Determination of Cyclopropenoid Fatty Acids in Ewe Milk Fat by GC-MS after Intravenous Administration of Sterculic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Milk Samples, and Standards
2.2. Derivatization, Identification, and Quantification of Sterculic Acid by GC-MS
2.2.1. Sterculic Acid Standard Derivatization
2.2.2. Ewe Milk Fat Extraction and Derivatization
2.2.3. GC-MS Analysis
3. Results
3.1. GC-MS Determination of Sterculic Acid Chemically Synthesized Standard
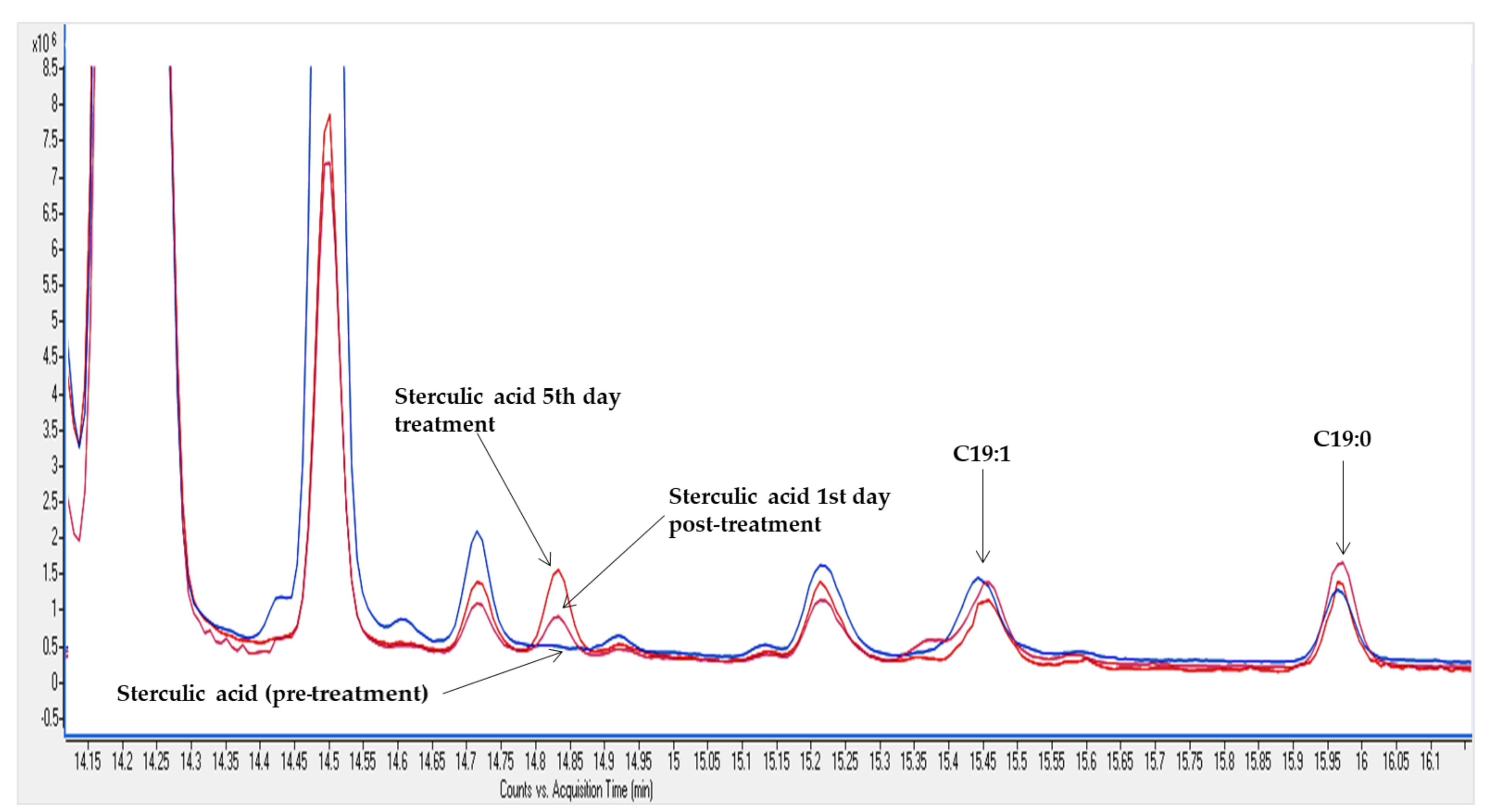
3.2. GC-MS Analysis of FAMEs in Ewe Milk Fat
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bao, X.; Katz, S.; Pollard, M.; John, O. Carbocyclic fatty acids in plants: Biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculia foetida. Plant Biol. 2002, 99, 7172–7177. [Google Scholar] [CrossRef] [PubMed]
- Aued-Pimentel, S.; Lago, J.H.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Adoree, A.A.R.; Blond, J.P.; Cao, J.; Gaydou, E.E.; Bezard, J. Influence of cyclopropene fatty acids (Baobab seed oil) feeding on the in vitro A9 desaturatlon of stearlc acid in rat liver microsomes. J. Nutr. Biochem. 1993, 4, 92–104. [Google Scholar]
- Montanari, C.; Sado Kamdem, S.L.; Serrazanetti, D.I.; Etoa, F.X.; Guerzoni, M.E. Synthesis of cyclopropane fatty acids in Lactobacillus helveticus and Lactobacillus sanfranciscensis and their cellular fatty acids changes following short term acid and cold stresses. Food Microbiol. 2010, 27, 493–502. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Palla, G. An Overview on the Presence of Cyclopropane Fatty Acids in Milk and Dairy Products. J. Agric. Food Chem. 2014, 62, 7828–7832. [Google Scholar] [CrossRef] [PubMed]
- Barb Mitchell, B.; Rozema, B.; Vennard, T.; Sabbatini, J. Determination of Nutritional and Cyclopropenoid Fatty Acids in Cottonseed by a Single GC Analysis. J. Am. Oil Chem. Soc. 2015, 92, 947–956. [Google Scholar] [CrossRef]
- Obert, J.C.; Hughes, D.; Sorenson, W.R.; McCann, M.; Ridley, W.P. A Quantitative Method for the Determination of Cyclopropenoid Fatty Acids in Cottonseed, Cottonseed Meal, and Cottonseed Oil (Gossypium hirsutum) by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2007, 55, 2062–2067. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Hassoun, P.; Bastianelli, D.; Lebas, F. Cottonseed Meal. Feedipedia, a Programme by INRA, CIRAD, AFZ and FAO. Available online: https://feedipedia.org/node/550 (accessed on 27 March 2020).
