Three Types of Beetroot Products Enriched with Lactic Acid Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacteria Strain
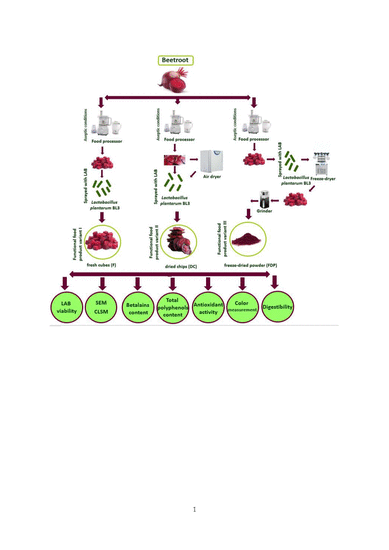
2.2. Sample Preparation
2.3. Lactic Acid Bacteria (LAB) Viability
2.4. Scanning Electron Microscopy (SEM)
2.5. Confocal Laser Scanning Microscopy
2.6. Hardness Measurement
2.7. Betalains Quantification
2.8. Color Measurement
2.9. Antioxidant Activity
2.10. Total Phenolic Content
2.11. Digestibility
2.12. Statistical Analysis of Data
3. Results and Discussion
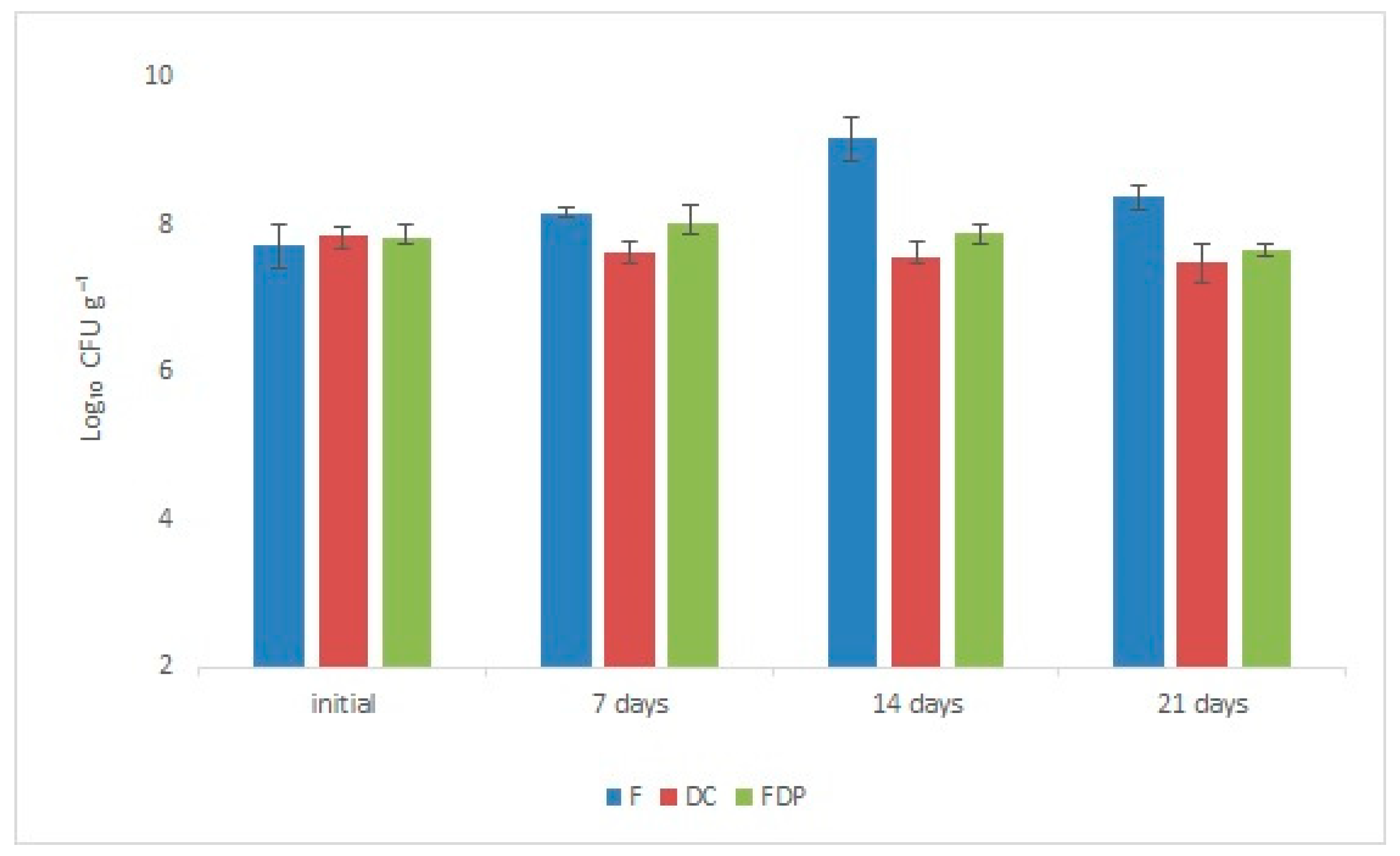
3.1. LAB Viability
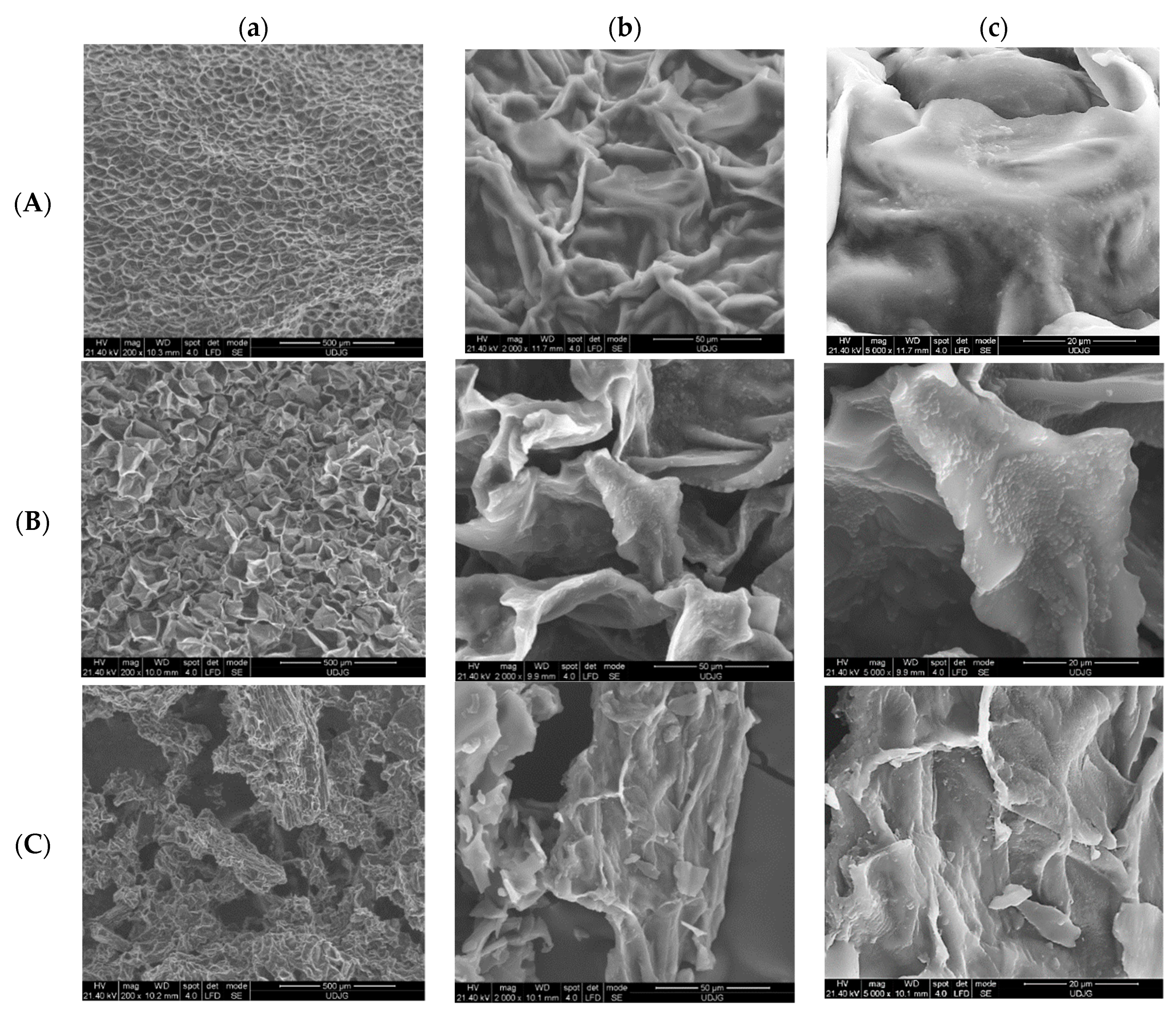
3.2. Scanning Electron Microscopy (SEM)
3.3. Confocal Laser Scanning Microscopy
3.4. Measurement of Hardness
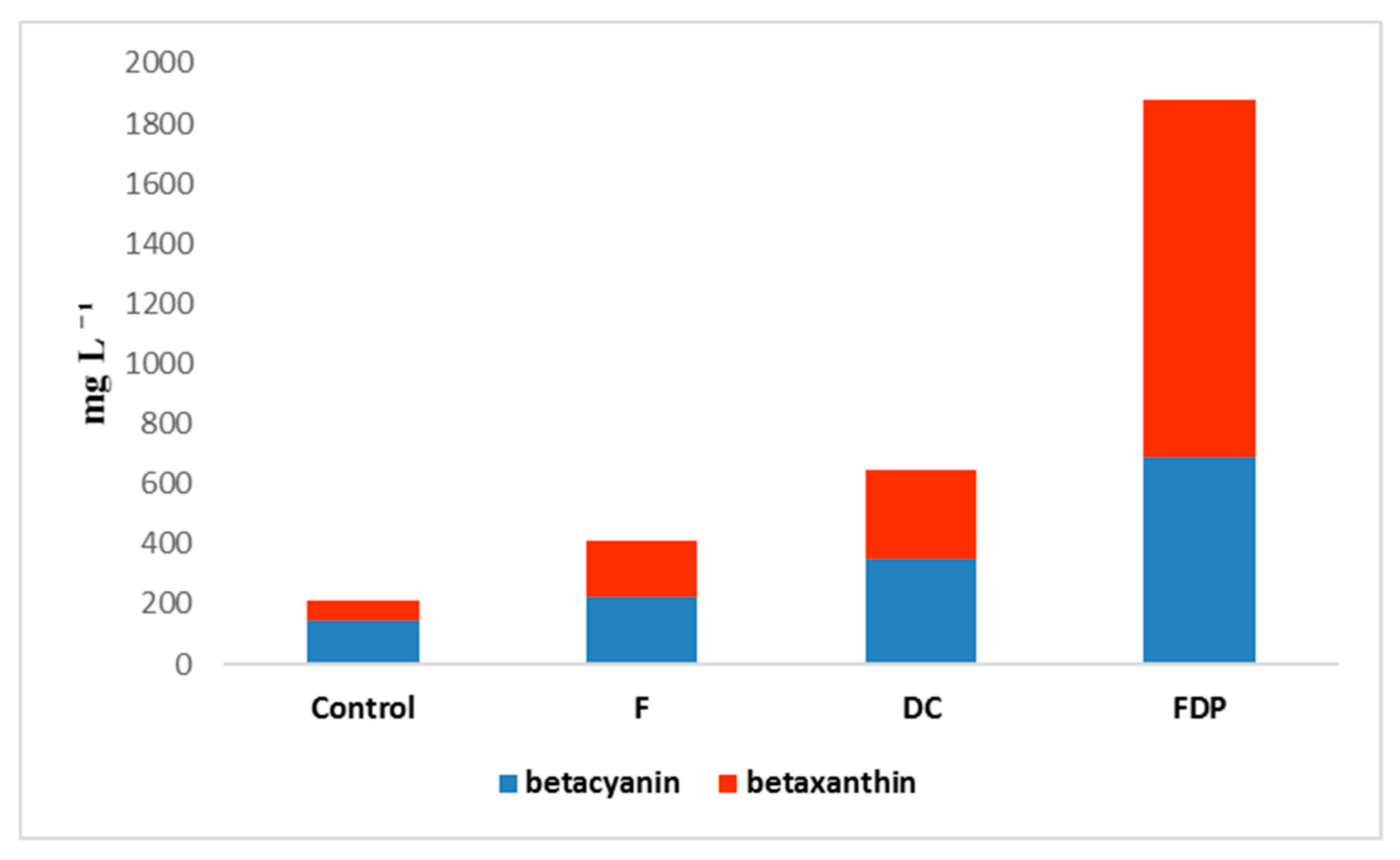
3.5. Betalains Quantification, Total Polyphenols Content, and Antioxidant Activity
3.6. Measurement of Color
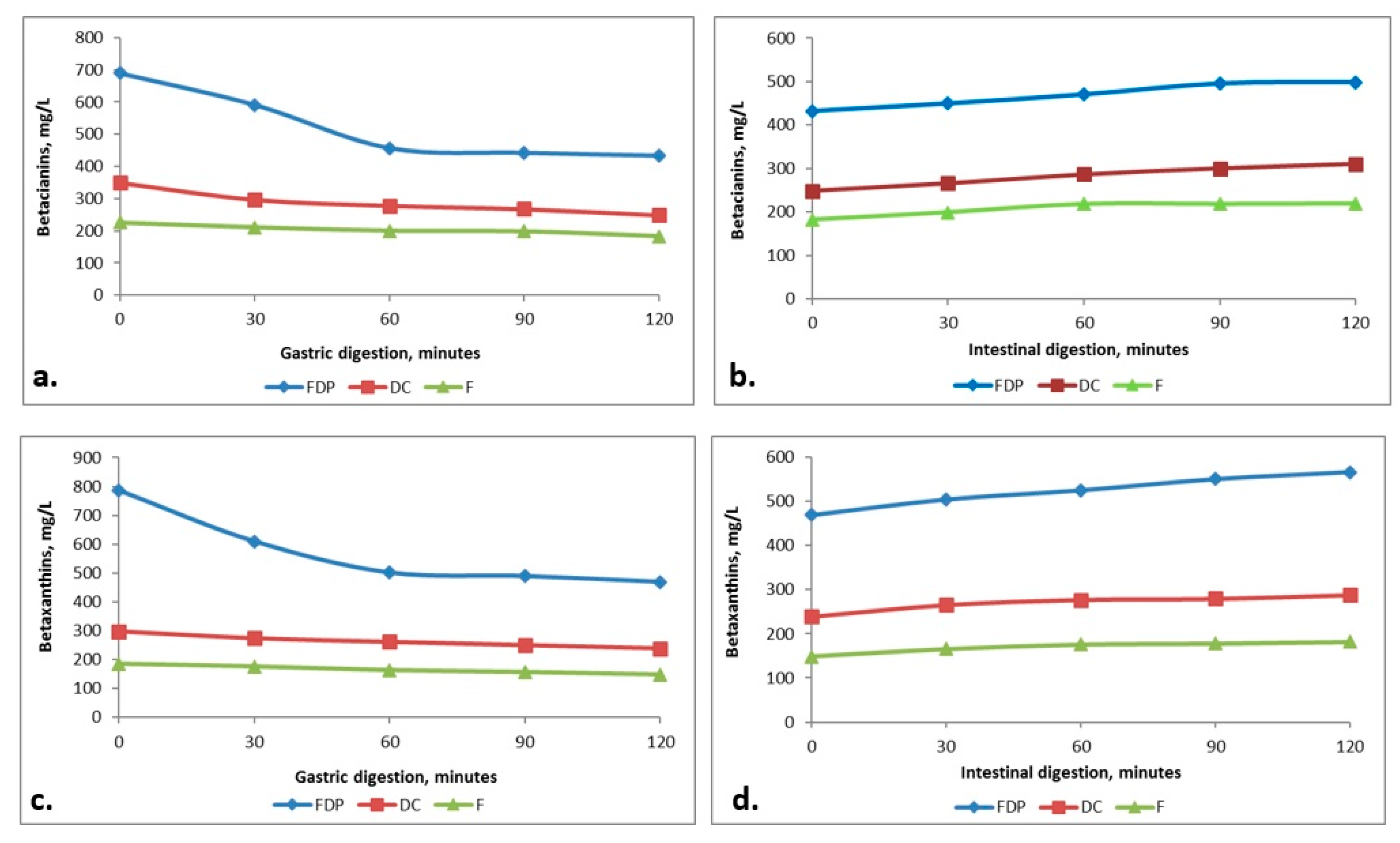
3.7. Digestibility of the Beetroot Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Šaponjac, V.T.; Čanadanović–Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Food Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother. Res. 2009, 23, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Butera, D.; D’Arpa, D.; Di Gaudio, F.; Allegra, M.; Gentile, C.; Livrea, M.A. Increased resistance to oxidation of betalain-enriched human low-density lipoproteins. Free Radic. Res. Commun. 