Antimicrobial Activity of Selected Red and White Wines against Escherichia coli: In Vitro Inhibition Using Fish as Food Matrix
Abstract
1. Introduction
2. Materials and Methods
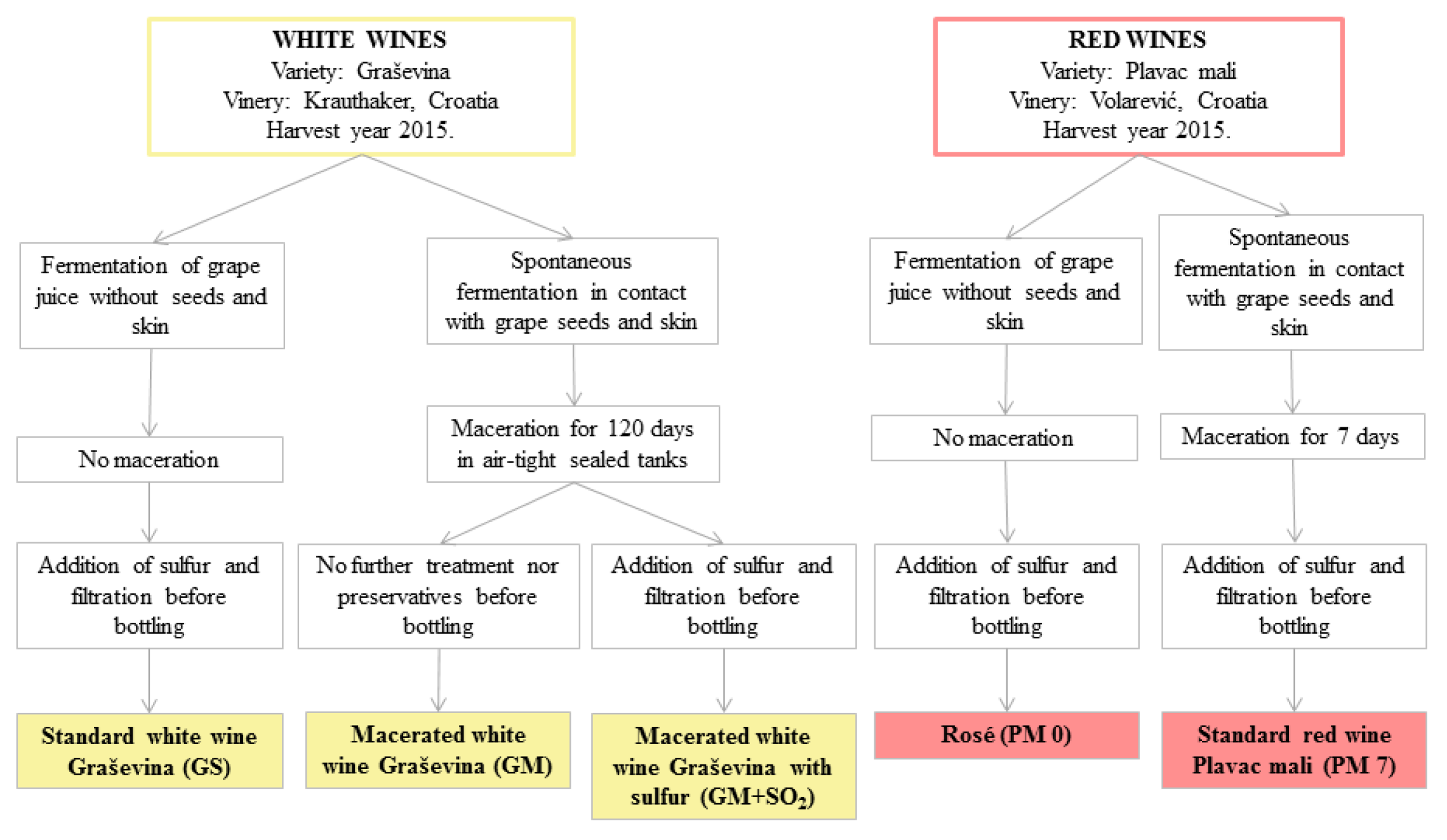
2.1. Wine Samples and Technology
2.2. Wine Chemistry Analyses
2.3. Analysis of Total Phenolics, Tannins and Anthocyanins
2.4. The Escherichia coli Strains and Inoculum Preparation
2.5. In Vitro Evaluation of Antimicrobial Activity of Wines
2.5.1. Agar-Disk and Agar-Well Diffusion Assays
2.5.2. Minimal Inhibitory Concentration (MIC)
2.6. Preparation of Fish Samples and Inoculation with E. coli
2.6.1. Enumeration of E. coli
2.6.2. pH Analyses
2.7. Statistical Analyses
3. Results and Discussion
3.1. Wine Chemistry
3.2. Antimicrobial Activity
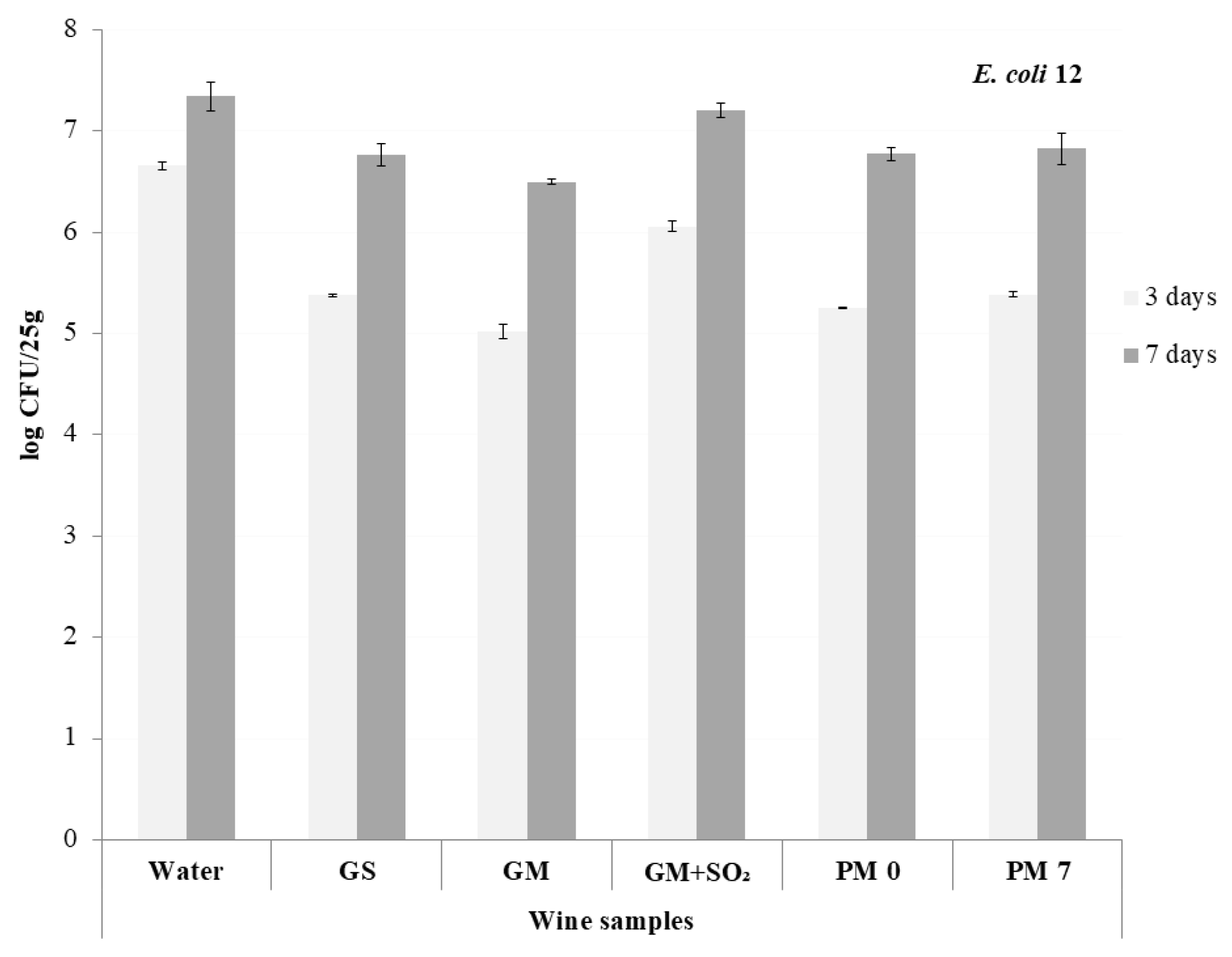
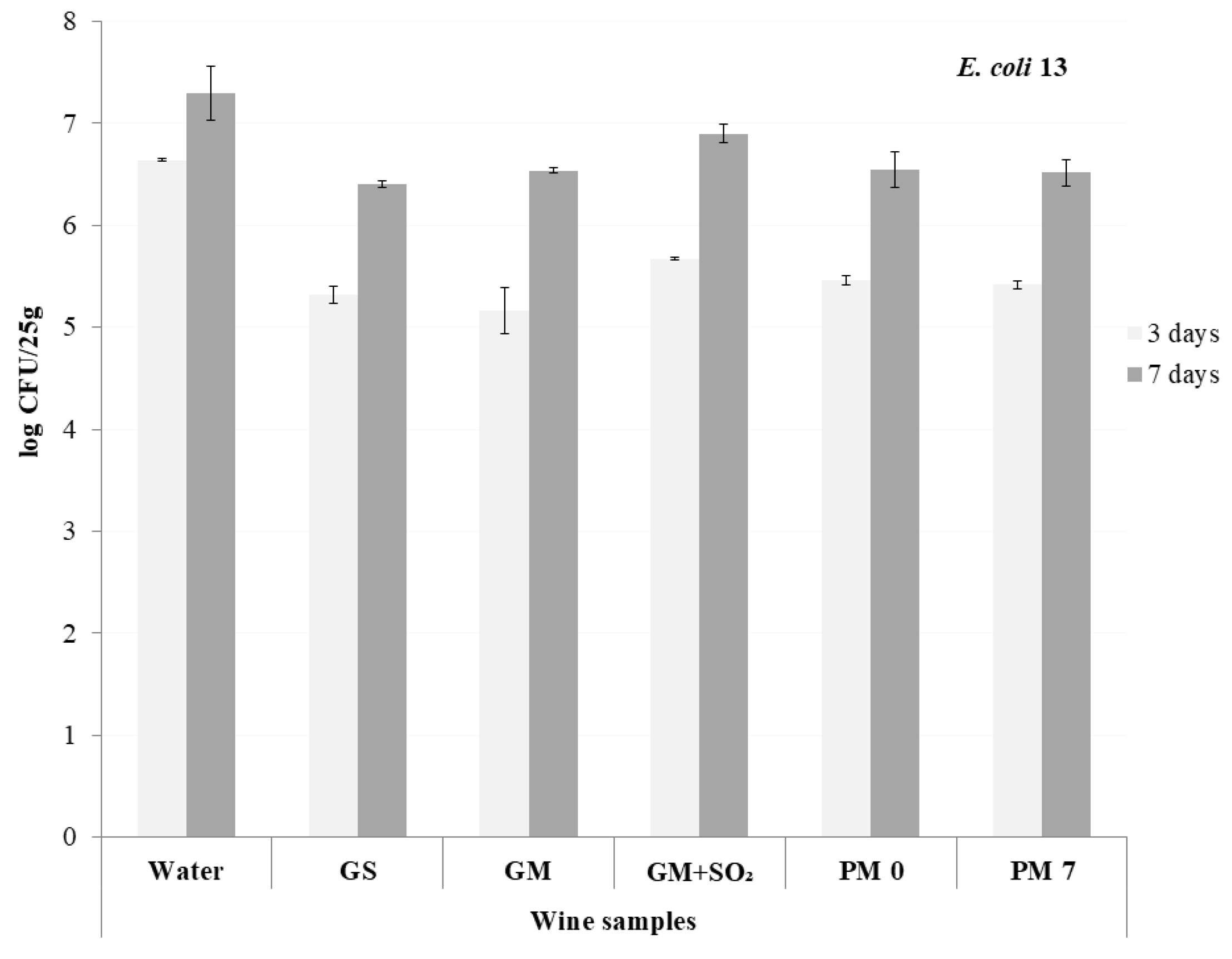
3.3. The Experiment on Fish Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fung, F.; Wang, H.S.; Menon, S. Food Safety in the 21st Century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Alali, W. Recent Discoveries in Human Serious Foodborne Pathogenic Bacteria: Resurgence, Pathogenesis, and Control Strategies. Front. Microbiol. 2018, 9, 2412. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Cheng, B.-K.; Jing, H.-Q. Escherichia Coli O157: H7 and Shiga-like-toxin-producing Escherichia Coli in China. World J. Gastroenterol. 