ± 0.0 cd | 0.5 ± 0.0 bc | 0.5 ± 0.0 cd | 0.5 ± 0.0 cd | 0.5 ± 0.0 bc | 0.5 ± 0.0 a | 0.5 ± 0.00 a | 0.5 ± 0.0 ab |
| ∆E* | - | 0.6 | 0.7 | 0.4 | 1.0 | 1.2 | 0.9 | 4.0 | 4.1 | 3.7 |
| Analysis | WW | High Hydrostatic Pressure Processing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | 200 MPa/5 min | 200 MPa/15 min | 200 MPa/25 min | 400 MPa/5 min | 400 MPa/15 min | 400 MPa/25 min | 600 MPa/5 min | 600 MPa/15 min | 600 MPa/25 min | |
| TP (mg/L) | 261.7 ± 0.3 a | 259.1 ± 0.5 abc | 258.7 ± 1.1 abcd | 256.5 ± 0.5 cd | 256.1 ± 0.3 d | 258.8 ± 0.8 abcd | 256.1 ± 0.5 cd | 259.7 ± 0.1 ab | 257.1 ± 1.1 bcd | 256.6 ± 1.4 cd |
| Pa (mg/L) | ||||||||||
| Gal | 2.56 ± 0.01 de | 2.66 ± 0.02 a | 2.63 ± 0.00 ab | 2.62 ± 0.02 abc | 2.64 ± 0.01 a | 2.61 ± 0.01 abcd | 2.57 ± 0.01 cde | 2.58 ± 0.02 bcde | 2.56 ± 0.01 de | 2.55 ± 0.01 e |
| Protocat | 5.67 ± 0.02 bc | 5.91 ± 0.11 a | 5.89 ± 0.02 a | 5.77 ± 0.03 ab | 5.75 ± 0.09 ab | 5.72 ± 0.01 ab | 5.64 ± 0.02 bc | 5.63 ± 0.03 bc | 5.61 ± 0.00 bc | 5.48 ± 0.00 c |
| Van | 0.78 ± 0.06 a | 0.72 ± 0.10 a | 0.52 ± 0.04 bc | 0.51 ± 0.05 c | 0.70 ± 0.06 ab | 0.51 ± 0.01 bc | 0.49 ± 0.00 c | 0.41 ± 0.03 c | 0.40 ± 0.00 c | 0.38 ± 0.00 c |
| Syr | 0.25 ± 0.02 a | 0.25 ± 0.03 a | 0.22 ± 0.03 ab | 0.19 ± 0.00 ab | 0.23 ± 0.01 ab | 0.20 ± 0.00 ab | 0.18 ± 0.03 ab | 0.19 ± 0.00 ab | 0.18 ± 0.01 ab | 0.17 ± 0.01 b |
| Caft | 30.61 ± 0.25 a | 30.47 ± 0.02 a | 29.69 ± 0.26 bcd | 29.25 ± 0.01 ef | 30.38 ± 0.02 ab | 29.47 ± 0.12 cde | 28.88 ± 0.03 ef | 30.21 ± 0.01 abc | 28.76 ± 0.40 ef | 28.59 ± 0.25 f |
| Chlo | 2.40 ± 0.00 b | 2.44 ± 0.00 a | 2.39 ± 0.02 b | 2.37 ± 0.01 b | 2.39 ± 0.02 b | 2.36 ± 0.00 b | 2.30 ± 0.00 c | 2.37 ± 0.00 b | 2.28 ± 0.02 c | 2.26 ± 0.01 c |
| Caf | 2.30 ± 0.01 b | 2.41 ± 0.01 a | 2.27 ± 0.01 bc | 2.26 ± 0.01 bc | 2.28 ± 0.01 bc | 2.25 ± 0.02 bc | 2.24 ± 0.02 cd | 2.24 ± 0.00 cd | 2.20 ± 0.02 d | 2.20 ± 0.00 d |
| p-Coum | 1.43 ± 0.01 bc | 1.49 ± 0.01 a | 1.47 ± 0.01 ab | 1.45 ± 0.01 abc | 1.49 ± 0.01 a | 1.46 ± 0.01 abc | 1.43 ± 0.01 bc | 1.45 ± 0.02 abc | 1.44 ± 0.01 bc | 1.42 ± 0.02 c |
| Fer | 0.57 ± 0.01 a | 0.58 ± 0.03 a | 0.56 ± 0.00 a | 0.56 ± 0.00 a | 0.56 ± 0.00 a | 0.56 ± 0.01 a | 0.55 ± 0.00 a | 0.56 ± 0.01 a | 0.56 ± 0.00 a | 0.55 ± 0.01 a |