2003, 37, 689–696. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Betalains in food: Occurrence, stability, and postharvest modifications. In Food Colourants: Chemical and Functional Properties; Socaciu, C., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 277–299. [Google Scholar]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Tsai, P.J.; Sheu, C.H.; Wu, P.H.; Sun, Y.F. Thermal and pH Stability of betacyanin pigment of djulis (Chenopodium formosanum) in Taiwan and their relation to antioxidant activity. J. Agric. Food Chem. 2010, 58, 1020–1025. [Google Scholar] [CrossRef]
- Gaertner, V.L.; Goldman, I.L. Pigment distribution and total dissolved solids of selected cycles of table beet from a recurrent selection program for increased pigment. J. Am. Soc. Hortic. Sci. 2005, 130, 424–433. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Hallmann, E.; Lipowski, J.; Drela, N.; Kowalik, A.; Pussa, T.; Matt, D.; Luik, A.; Gozdowski, D.; Rembiałkowska, E. Beetroot (Beta vulgaris L.) and naturally fermented beetroot juices from organic and conventional production: Metabolomics, antioxidant levels and anti-cancer activity. J. Sci. Food Agric. 2014, 94, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.S.; Bird, A.R.; Mc-Orist, A.I. Comparison of two selective media for the detection and enumeration of lactobacilli in human faeces. J. Microbiol. Methods 2002, 51, 313–321. [Google Scholar] [CrossRef]