1999, 5, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Mylius, S.D.; Nauta, M.J.; Havelaar, A.H. Cross-contamination during food preparation: A mechanistic model applied to chicken-borne Campylobacter. Risk Anal. Int. J. 2007, 27, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- Waite, J.G.; Daeschel, M.A. Contribution of wine components to inactivation of food-borne pathogens. J. Food Sci. 2007, 7, M286–M291. [Google Scholar] [CrossRef]
- Isohanni, P.; Alter, T.; Saris, P.; Lyhs, U. Wines as possible meat marinade ingredients possess antimicrobial potential against Campylobacter. Poult. Sci. 2010, 89, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Kargiotou, C.; Katsanidis, E.; Rhoades, J.; Kontominas, M.; Koutsoumanis, K. Efficacies of soy sauce and wine base marinades for controlling spoilage of raw beef. Food Microbiol. 2011, 28, 158–163. [Google Scholar] [CrossRef]
- Tenore, G.C.; Basile, A.; Novellino, E. Antioxidant and antimicrobial properties of polyphenolic fractions from selected Moroccan red wines. J. Food Sci. 2011, 76, C1342–C1348. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Soulti, K.; Roussis, I.G. Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol. Biotechnol. 2005, 43, 41–46. [Google Scholar]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.W. Antimicrobial wine formulations against the foodborne pathogens Escherichia coli O157:H7 and Salmonella enterica. J. Food. Sci. 2006, 71, M245–M251. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef] [PubMed]
- Moretro, T.; Daeschel, M.A. Wine is bactericidal to food-borne pathogens. J. Food Sci. 2004, 69, M250–M257. [Google Scholar]
