Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Bean Preparation
2.2. Reagents
2.3. Coffee Brewing and Storage
2.4. HPLC Analysis
2.5. pH and Total Titratable Acidity Measurement
2.6. Total Antioxidant Capacity (TAC) Measurements
2.7. Browned Compounds (Abs 420 nm)
2.8. Total Dissolved Solids
2.9. Statistical Analysis
3. Results and Discussion
3.1. Acidity
3.2. Total Dissolved Solids (TDS)
3.3. Browned Compounds, CQA Concentration, and Total Antioxidant Capacity (TAC)
3.4. Caffeine
3.5. Roasting and Extraction Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schenker, S.; Rothgeb, T. The roast—Creating the Beans’ Signature. In The Craft and Science of Coffee; Academic Press: Cambridge, MA, USA, 2017; pp. 245–271. [Google Scholar]
- Schenker, S.; Handschin, S.; Frey, B.; Perren, R.; Escher, F. Pore Structure of Coffee Beans Affected by Roasting Conditions. J. Food Sci. 2000, 65, 452–457. [Google Scholar] [CrossRef]
- Clarke, R.J.; Macrae, R. (Eds.) Coffee: Volume 1 Chemistry; Elsevier Applied Science: London, UK; New York, NY, USA, 1985. [Google Scholar]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins Produced by the Maillard Reaction: Structure and Biological Activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Liu, Y.; Kitts, D.D. Confirmation That the Maillard Reaction Is the Principle Contributor to the Antioxidant Capacity of Coffee Brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; de Toledo Benassi, M. Roasting Process Affects Differently the Bioactive Compounds and the Antioxidant Activity of Arabica and Robusta Coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Fuller, M.; Rao, N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of Grinding, Extraction Time and Type of Coffee on the Physicochemical and Flavour Characteristics of Cold Brew Coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and Comparison of Cold Brew and Cold Drip Coffee Extraction Methods. J. Sci. Food Agric. 2019, 99, 391–399. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and Antioxidant Activity of Cold Brew Coffee. Sci. Rep. 2018, 8, 16030. [Google Scholar] [CrossRef]
- Ginz, M.; Balzer, H.H.; Bradbury, A.G.W.; Maier, H.G. Formation of Aliphatic Acids by Carbohydrate Degradation during Roasting of Coffee. Eur. Food Res. Technol. 2000, 211, 404–410. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Role of Roasting Conditions in the Profile of Volatile Flavor Chemicals Formed from Coffee Beans. J. Agric. Food Chem. 2009, 57, 5823–5831. [Google Scholar] [CrossRef]
- Uman, E.; Colonna-Dashwood, M.; Colonna-Dashwood, L.; Perger, M.; Klatt, C.; Leighton, S.; Miller, B.; Butler, K.T.; Melot, B.C.; Speirs, R.W.; et al. The Effect of Bean Origin and Temperature on Grinding Roasted Coffee. Sci. Rep. 2016, 6, 24483. [Google Scholar] [CrossRef]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of Caffeoylquinic Acids in Coffee Brews by HPLC-DAD. J. Anal. Methods Chem. 2014, 2014, 965353. [Google Scholar] [CrossRef]
- Tanaka, Y. Analysis of Chlorogenic Acid in Coffee by HPLC; LC Technical Note 67; GL Science Inc.: Tokyo, Japan, 2016. [Google Scholar]
- Trugo, L.C.; Macrae, R. Chlorogenic Acid Composition of Instant Coffees. Analyst 1984, 109, 263–266. [Google Scholar] [CrossRef]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of Roasting on the Formation of Chlorogenic Acid Lactones in Coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef]
- Monente, C.; Ludwig, I.A.; Irigoyen, A.; De Peña, M.-P.; Cid, C. Assessment of Total (Free and Bound) Phenolic Compounds in Spent Coffee Extracts. J. Agric. Food Chem. 2015, 63, 4327–4334. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Meydav, S.; Saguy, I.; Kopelman, I.J. Browning Determination in Citrus Products. J. Agric. Food Chem. 1977, 25, 602–604. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Podsędek, A.; Żyżelewicz, D.; Materska, M.; Jankowski, S.; Janda, B. Effect of Different Extraction Methods on the Recovery of Chlorogenic Acids, Caffeine and Maillard Reaction Products in Coffee Beans. Eur. Food Res. Technol. 2009, 228, 913–922. [Google Scholar] [CrossRef]
- Moreno, F.L.; Raventós, M.; Hernández, E.; Santamaría, N.; Acosta, J.; Pirachican, O.; Torres, L.; Ruiz, Y. Rheological Behaviour, Freezing Curve, and Density of Coffee Solutions at Temperatures Close to Freezing. Int. J. Food Prop. 2015, 18, 426–438. [Google Scholar] [CrossRef]
- Wessa, P. Free Statistics Software, Office for Research Development and Education, Version 1.2.1. Available online: https://www.wessa.net (accessed on 20 May 2020).