- Nguyen, T.D.T.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Tanomand, A.; Reza Ashoori, M.; Asgharzadeh, A.; Zarghami, N. Investigating the effects of Lactobacillus casei on some biochemical parameters in diabetic mice. JEMDSA 2017, 22, 47–50. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Antioxidant activity and inhibition of key enzymes linked to type-2 diabetes and hypertension by Azadirachta indica-yogurt. J. Saudi Chem. Soc. 2013, 17, 295–301. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.Q.; Li, L. The Potential Role of Probiotics in Cancer Prevention and Treatment. Nutr. Cancer 2016, 68, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- De LeBlanc, A.D.M.; Chaves, S.; Carmuega, E.; Weill, R.; Antóine, J.; Perdigón, G. Effect of long-term continuous consumption of fermented milk containing probiotic bacteria on mucosal immunity and the activity of peritoneal macrophages. Immunobiology 2008, 213, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Kassayová, M.; Bobrov, N.; Strojný, L.; Kisková, T.; Mikeš, J.; Demečková, V.; Bojková, B.; Péč, M.; Kubatka, P.; Bomba, A. Preventive Effects of Probiotic Bacteria Lactobacillus plantarum and Dietary Fiber in Chemically-induced Mammary Carcinogenesis. Anticancer Res. 2014, 34, 4969–4976. [Google Scholar] [PubMed]