- Radovanović, A.N.; Jovančičević, B.S.; Radovanović, B.C.; Mihajilov-Krstev, T. Antibmicrobial effectiveness of selected Vranac wines aginst six Gram-positive and six Gram-negative bacterial strains. Trop. J. Pharm. Res. 2014, 13, 819–824. [Google Scholar] [CrossRef]
- Birk, T.; Knøchel, S. Fate of food-associated bacteria in pork as affected by marinade, temperature, and ultrasound. J. Food Prot. 2009, 72, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Nisiotou, A.; Chorianopoulos, N.G.; Gounadaki, A.; Panagou, E.Z.; Nychas, G.J. Effect of wine-based marinades on the behavior of Salmonella Typhimurium and background flora in beef fillets. Int. J. Food Microbiol. 2013, 164, 119–127. [Google Scholar] [CrossRef]
- Rhoades, J.; Kargiotou, C.; Katsanidis, E.; Koutsoumanis, K.P. Use of marination for controlling Salmonella enterica and Listeria monocytogenes in raw beef. Food Microbiol. 2013, 36, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.G.; Patarata, L. Behavior of Salmonella spp., Listeria monocytogenes, and Staphylococcus aureus in Chourico de Vinho, a dry fermented sausage made from wine-marinated meat. J. Food Prot. 2013, 76, 588–594. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Henika, P.R. Addition of phytochemical-rich plant extracts mitigate the antimicrobial activity of essential oil/wine mixtures against Escherichia coli O157:H7 but not against Salmonella enterica. Food Control 2017, 73, 562–565. [Google Scholar] [CrossRef][Green Version]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramović, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitisvinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Milat, A.M.; Boban, M.; Teissedre, P.