- Bilge, G. Investigating the Effects of Geographical Origin, Roasting Degree, Particle Size and Brewing Method on the Physicochemical and Spectral Properties of Arabica Coffee by PCA Analysis. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Moon, J.-K.; Yoo, H.S.; Shibamoto, T. Role of Roasting Conditions in the Level of Chlorogenic Acid Content in Coffee Beans: Correlation with Coffee Acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef]
- Asiah, N.; Aqil, M.; Dwiranti, N.S.; David, W.; Ardiansyah, A. Sensory and Chemical Changes of Cold and Hot Brew Arabica Coffee at Various Resting Time. Asia Pac. J. Sustain. Agric. Food Energy 2019, 7, 23–26. [Google Scholar] [CrossRef]
- Bicho, N.C.; Leitão, A.E.; Ramalho, J.C.; Lidon, F.C. Identification of Chemical Clusters Discriminators of the Roast Degree in Arabica and Robusta Coffee Beans. Eur. Food Res. Technol. 2011, 233, 303–311. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant Activity, Polyphenols, Caffeine and Melanoidins in Soluble Coffee: The Influence of Processing Conditions and Raw Material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Mendonça, L.; Pereira, R. Parâmetro Bromatológicos de Grãos Crus E Torrados de Cultivares de Café (Coffea Arabica L.). Food Sci. Technol. 2005, 25, 239–243. [Google Scholar] [CrossRef][Green Version]
- Spiro, M.; Hunter, J.E. The Kinetics and Mechanism of Caffeine Infusion from Coffee: The Effect of Roasting. J. Sci. Food Agric. 1985, 36, 871–876. [Google Scholar] [CrossRef]
- Sivetz, M.; Desrosier, N.W. Coffee Technology; AVI Publishing Co.: Westport, CT, USA, 1979; pp. 248–259. [Google Scholar]
- Morales, F.J. Assessing the Non-Specific Hydroxyl Radical Scavenging Properties of Melanoidins in a Fenton-Type Reaction System. Anal. Chim. Acta 2005, 534, 171–176. [Google Scholar] [CrossRef]