- Liu, C.F.; Pan, T.M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010, 18, 77–86. [Google Scholar]
- Moreno, Y.; Collado, M.C.; Ferrús, M.A.; Cobo, J.M.; Hernández, E.; Hernández, M. Viability assessment of lactic acid bacteria in commercial dairy products stored at 4 °C using LIVE/DEAD® BacLightTM staining and conventional plate counts. Int. J. Food Sci. Technol. 2005, 41, 275–280. [Google Scholar] [CrossRef]
- Neagu, C.; Barbu, V. Principal component analysis of the factors involved in the extraction of beetroot betalains. J. Agroaliment. Process Technol. 2014, 20, 311–318. [Google Scholar]
- Bucur, L.; Ţarălungă, G.; Schroder, V. The betalains content and antioxidant capacity of red beet (Beta vulgaris l. subsp. vulgaris) root. Farmacia 2016, 64, 198–201. [Google Scholar]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Chandran, J.; Nisha, P.; Singhal, R.S.; Pandit, A.B. Degradation of colour in beetroot (Beta vulgaris L.): A kinetics study. J. Food Sci. Technol. 2014, 51, 2678–2684. [Google Scholar] [CrossRef]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of thermal treatment on phenolic compounds from plum (Prunus domestica) extracts—A kinetic study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]