-L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of oxidation and browning of macerated white wine on its antioxidant and direct vasodilatory activity. J. Funct. Foods 2019, 15, 138–147. [Google Scholar] [CrossRef]
- Boban, N.; Tonkic, M.; Budimir, D.; Modun, D.; Sutlovic, D.; Punda-Polic, V.; Boban, M. Antimicrobial effects of wine: Separating the role of polyphenols, pH, ethanol, and other wine components. J. Food Sci. 2010, 75, M322–M326. [Google Scholar] [CrossRef]
- Rodríguez-Vaquero, M.J.; Aredes-Fernández, P.A.; Manca de Nadra, M.C. Phenolic compounds from wine as natural preservatives of fish meat. Food Technol. Biotechnol. 2013, 51, 376–382. [Google Scholar]
- Lee, J.H.; Cho, B.K. Effects of grape, wild grape and black raspberry wines on ground pork during refrigerated storage. Acta Aliment. 2014, 43, 553–563. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines, 1st ed.; John Wiley and Sons: New York, NY, USA, 1980; pp. 181–185. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Dosage des tanins du vin rouge et détermination de leur structure. Chim. Anal. 1966, 48, 188–196. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreetet, E. Le dosage des anthocyanes dans le vin rouge. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—8th ed. CLSI document M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; p. 226. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0, 2020. Available online: http://www.eucast.org (accessed on 7 May 2020).