- Nunes, F.M.; Coimbra, M.A. Melanoidins from Coffee Infusions. Fractionation, Chemical Characterization, and Effect of the Degree of Roast. J. Agric. Food Chem. 2007, 55, 3967–3977. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the Antioxidant Activity of Melanoidins from Coffee Brews by Different Antioxidant Methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Bartel, C.; Mesias, M.; Morales, F.J. Investigation on the Extractability of Melanoidins in Portioned Espresso Coffee. Food Res. Int. 2015, 67, 356–365. [Google Scholar] [CrossRef]
- Smrke, S.; Opitz, S.E.W.; Vovk, I.; Yeretzian, C. How Does Roasting Affect the Antioxidants of a Coffee Brew? Exploring the Antioxidant Capacity of Coffee via On-Line Antioxidant Assays Coupled with Size Exclusion Chromatography. Food Funct. 2013, 4, 1082–1092. [Google Scholar] [CrossRef]
- Cämmerer, B.; Kroh, L.W. Antioxidant Activity of Coffee Brews. Eur. Food Res. Technol. 2006, 223, 469–474. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical Characterization and Antioxidant Properties of Coffee Melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interactions between Major Chlorogenic Acid Isomers and Chemical Changes in Coffee Brew That Affect Antioxidant Activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Bravo, J.; De Peña, M.P.; Cid, C. Effect of Sugar Addition (Torrefacto) during Roasting Process on Antioxidant Capacity and Phenolics of Coffee. LWT Food Sci. Technol. 2013, 51, 553–559. [Google Scholar] [CrossRef]
- del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of Roasting on the Antioxidant Activity of Coffee Brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef]
- Opitz, S.E.W.; Smrke, S.; Goodman, B.A.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods 2014, 3, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, M.C.; Anese, M.; Manzocco, L.; Lerici, C.R. Antioxidant Properties of Coffee Brews in Relation to the Roasting Degree. LWT Food Sci. Technol. 1997, 30, 292–297. [Google Scholar] [CrossRef]
- Sacchetti, G.; Di Mattia, C.; Pittia, P.; Mastrocola, D. Effect of Roasting Degree, Equivalent Thermal Effect and Coffee Type on the Radical Scavenging Activity of Coffee Brews and Their Phenolic Fraction. J. Food Eng. 2009, 90, 74–80. [Google Scholar] [CrossRef]
