Application of Autoclave Treatment for Development of a Natural Wheat Bran Antioxidant Ingredient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Preparation
2.3. Physicochemical Properties of WB
2.3.1. Proximal Composition
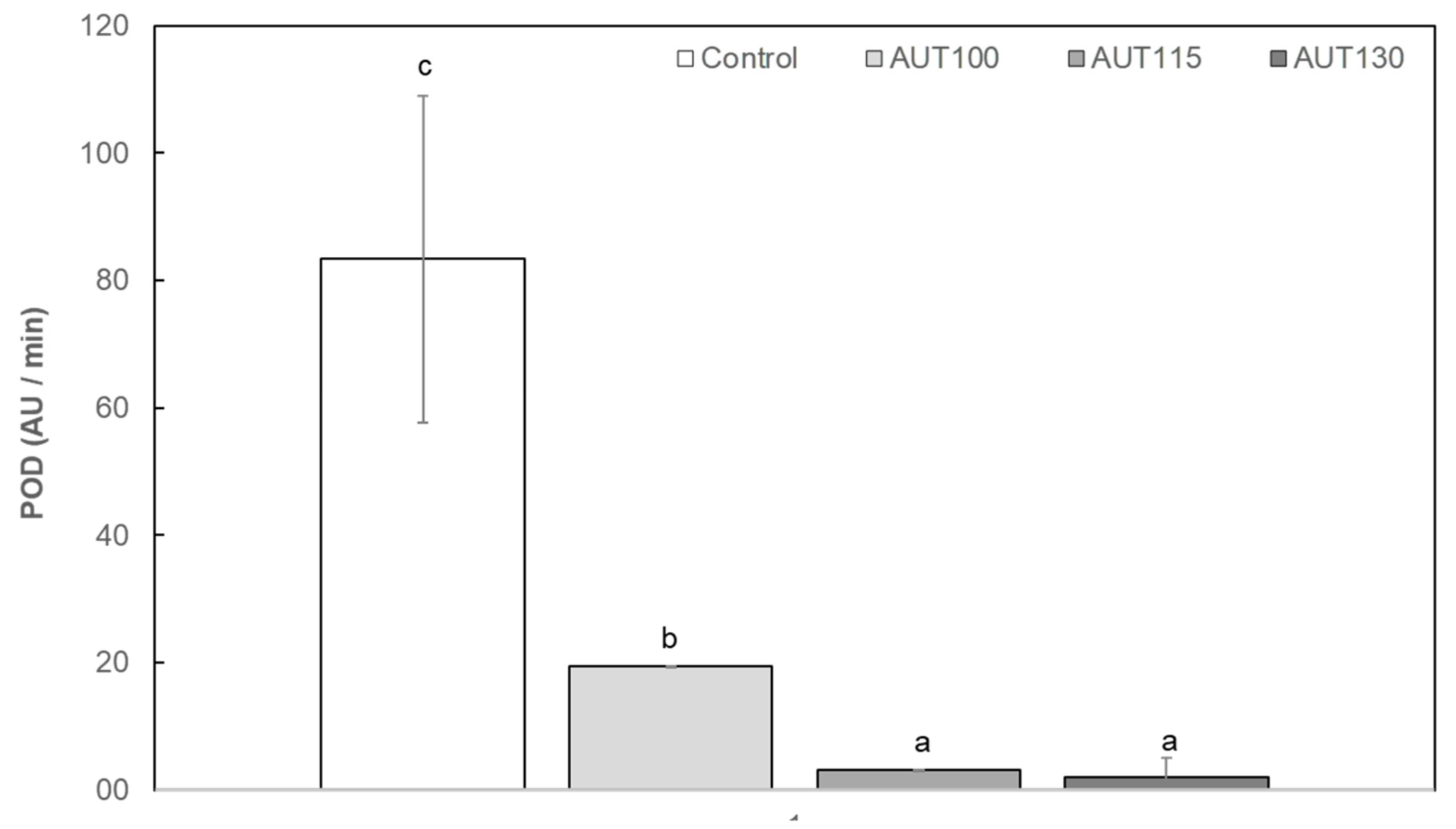
2.3.2. Peroxidase (POD) Activity
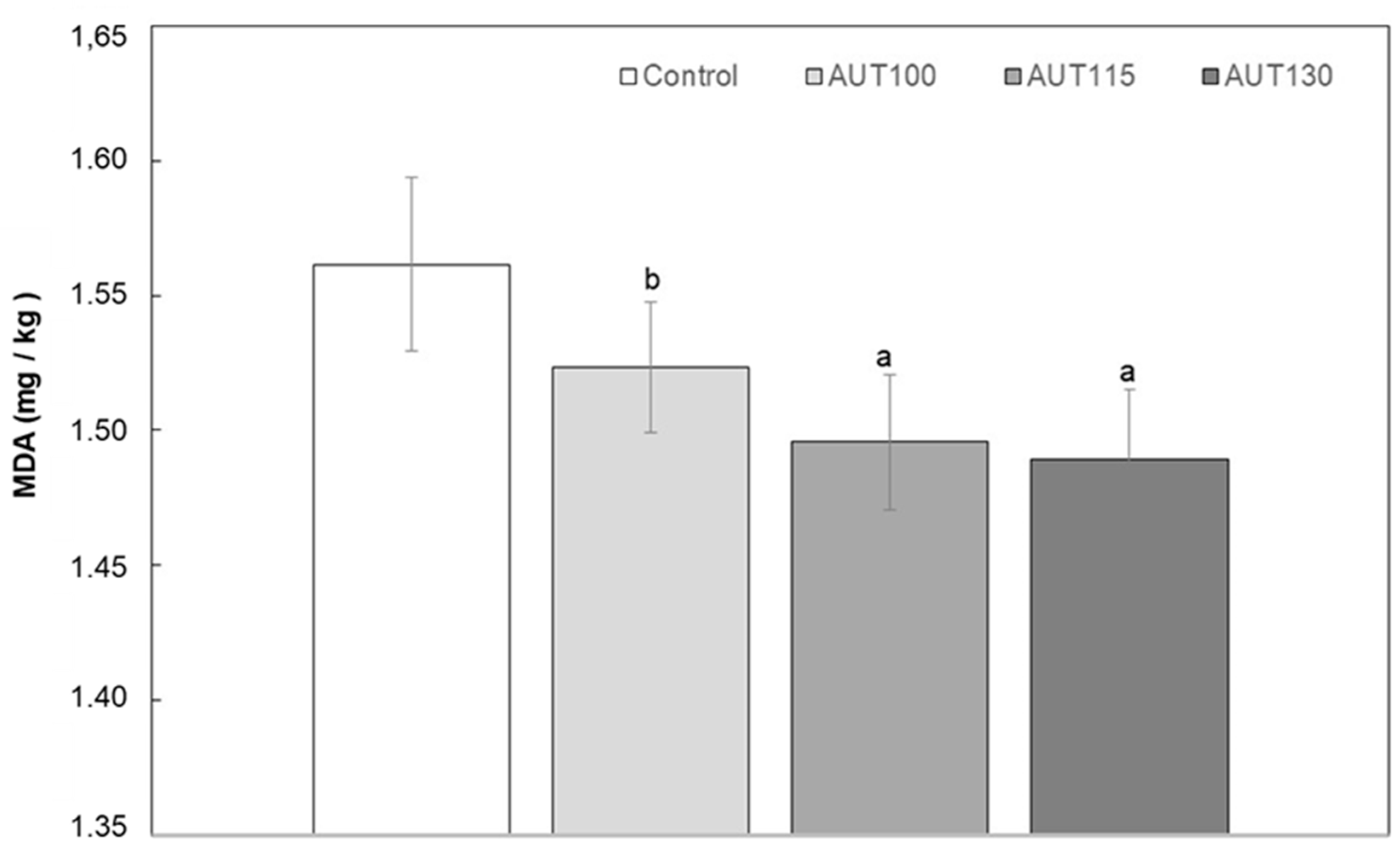
2.3.3. Determination of Malondialdehyde (MDA)