| ∑ Pa | 46.6 ± 0.3 a | 46.9 ± 0.1 a | 45.6 ± 0.3 b | 45.0 ± 0.1 bc | 46.4 ± 0.1 a | 45.1 ± 0.2 b | 44.3 ± 0.0 cd | 45.7 ± 0.1 b | 44.0 ± 0.3 d | 43.6 ± 0.2 d |
| Fl (mg/L) | ||||||||||
| Pro B1 | 11.47 ± 0.01 a | 11.30 ± 0.00 ab | 11.18 ± 0.01 abc | 11.14 ± 0.06 abc | 11.29 ± 0.01 ab | 11.03 ± 0.01 bc | 10.84 ± 0.19 cd | 10.81 ± 0.10 cd | 10.64 ± 0.17 d | 10.55 ± 0.13 d |
| ProB2 | 2.66 ± 0.12 a | 2.57 ± 0.16 a | 2.28 ± 0.09 ab | 2.00 ± 0.15 bc | 1.83 ± 0.02 c | 1.76 ± 0.14 c | 1.67 ± 0.09 c | 1.77 ± 0.09 c | 1.67 ± 0.08 c | 1.58 ± 0.02 c |
| Cat | 6.83 ± 0.04 a | 6.02 ± 0.21 b | 4.41 ± 0.22 c | 3.40 ± 0.05 de | 3.70 ± 0.05 d | 3.21 ± 0.05 ef | 3.12 ± 0.06 ef | 2.97 ± 0.02 f | 2.95 ± 0.06 f | 2.92 ± 0.04 f |
| Epicat | 10.53 ± 0.11 a | 10.24 ± 0.03 ab | 9.89 ± 0.14 bc | 9.50 ± 0.01 c | 9.67 ± 0.05 c | 8.88 ± 0.01 d | 8.77 ± 0.22 d | 8.68 ± 0.29 d | 8.55 ± 0.03 d | 7.83 ± 0.03 e |
| ∑ Fl | 31.5 ± 0.0 a | 30.1 ± 0.4 b | 27.8 ± 0.0 c | 26.1 ± 0.1 d | 26.5 ± 0.1 d | 24.9 ± 0.2 e | 24.4 ± 0.1 ef | 24.2 ± 0.1 ef | 23.8 ± 0.1 f | 22.9 ± 0.2 g |
| Color | ||||||||||
| L* | 101.8 ± 0.0 a | 101.2 ± 0.6 a | 101.1 ± 0.0 a | 100.1 ± 0.0 b | 100.0 ± 0.0 b | 99.9 ± 0.1 b | 100.0 ± 0.0 b | 98.2 ± 0.1 c | 98.1 ± 0.0 c | 98.0 ± 0.0 c |
| a* | −0.2 ± 0.0 c | −0.2 ± 0.1 bc | −0.1 ± 0.0 bc | −0.0 ± 0.0 ab | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | −0.6 ± 0.1 d | −0.6 ± 0.0 d | −0.5 ± 0.1 d |
| b* | −0.8 ± 0.0 c | −0.5 ± 0.3 bc | −0.5 ± 0.0 b | −0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.1 a | −0.0 ± 0.0 a | −0.6 ± 0.0 bc | −0.6 ± 0.0 bc | −0.6 ± 0.0 bc |
| C* | 0.9 ± 0.0 a | 0.5 ± 0.3 ab | 0.5 ± 0.0 bc | 0.0 ± 0.0 d | 0.0 ± 0.0 d | 0.1 ± 0.0 cd | 0.1 ± 0.0 d | 0.6 ± 0.0 ab | 0.6 ± 0.0 ab | 0.6 ± 0.0 ab |
| H* | 1.3 ± 0.0 a | 1.3 ± 0.0 a | 1.3 ± 0.0 a | 1.0 ± 0.2 a | 0.1 ± 0.7 ab | 1.0 ± 0.4 a | −0.8 ± 0.9 ab | −1.5 ± 0.0 b | −1.5 ± 0.0 b | −1.5 ± 0.0 b |
| ∆E* | - | 0.7 | 0.9 | 1.9 | 2.0 | 2.2 | 2.0 | 3.7 | 3.7 | 3.9 |
| Time (months) | Red Wine | Color | Odor | Taste |
| 0 | standard SO2 | 8.7 ± 0.5 a | 8.6 ± 0.5 a | 8.5 ± 0.5 a |
| low SO2+GSH | 8.6 ± 0.5 a | 8.5 ± 0.5 a | 8.4 ± 0.5 a | |
| low SO2 | 8.3 ± 0.5 a | 8.3 ± 0.5 a | 8.4 ± 0.5 a | |
| 3 | standard SO2 | 8.1 ± 0.3 a | 8.0 ± 0.5 a | 7.9 ± 0.3 a |