- Croitoru, C.; Mureşan, C.; Turturică, M.; Stănciuc, N.; Andronoiu, D.G.; Dumitraşcu, L.; Barbu, V.; Enachi, E. (Ioniţă); Horincar, G. (Parfene); Râpeanu, G. Improvement of Quality Properties and Shelf Life Stability of New Formulated Muffins Based on Black Rice. Molecules 2018, 23, 3047. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- EU. A2 Date of publication of application: 14.04.93 Bulletin 93/15. Method of vegetable processing. Patent 0 536 851. Application number: 92203124.0; Applicant: Matforsk Norwegien Food Research Institute; Osloveien 1N-1329 As(NO).
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Niu, L.Y.; Li, D.-J.; Liu, C.-G.; Liu, Y.-P.; Liu, C.-J.; Song, J.-F. Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks. J. Integr. Agric. 2018, 17, 247–255. [Google Scholar] [CrossRef]
- Kushwaha, R.; Kumar, V.; Vyas, G.; Kaur, J. Optimization of Different Variable for Eco-friendly Extraction of Betalains and Phytochemicals from Beetroot Pomace. Waste Biomass Valorization 2017, 8, 3–12. [Google Scholar] [CrossRef]
- Molina, G.A.; Hernández-Martínez, A.R.; Cortez-Valadez, M.; García-Hernández, F.; Estevez, M. Effects of tetraethyl orthosilicate (TEOS) on the light and temperature stability of a pigment from Beta vulgaris and its potential food industry applications. Molecules 2014, 19, 17985–18002. [Google Scholar] [CrossRef]
- Sekiguchi, H.; Ozeki, Y.; Sasaki, N. Red Beet Biotechnology: Food and Pharmaceutical Applications; Neelwarne, B., Ed.; Springer Science & Business Media: Boston, MA, USA, 2012; pp. 60–63. [Google Scholar]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.; Gabr, A.