2.4. Phenol Quantification and Characterisation
2.4.1. Extracts
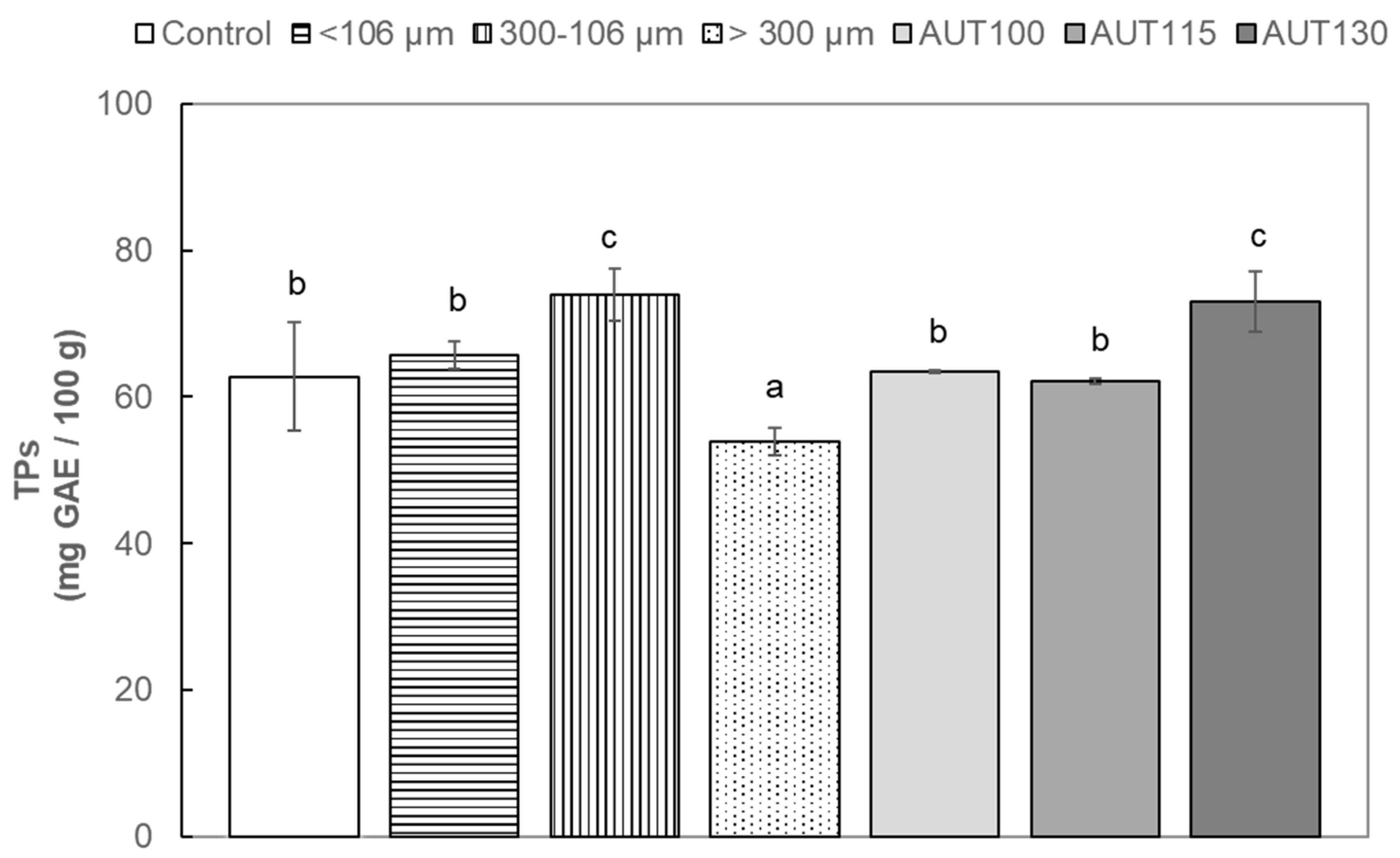
2.4.2. Total Phenols (TP)
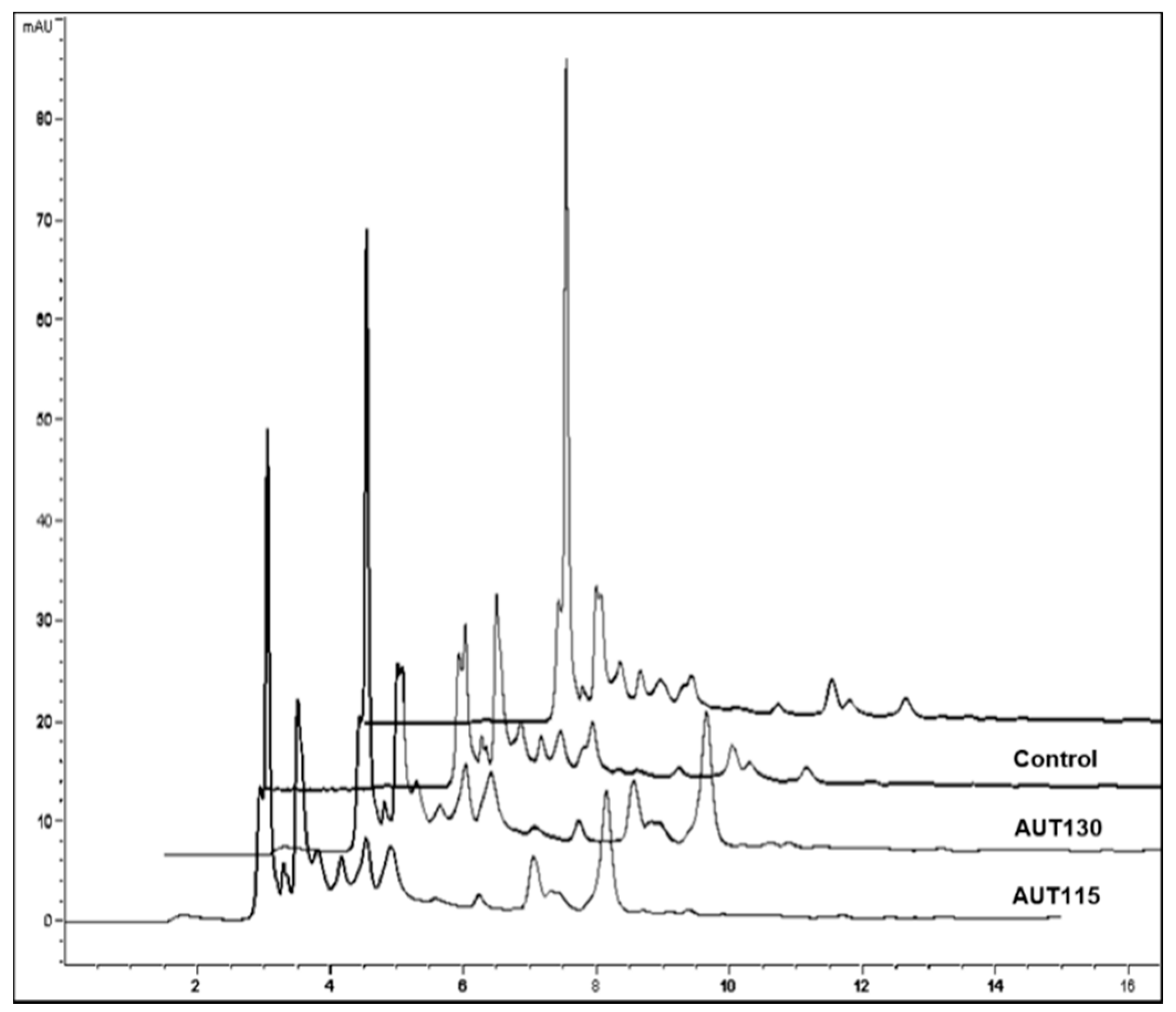
2.4.3. Determination of Ferulic Acid and Soluble Phenolic Compounds
2.5. Total Antioxidant Capacity (TAC)
2.5.1. DPPH and Q-DPPH Assay
2.5.2. ORAC Assay
2.5.3. ABTS and Q-ABTS Assay
2.5.4. FRAP Assay
2.6. Glycemic Index (GI) of WB