- Yu, X.H.; Rawat, R.; Shanklin, J. Characterization and analysis of the cotton cyclopropane fatty acid synthase family and their contribution to cyclopropane fatty acid synthesis. BMC Plant Biol. 2011, 11, 97. [Google Scholar] [CrossRef]
- Schneider, A.C.; Beguine, P.; Bourez, S.; Perfield, J.W.; Mignolet, E.; Debier, C.; Schneider, Y.J.; Larondelle, Y. Conversion of t11t13 CLA into c9t11 CLA in Caco-2 Cells and Inhibition by Sterculic Oil. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.; Burns, T.A.; Pratt, S.L.; Duckett, S.K. Inhibition of stearoyl-CoA desaturase 1 reduces lipogenesis in primary bovine adipocytes. Lipids 2013, 48, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Bichi, E.; Toral, P.G.; Hervás, G.; Frutos, P.; Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Inhibition of Δ9-desaturase activity with sterculic acid: Effect on the endogenous synthesis of cis-9 18:1 and cis-9, trans-11 18:2 in dairy sheep. J. Dairy Sci. 2012, 95, 5242–5252. [Google Scholar] [CrossRef] [PubMed]
- Ortinau, L.C.; Nickelson, K.J.; Stromsdorfer, K.L.; Naik, C.Y.; Pickering, R.T.; Haynes, R.A.; Fritsche, K.L.; Perfield, J.W., II. Sterculic Oil, a natural inhibitor of SCD1, improves the metabolic state of obese OLETF rats. Obesity 2013, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Peláez, R.; Pariente, A.; Pérez-Sala, Á.; Larráyoz, I.M. Sterculic Acid: The Mechanisms of Action beyond Stearoyl-CoA Desaturase Inhibition and Therapeutic Opportunities in Human Diseases. Cells 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Brondz, I. Development of fatty acid analysis by high-performance liquid chromatography, gas chromatography, and related techniques. Anal. Chim. Acta 2002, 465, 1–37. [Google Scholar] [CrossRef]
- Caligiani, A.; Nocetti, M.; Lolli, V.; Marseglia, A.; Palla, G. Development of a Quantitative GC–MS Method for the Detection of Cyclopropane Fatty Acids in Cheese as New Molecular Markers for Parmigiano Regiano Authentication. J. Agric. Food Chem. 2016, 64, 4158–4164. [Google Scholar] [CrossRef]
- Dobson, G. Cyclic fatty acids: Qualitative and quantitative analysis. In Lipid Analysis in Oils and Fats; Hamilton, R.J., Ed.; Springer: Boston, MA, USA, 1998; pp. 136–180. [Google Scholar]