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsoudaet, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Koohsari, H.; Ghaemi, E.A.; Shesh Poli, M.S.; Sadegh, A. Evaluation of antibacterial activity of Lemon verbena (Lippiacitriodora) leaves. Ann. Biol. Res. 2013, 4, 52–55. [Google Scholar]
- Skroza, D.; Šimat, V.; SmoleMožina, S.; Katalinić, V.; Boban, N.; Generalić Mekinić, I. Interactions of resveratrol with other phenolics and activity against food-borne pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef]
- ISO. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive E. coli—Part 2: Colony Count Technique at 44 °C Using 5-Bromo-4-Chloro-3-Indolyl-Beta-D-Glucuronic Acid; ISO 16649–2; International Organization for Standardization: Geneva, Switzerland, 2001; p. 8. [Google Scholar]
- Šimat, V.; Bogdanović, T.; Krželj, M.; Soldo, A.; Maršić-Lučić, J. Differences in chemical, physical and sensory properties during shelf life assessment of wild and farmed gilthead sea bream (Sparus aurata, L.). J. Appl. Ichtyol. 2012, 28, 95–101. [Google Scholar] [CrossRef]
- L 149/1 Official Journal of the European Union. Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Wine-Growing Areas where the Alcoholic Strength may be Increased, Authorised Oenological Practices and Restrictions Applicable to the Production and Conservation of Grapevine Products, the Minimum Percentage of Alcohol for By-Products and Their Disposal, and Publication of OIV Files. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2019:149:FULL&from=IT (accessed on 15 March 2020).