2.7. Techno-Functional Properties of WB
2.7.1. Hydration, Foaming and Emulsifying Properties
2.7.2. Least Gelation Concentration (LGC)
2.7.3. Pasting Properties
2.7.4. Rheological Properties: Viscoelastic Behavior of Gels
2.7.5. Scanning Electron Microscopy (SEM)
2.8. Organoleptic Properties of WB
2.8.1. Colorimetric Analysis
2.8.2. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Thermal Treatment and Particle Size on Physicochemical Properties of WB
3.2. Effect of Thermal Treatment on Phenolic Profile of WB
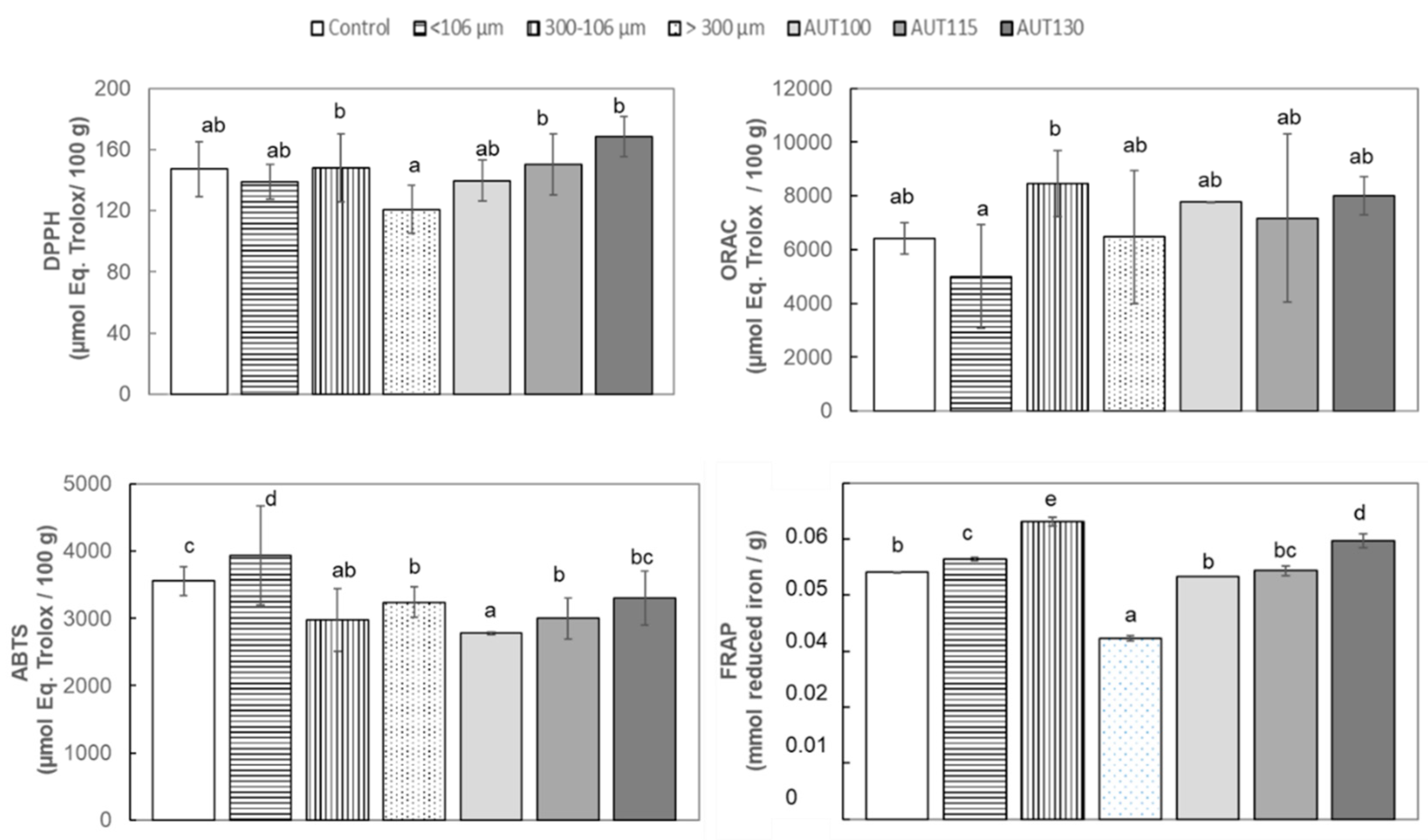
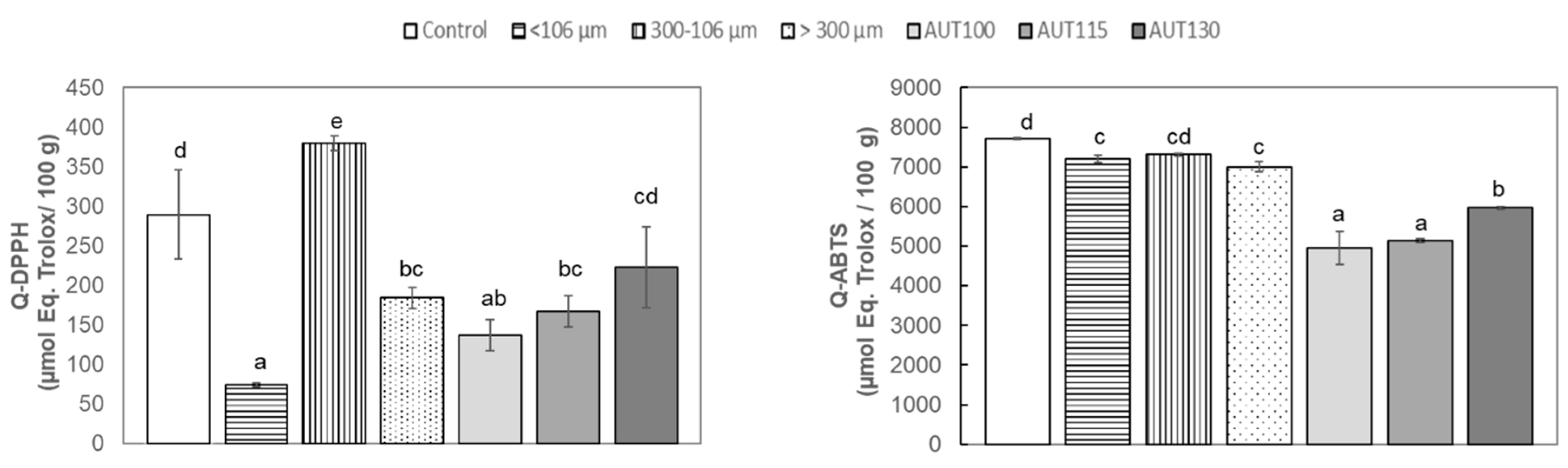
3.3. Effect of Thermal Treatment and Particle Size on Total Antioxidant Capacity (TAC) of WB
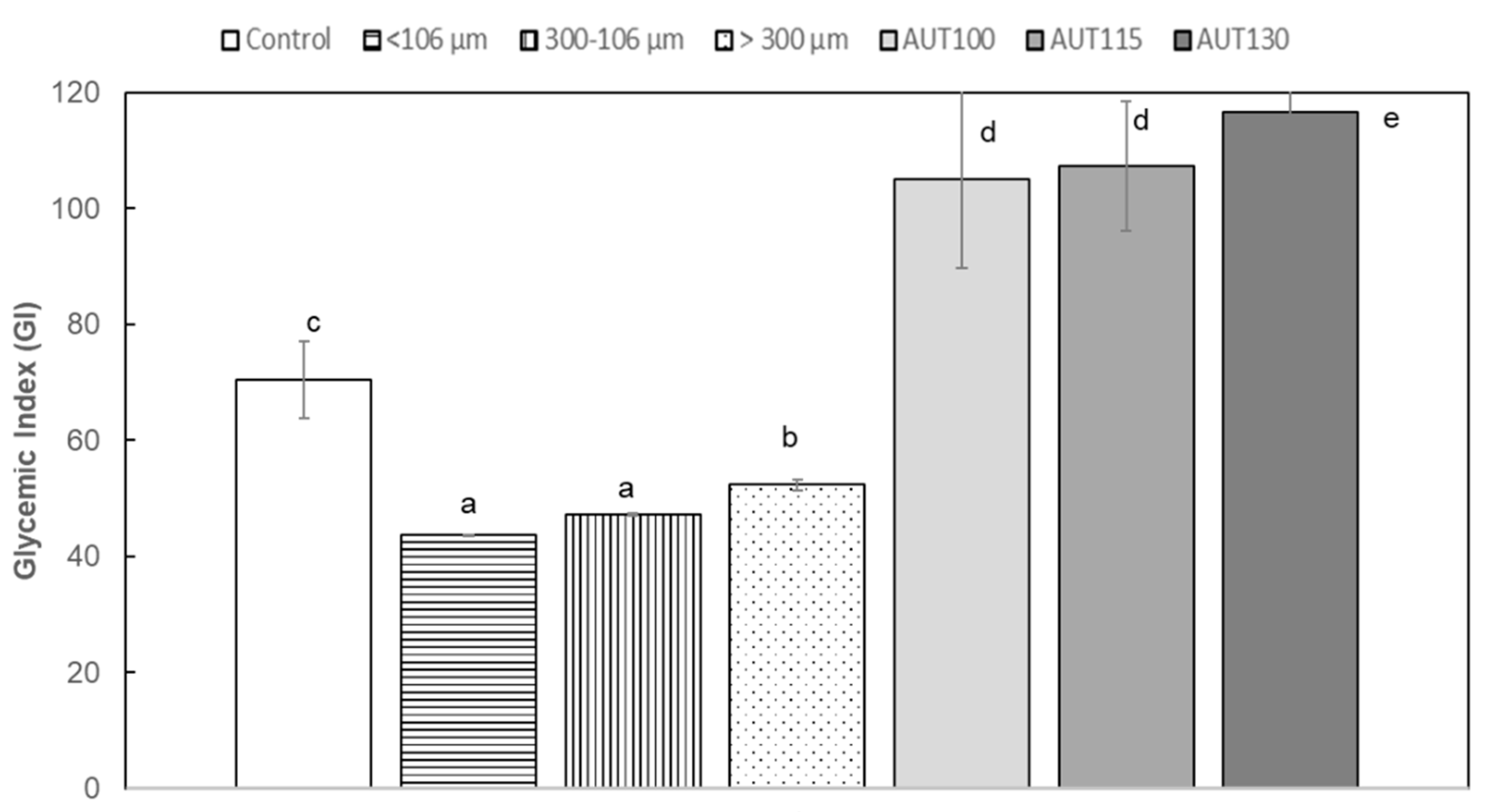
3.4. Effect of Thermal Treatment and Particle Size on Glycemic Index (GI) of WB