- Lolli, V.; Marseglia, A.; Palla, G.; Zanardi, E.; Caligiani, A. Determination of cyclopropane fatty acids in food of animal origin by 1HNMR. J. Anal. Methods Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Lolli, V.; Dall’Asta, M.; Del Rio, D.; Palla, G.; Caligiani, A. Presence of cyclopropane fatty acids in foods and estimation of dietary intake in the Italian population. Int. J. Food Sci. Nutr. 2019, 70, 467–473. [Google Scholar] [CrossRef]
- Hernando, J.; Matía, M.P.; Novella, J.L.; Alvarez-Builla, J. Synthesis of Sterculic Acid; ARKIVOC: Gainesville, FL, USA, 2002; pp. 26–30. [Google Scholar]
- Christie, W.W.; Han, X. Preparation of derivatives of fatty acids. In Lipid Analysis, 4th ed.; Woodhead Publishing: Sawston, UK; Elsevier: Cambridge, MA, USA, 2012; pp. 145–158. [Google Scholar]
- Gómez-Cortés, P.; Toral, P.G.; Frutos, P.; Juárez, M.; De la Fuente, M.A.; Hervás, G. Effect of the supplementation of dairy sheep diet with incremental amounts of sunflower oil on animal performance and milk fatty acid profile. Food Chem. 2011, 125, 644–651. [Google Scholar] [CrossRef]
- Rao, K.; Jones, G.; Rivett, D.; Tucker, D. Cyclopropene fatty acids of six seed oils from malvaceae. J. Am. Oil Chem. Soc. 1989, 66, 360–361. [Google Scholar] [CrossRef]
- Gomez, F.; Bauman, D.; Ntambi, J.; Fox, B. Effects of sterculic acid on stearoyl-CoA desaturase in differentiating 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2003, 300, 316–326. [Google Scholar] [CrossRef]
- Dallaire, M.P.; Taga, H.; Ma, L.; Corl, B.A.; Gervais, R.; Lebeuf, Y.; Richard, F.J.; Chouinard, P.Y. Effects of abomasal infusion of conjugated linoleic acids, Sterculia foetida oil, and fish oil on production performance and the extent of fatty acid Delta(9)-desaturation in dairy cows. J. Dairy Sci. 2014, 97, 6411–6425. [Google Scholar] [CrossRef] [PubMed]
- Griinari, J.M.; Corl, B.A.; Lacy, S.H.; Chouinard, P.Y.; Nurmela, K.V.V.; Bauman, D.E. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Delta (9)-desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar] [CrossRef]
- Corl, B.A.; Baumgard, L.H.; Griinari, J.M.; Delmonte, P.; Morehouse, K.M.; Yuraweczc, M.P.; Bauman, D.E. Trans-7, cis-9 CLA is synthesized endogenously by delta (9)-desaturase in dairy cows. Lipids 2002, 37, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Bickerstaffe, R.; Johnson, A.R. Effect of intravenous infusions of sterculic acid on milk-fat synthesis. Br. J. Nutr. 1972, 27, 561–570. [Google Scholar] [CrossRef]