- Catelani, T.A.; Páscoa, R.N.M.J.; Santos, J.R.; Pezza, L.; Pezza, H.R.; Lima, J.L.F.C.; Lopes, J.A. A Non-Invasive Real-Time Methodology for the Quantification of Antioxidant Properties in Coffee during the Roasting Process Based on Near-Infrared Spectroscopy. Food Bioprocess. Technol. 2017, 10, 630–638. [Google Scholar] [CrossRef]
- Herawati, D.; Giriwono, P.E.; Dewi, F.N.A.; Kashiwagi, T.; Andarwulan, N. Critical Roasting Level Determines Bioactive Content and Antioxidant Activity of Robusta Coffee Beans. Food Sci. Biotechnol. 2019, 28, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Moon, J.-K.; Jang, H.W.; Shibamoto, T. Role of Degradation Products of Chlorogenic Acid in the Antioxidant Activity of Roasted Coffee. J. Agric. Food Chem. 2015, 63, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative Study of Polyphenols and Caffeine in Different Coffee Varieties Affected by the Degree of Roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of Coffee Antioxidants: Impact of Brewing Time and Method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Caemmerer, B.; De Peña, M.P.; Cid, C.; Kroh, L.W. Influence of Brewing Method and Acidity Regulators on the Antioxidant Capacity of Coffee Brews. J. Agric. Food Chem. 2010, 58, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Manzocco, L.; Nicoli, M.C.; Lerici, C.R. Antioxidant Properties of Tomato Juice as Affected by Heating. J. Sci. Food Agric. 1999, 79, 750–754. [Google Scholar] [CrossRef]
- Pilipczuk, T.; Kusznierewicz, B.; Zielińska, D.; Bartoszek, A. The Influence of Roasting and Additional Processing on the Content of Bioactive Components in Special Purpose Coffees. J. Food Sci. Technol. 2015, 52, 5736–5744. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.J.; Crozier, A. Variations in Caffeine and Chlorogenic Acid Contents of Coffees: What Are We Drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef]
- Tfouni, S.A.V.; Carreiro, L.B.; Teles, C.R.A.; Furlani, R.P.Z.; Cipolli, K.M.V.A.B.; Camargo, M.C.R. Caffeine and Chlorogenic Acids Intake from Coffee Brew: Influence of Roasting Degree and Brewing Procedure. Int. J. Food Sci. Technol. 2014, 49, 747–752. [Google Scholar] [CrossRef]