- Jamir, L.; Kumar, V.; Gat, Y.; Kumar, A.; Kaur, S. Wine: A potential source of antimicrobial compounds. J. Wine Res. 2019, 30, 220–237. [Google Scholar] [CrossRef]
- Just, J.R.; Daeschel, M.A. Antimicrobial effects of wine on Escherichia coli O157: H7 and Salmonella typhimurium in a model stomach system. J. Food Sci. 2003, 68, 285–290. [Google Scholar] [CrossRef]
- Weisse, M.E.; Eberly, B.; Person, D.A. Wine as a digestive aid: Comparative antimicrobial effects of bismuth salicylate and red and white wine. BMJ 1995, 311, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 2002, 63, 99–104. [Google Scholar] [CrossRef]
- Boban, N.; Tonkić, M.; Modun, D.; Budimir, D.; Mudnić, I.; Sutlović, D.; Punda-Polić, V.; Boban, M. Thermally treated wine retains antibacterial effects to food-borne pathogens. Food Control 2010, 21, 1161–1165. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Chouliara, I.; Badeka, A.; Savvaidis, I.N.; Kontominas, M.G. Effect of gutting on microbiological, chemical, and sensory properties of aquacultured sea bass (Dicentrarchus labrax) stored in ice. Food Microbiol. 2003, 20, 411–420. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Cushnie, T.T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
| Wine Samples * | pH | Acidity (g/L) ** | Alcohol (%) | Total Phenolics (mg GAE/L) | Anthocyanins (mg/L) | Tannins (mg/L) | Free SO2 (mg/L) | Total SO2 (mg/L) |
|---|---|---|---|---|---|---|---|---|
| GS | 3.54 | 4.9 | 13.0 | 305 ± 3.5 a | 0 a | 0.26 ± 0.01 a | 27 | 124 |
| GM | 3.94 | 4.8 | 13.3 | 2699 ± 8.2 b | 0 b | 3.34 ± 0.10 b | 1 | 3 |
| GM + SO2 | 3.90 | 5.4 | 12.8 | 2850 ± 34.6 b | 0 c | 4.93 ± 0.19 c | 5 | 99 |
| PM 0 | 3.54 | 3.8 | 14.3 | 539 ± 22.2 c | 1.97 ± 0.04 b | 1.08 ± 0.07 d | 9 | 173 |
| PM 7 | 3.61 | 5.7 | 13.7 | 1988.96 ± 35.0 d | 30.14 ± 6.52 d | 4.93 ± 0.46 e | 6 | 89 |
| MIC (mg GAE/L) | Well (mm) | |||
|---|---|---|---|---|
| Wine Samples * | E. coli 12 | E. coli 13 | E. coli 12 | E. coli 13 |
| GS | 122.0 | 122.0 | <8 | <8 |
| GM | 1079.6 | 1079.6 | <8 | <8 |
| GM + SO2 | 570.0 | 570.0 | <8 | <8 |
| PM 0 | 215.6 | 215.6 | <8 | <8 |
| PM 7 | 397.8 | 795.6 | <8 | <8 |
| Ethanol (13–15%) | 0 | 0 | <8 | <8 |
| Wine Samples * | pH |
|---|---|
| Water | 6.58 ± 0.01 a |
| GS | 6.27 ± 0.02 b |
| GM | 6.32 ± 0.01 b |
| GM + SO2 | 6.40 ± 0.01 c |
| PM 0 | 6.25 ± 0.01 b |
| PM 7 | 6.28 ± 0.01 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, H.C.; Skroza, D.; Dugandžić, A.; Boban, M.; Šimat, V. Antimicrobial Activity of Selected Red and White Wines against Escherichia coli: In Vitro Inhibition Using Fish as Food Matrix. Foods 2020, 9, 936. https://doi.org/10.3390/foods9070936
Santoro HC, Skroza D, Dugandžić A, Boban M, Šimat V. Antimicrobial Activity of Selected Red and White Wines against Escherichia coli: In Vitro Inhibition Using Fish as Food Matrix. Foods. 2020; 9(7):936. https://doi.org/10.3390/foods9070936
Chicago/Turabian StyleSantoro, Heidi Christine, Danijela Skroza, Anđela Dugandžić, Mladen Boban, and Vida Šimat. 2020. "Antimicrobial Activity of Selected Red and White Wines against Escherichia coli: In Vitro Inhibition Using Fish as Food Matrix" Foods 9, no. 7: 936. https://doi.org/10.3390/foods9070936
APA StyleSantoro, H. C., Skroza, D., Dugandžić, A., Boban, M., & Šimat, V. (2020). Antimicrobial Activity of Selected Red and White Wines against Escherichia coli: In Vitro Inhibition Using Fish as Food Matrix. Foods, 9(7), 936. https://doi.org/10.3390/foods9070936