- Toral, P.G.; Carreño, F.D.; Hervás, G. Endogenous synthesis of milk oleic acid in dairy ewes: In vivo measurement using 13C-labeled stearic acid. J. Dairy Sci. 2017, 100, 5880–5887. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Toral, P.G. Endogenous synthesis of milk cis-9, trans-11 conjugated linoleic acid in dairy ewes: Quantification using 13C-labeled vaccenic acid and comparison with estimates based on cobalt administration. J. Dairy Sci. 2020, 103, 368–378. [Google Scholar] [CrossRef]




| Relative (%) 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | M+ (m/z) | RT (min) | pH = 5 | pH = 7 | ||||
| Sterculene | 266 | 16.2 | 12.4 | ± | 17.0 | 3.6 | ± | 2.7 |
| Sterculic acid | 366 | 18.0 | 78.4 | ± | 9.4 | 86.3 | ± | 4.5 |
| Dihydrosterculic acid | 368 | 18.2 | 2.0 | ± | 1.3 | 3.7 | ± | 0.4 |
| Sterculic acid isomer | 366 | 18.4 | 7.2 | ± | 6.3 | 6.3 | ± | 1.7 |
| Timepoints | Sterculic Acid Concentration (mg/kg Total Fat) | |||||
|---|---|---|---|---|---|---|
| Ewe 1 | Ewe 2 | Ewe 3 | Ewe 4 | Ewe 5 | Ewe 6 | |
| 1st day-pre-treatment | <LOD 2 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 5th day-pre-treatment | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 4th day-treatment | 500 ± 40 | 760 ± 60 | 394 ± 1 | 500 ± 20 | 573 ± 23 | 654.1 ± 0.3 |
| 5th day-treatment | 620 ± 50 | 740 ± 70 | 555 ± 47 | 580 ± 40 | 620 ± 36 | 825.9 ± 0.1 |
| 1st day-post-treatment | 210 ± 33 | 281 ± 48 | 219 ± 4 | 296 ± 7 | 256 ± 5 | 309 ± 11 |
| 5th day-post-treatment | <LOD | <LOD | <LOD | <LOQ 3 | <LOQ | <LOQ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lolli, V.; Toral, P.G.; Caligiani, A.; Gómez-Cortés, P. Determination of Cyclopropenoid Fatty Acids in Ewe Milk Fat by GC-MS after Intravenous Administration of Sterculic Acid. Foods 2020, 9, 901. https://doi.org/10.3390/foods9070901
Lolli V, Toral PG, Caligiani A, Gómez-Cortés P. Determination of Cyclopropenoid Fatty Acids in Ewe Milk Fat by GC-MS after Intravenous Administration of Sterculic Acid. Foods. 2020; 9(7):901. https://doi.org/10.3390/foods9070901
Chicago/Turabian StyleLolli, Veronica, Pablo G. Toral, Augusta Caligiani, and Pilar Gómez-Cortés. 2020. "Determination of Cyclopropenoid Fatty Acids in Ewe Milk Fat by GC-MS after Intravenous Administration of Sterculic Acid" Foods 9, no. 7: 901. https://doi.org/10.3390/foods9070901
APA StyleLolli, V., Toral, P. G., Caligiani, A., & Gómez-Cortés, P. (2020). Determination of Cyclopropenoid Fatty Acids in Ewe Milk Fat by GC-MS after Intravenous Administration of Sterculic Acid. Foods, 9(7), 901. https://doi.org/10.3390/foods9070901