- Purdon, M.P.; McCamey, D.A. Use of a 5-Caffeoylquinic Acid/Caffeine Ratio to Monitor the Coffee Roasting Process. J. Food Sci. 1987, 52, 1680–1683. [Google Scholar] [CrossRef]
- Wanyika, H.N.; Gatebe, E.G.; Gitu, L.M.; Ngumba, E.K.; Maritim, C.W. Determination of Caffeine Content of Tea and Instant Coffee Brands Found in the Kenyan Market. Afr. J. Food Sci. 2010, 4, 353–358. [Google Scholar]
- Lane, S.; Palmer, J.; Christie, B.; Ehlting, J.; Le, C. Can Cold Brew Coffee Be Convenient? A Pilot Study for Caffeine Content in Cold Brew Coffee Concentrate Using High Performance Liquid Chromatography. Arbutus Rev. 2017, 8, 15–23. [Google Scholar] [CrossRef]
- Mateus, M.-L.; Champion, D.; Liardon, R.; Voilley, A. Characterization of Water Mobility in Dry and Wetted Roasted Coffee Using Low-Field Proton Nuclear Magnetic Resonance. J. Food Eng. 2007, 81, 572–579. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Loots, M.J.; Schols, H.A.; Van Boekel, M.A.; Smit, G. Roasting Effects on Formation Mechanisms of Coffee Brew Melanoidins. J. Agric. Food Chem. 2008, 56, 7138–7145. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.M.; Coimbra, M.A. Role of Hydroxycinnamates in Coffee Melanoidin Formation. Phytochem. Rev. 2010, 9, 171–185. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.-G.; Shibamoto, T. Antioxidative Activities of Fractions Obtained from Brewed Coffee. J. Agric. Food Chem. 2004, 52, 592–596. [Google Scholar] [CrossRef]
- Degenhardt, A.; Mendl, C.; Ceriali, S.; Ullrich, F. Modified Coffee Roasting as a Means of Acidity Increase. In Proceedings of the 21st international Conference on Coffee Science, Montpellier, France, 11–15 September 2006. [Google Scholar]
- Voilley, A.; Simatos, D. Modeling the Solubilization Process during Coffee Brewing. J. Food Process Eng. 1979, 3, 185–198. [Google Scholar] [CrossRef]
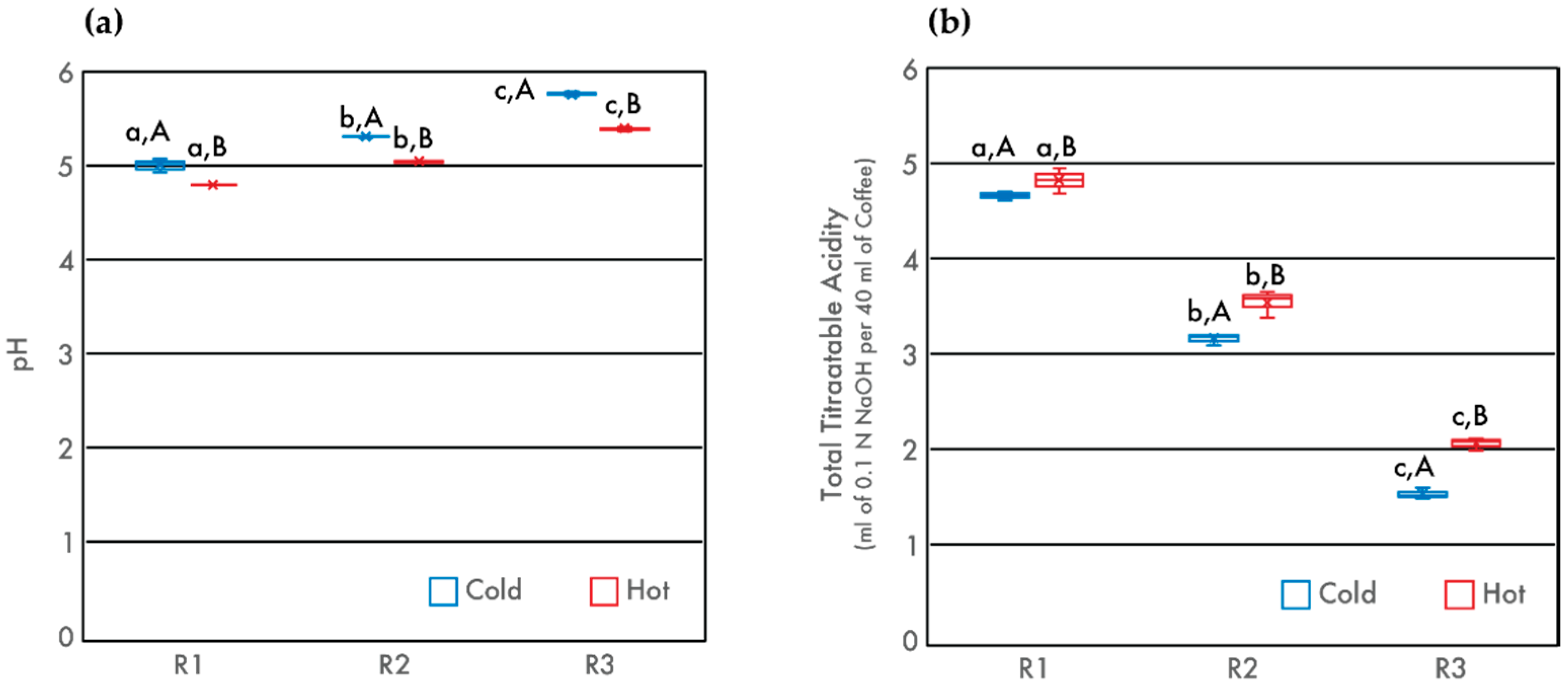
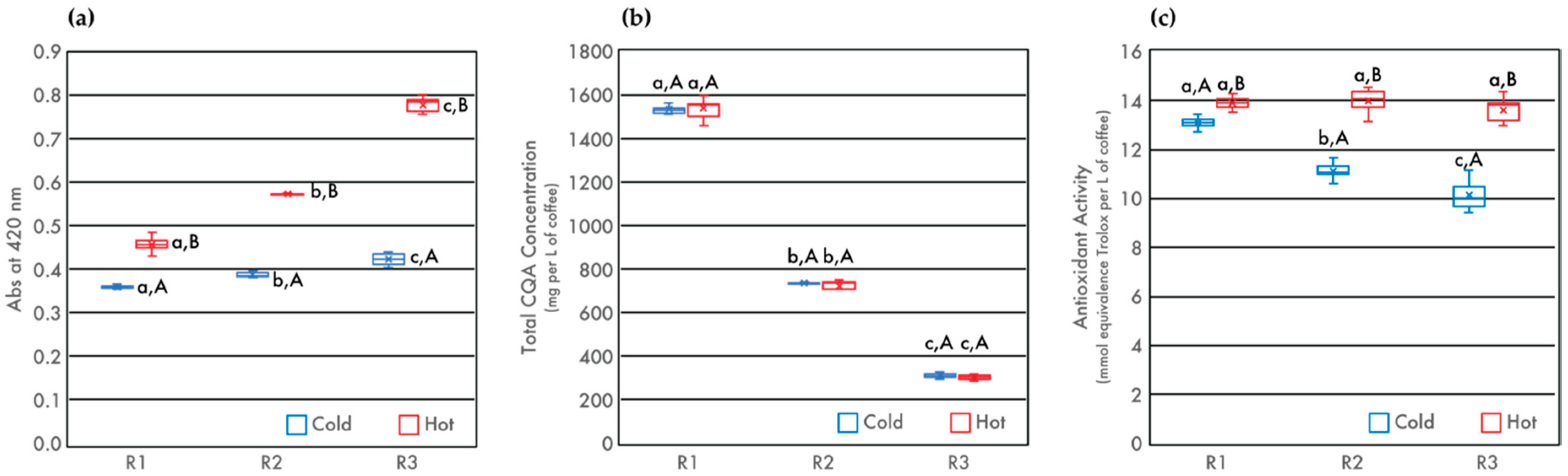