3.5. Effect of Thermal Treatment and Particle Size on Techno-Functional Properties of WB
3.5.1. Hydration, Foaming and Emulsifying Properties
3.5.2. Least Gelation Concentration (LGC)
3.5.3. Pasting Properties
3.5.4. Rheological Properties: Viscoelastic Behavior of Gels
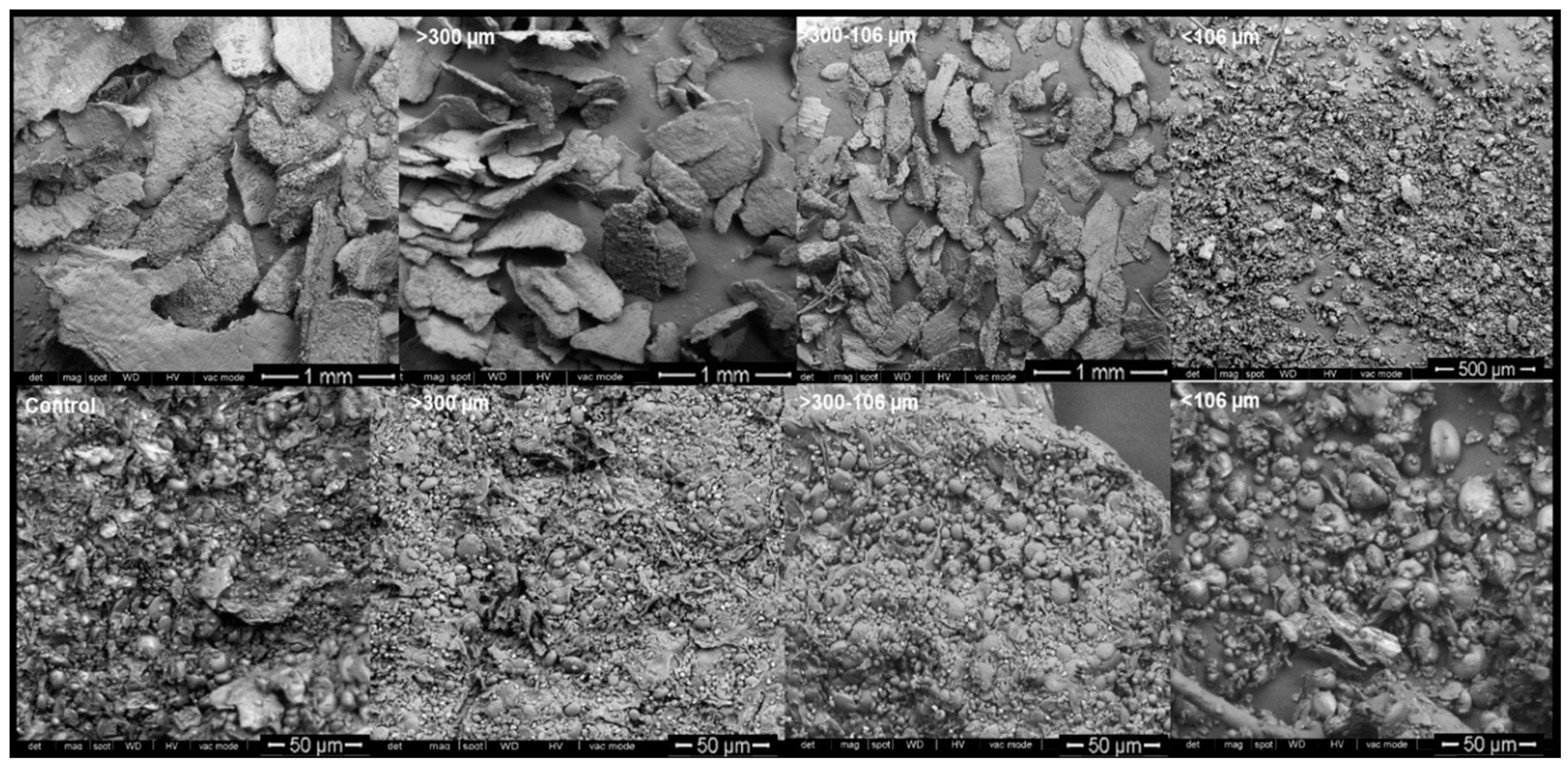
3.5.5. Scanning Electron Microscopy (SEM)
3.6. Effect of Thermal Treatment and Particle Size on Colorimetric Properties of WB