| low SO2+GSH | 7.8 ± 0.3 ab | 7.7 ± 0.5 ab | 7.5 ± 0.3 a | |
| low SO2 | 7.4 ± 0.5 b | 7.4 ± 0.3 b | 7.2 ± 0.5 a | |
| 6 | standard SO2 | 7.8 ± 0.5 a | 7.7 ± 0.5 a | 7.6 ± 0.5 a |
| low SO2+GSH | 7.6 ± 0.4 ab | 7.4 ± 0.3 ab | 7.3 ± 0.3 ab | |
| low SO2 | 7.2 ± 0.3 b | 7.0 ± 0.3 b | 6.9 ± 0.3 b | |
| 12 | standard SO2 | 7.4 ± 0.5 a | 7.2 ± 0.4 a | 6.8 ± 0.3 a |
| low SO2+GSH | 7.1 ± 0.3 a | 6.9 ± 0.3 ab | 6.6 ± 0.4 ab | |
| low SO2 | 6.6 ± 0.5 b | 6.5 ± 0.5 b | 6.1 ± 0.4 b | |
| Time (months) | White Wine | Color | Odor | Taste |
| 0 | standard SO2 | 8.5 ± 0.5 a | 8.3 ± 0.5 a | 8.2 ± 0.3 a |
| low SO2+GSH | 8.0 ± 0.4 b | 7.8 ± 0.4 ab | 7.7 ± 0.4 a | |
| low SO2 | 7.9 ± 0.3 b | 7.7 ± 0.5 b | 7.6 ± 0.5 a | |
| 3 | standard SO2 | 7.5 ± 0.5 a | 7.3 ± 0.5 a | 7.2 ± 0.4 a |
| low SO2+GSH | 6.9 ± 0.3 b | 6.7 ± 0.5 b | 6.6 ± 0.5 b | |
| low SO2 | 6.8 ± 0.4 b | 6.6 ± 0.3 b | 6.3 ± 0.5 b | |
| 6 | standard SO2 | 7.3 ± 0.3 a | 7.2 ± 0.4 a | 7.0 ± 0.3 a |
| low SO2+GSH | 6.7 ± 0.4 b | 6.6 ± 0.5 b | 6.5 ± 0.5 ab | |
| low SO2 | 6.6 ± 0.5 b | 6.5 ± 0.5 b | 6.2 ± 0.4 b | |
| 12 | standard SO2 | 6.9 ± 0.4 a | 6.6 ± 0.3 a | 6.5 ± 0.5 a |
| low SO2+GSH | 6.4 ± 0.5 ab | 6.0 ± 0.4 ab | 5.8 ± 0.4 b | |
| low SO2 | 6.1 ± 0.3 b | 5.8 ± 0.3 b | 5.8 ± 0.4 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukić, K.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K. Phenolic and Aroma Changes of Red and White Wines during Aging Induced by High Hydrostatic Pressure. Foods 2020, 9, 1034. https://doi.org/10.3390/foods9081034
Lukić K, Ćurko N, Tomašević M, Kovačević Ganić K. Phenolic and Aroma Changes of Red and White Wines during Aging Induced by High Hydrostatic Pressure. Foods. 2020; 9(8):1034. https://doi.org/10.3390/foods9081034
Chicago/Turabian StyleLukić, Katarina, Natka Ćurko, Marina Tomašević, and Karin Kovačević Ganić. 2020. "Phenolic and Aroma Changes of Red and White Wines during Aging Induced by High Hydrostatic Pressure" Foods 9, no. 8: 1034. https://doi.org/10.3390/foods9081034
APA StyleLukić, K., Ćurko, N., Tomašević, M., & Kovačević Ganić, K. (2020). Phenolic and Aroma Changes of Red and White Wines during Aging Induced by High Hydrostatic Pressure. Foods, 9(8), 1034. https://doi.org/10.3390/foods9081034