| Roasted Samples | pH | TTA (mL of 0.1 N NaOH) | TDS (%) | Browned Compounds (Abs420) | Caffeine (mg/L) | Total CQA (mg/L) | TAC (mmol Trolox/L Coffee) |
|---|---|---|---|---|---|---|---|
| Cold Brew | |||||||
| R1 | 5.00 ± 0.08 a,A | 4.66 ± 0.05 a,A | 1.88 ± 0.06 a,A | 0.358 ± 0.004 a,A | 1114 ± 56 a,A | 1535 ± 28 a,A | 13.09 ± 0.22 a,A |
| R2 | 5.30 ± 0.01 b,A | 3.15 ± 0.06 b,A | 2.06 ± 0.04 b,A | 0.386 ± 0.006 b,A | 1036 ± 19 b,A | 1733 ± 11 b,A | 11.11 ± 0.33 b,A |
| R3 | 5.75 ± 0.02 c,A | 1.53 ± 0.06 c,A | 2.05 ± 0.05 b,A | 0.422 ± 0.015 c,A | 1962 ± 41 c,A | 1308 ± 10 c,A | 10.13 ± 0.59 c,A |
| Hot Brew | |||||||
| R1 | 4.80 ± 0.01 a,B | 4.82 ± 0.13 a,B | 1.96 ± 0.05 a,B | 0.456 ± 0.018 a,B | 1095 ± 65 a,A | 1536 ± 46 a,A | 13.89 ± 0.24 a,B |
| R2 | 5.04 ± 0.01 b,B | 3.55 ± 0.14 b,B | 2.12 ± 0.04 b,A | 0.572 ± 0.004 b,B | 1056 ± 47 a,A | 1719 ± 33 b,A | 13.99 ± 0.44 a,B |
| R3 | 5.39 ± 0.03 c,B | 2.06 ± 0.06 c,B | 2.23 ± 0.04 c,B | 0.777 ± 0.018 c,B | 1035 ± 39 a,A | 1301 ± 11 c,A | 13.60 ± 0.50 a,B |
| Roasted Samples | Total CQA (mg/L) | 5-CQA (mg/L) | 4-CQA (mg/L) | 3-CQA (mg/L) |
|---|---|---|---|---|
| Cold Brew | ||||
| R1 | 1535 ± 28 a,A | 757 ± 27 a,A | 419 ± 12 a,A | 359 ± 14 a,A |
| R2 | 1733 ± 11 b,A | 353 ± 15 b,A | 210 ± 13 b,A | 170 ± 13 b,A |
| R3 | 1308 ± 10 c,A | 147 ± 14 c,A | 188 ± 14 c,A | 173 ± 14 c,A |
| Hot Brew | ||||
| R1 | 1536 ± 46 a,A | 787 ± 20 a,B | 413 ± 20 a,A | 336 ± 9 a,B |
| R2 | 1719 ± 33 b,A | 350 ± 15 b,A | 204 ± 19 b,A | 165 ± 9 b,A |
| R3 | 1301 ± 11 c,A | 144 ± 16 c,A | 186 ± 13 c,A | 171 ± 3 c,A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. https://doi.org/10.3390/foods9070902
Rao NZ, Fuller M, Grim MD. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods. 2020; 9(7):902. https://doi.org/10.3390/foods9070902
Chicago/Turabian StyleRao, Niny Z., Megan Fuller, and Meghan D. Grim. 2020. "Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction" Foods 9, no. 7: 902. https://doi.org/10.3390/foods9070902
APA StyleRao, N. Z., Fuller, M., & Grim, M. D. (2020). Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods, 9(7), 902. https://doi.org/10.3390/foods9070902