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Slavov, A.; Karagyozov, V.; Denev, P.; Kratchanova, M.; Kratchanov, C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech J. Food Sci. 2013, 31, 139–147. [Google Scholar] [CrossRef]
- Roy, K.; Gullapalli, S.; Chaudhuri, U.R.; Chakraborty, R. The use of a natural colorant based on betalain in the manufacture of sweet products in India. Int. J. Food Sci. Technol. 2004, 39, 1087–1091. [Google Scholar] [CrossRef]
- Gokhale, S.V.; Le, S.S. Dehydration of Red Beet Root (Beta vulgaris) by Hot Air Drying: Process Optimization and Mathematical Modeling. Food Sci. Biotechnol. 2011, 20, 955–964. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Espin, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]

| Texture Parameter | 7 Days | 14 Days | 21 Days |
|---|---|---|---|
| Hardness, N | 0.73 ± 0.008 | 0.75 ± 0.008 | 0.77 ± 0.008 |
| Springiness, mm | 1.57 ± 0.005 | 1.56 ± 0.008 | 1.52 ± 0.005 |
| Variants | BC (mg L−1) | BX (mg L−1) | TPC (mg Gallic Acid L−1) | Antioxidant Activity (%) |
|---|---|---|---|---|
| Control | 147.90 ± 5.204 | 64.68 ± 1.004 | 225.70 ± 0.034 | 20.19 |
| F | 226.18 ± 2.002 | 185.12 ± 1.229 | 418.30 ± 0.045 | 22.13 |
| DC | 349.25 ± 1.082 | 298.38 ± 5.854 | 635.80 ± 0.005 | 33.51 |
| FDP | 689.79 ± 4.321 | 786.69 ± 5.625 | 1314.70 ± 0.025 | 56.85 |
| L | a | b | x | BI | a/b | b/a | Hue Value (tg−) | |
|---|---|---|---|---|---|---|---|---|
| F | 35.64 ± 0.34 | 62.76 ± 0.23 | 19.58 ± 0.40 | 0.485 ± 0.18 | 114.117 ± 0.32 | 3.20 | 0.3119 | 17.32° |
| DC | 32.83 ± 0.18 | 45.25 ± 0.50 | 16.51 ± 0.40 | 0.455 ± 0.26 | 85.58 ± 0.31 | 2.70 | 0.3907 | 21.34° |
| FDP | 34.63 ± 0.32 | 57.61 ± 0.49 | 18.73 ± 0.10 | 0.477 ± 0.17 | 98.235 ± 0.21 | 3.07 | 0.3251 | 18.00° |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbu, V.; Cotârleț, M.; Bolea, C.A.; Cantaragiu, A.; Andronoiu, D.G.; Bahrim, G.E.; Enachi, E. Three Types of Beetroot Products Enriched with Lactic Acid Bacteria. Foods 2020, 9, 786. https://doi.org/10.3390/foods9060786
Barbu V, Cotârleț M, Bolea CA, Cantaragiu A, Andronoiu DG, Bahrim GE, Enachi E. Three Types of Beetroot Products Enriched with Lactic Acid Bacteria. Foods. 2020; 9(6):786. https://doi.org/10.3390/foods9060786
Chicago/Turabian StyleBarbu, Vasilica, Mihaela Cotârleț, Carmen Alina Bolea, Alina Cantaragiu, Doina Georgeta Andronoiu, Gabriela Elena Bahrim, and Elena Enachi. 2020. "Three Types of Beetroot Products Enriched with Lactic Acid Bacteria" Foods 9, no. 6: 786. https://doi.org/10.3390/foods9060786
APA StyleBarbu, V., Cotârleț, M., Bolea, C. A., Cantaragiu, A., Andronoiu, D. G., Bahrim, G. E., & Enachi, E. (2020). Three Types of Beetroot Products Enriched with Lactic Acid Bacteria. Foods, 9(6), 786. https://doi.org/10.3390/foods9060786