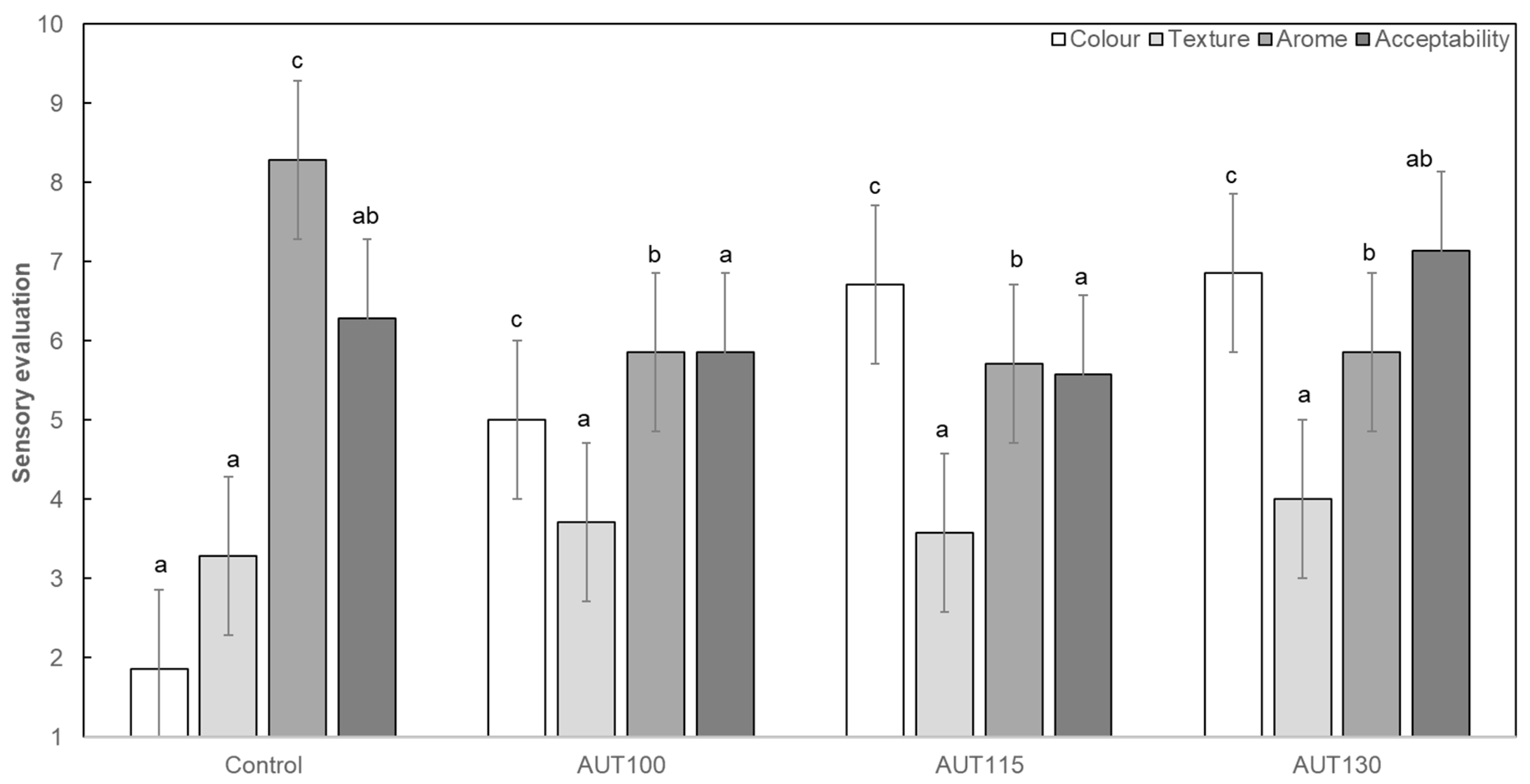
3.7. Effect of Thermal Treatment on Sensorial Properties of WB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019. In Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; ISBN 978-92-5-131570-5. [Google Scholar]
- Storey, M.; Anderson, P. Income and race/ethnicity influence dietary fiber intake and vegetable consumption. Nutr. Res. 2014, 34, 844–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, O.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat Bran-based biorefinery 2: Valorization of products. LWT Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Dimberg, L.; Aman, P.; Langberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- De Brier, N.; Gomand, S.V.; Joye, I.J.; Pareyt, B.; Courtin, C.M.; Delcour, J.A. The impact of pearling as a treatment prior to wheat roller milling on the texture and structure of bran-rich breakfast flakes. Lwt Food Sci. Technol. 2014, 62, 668–674. [Google Scholar] [CrossRef]
- Curti, E.; Carini, E.; Bonacini, G.; Tribuzio, G.; Vittadini, E. Effect of the addition of bran fractions on bread properties. J. Cereal Sci. 2013, 57, 325–332. [Google Scholar] [CrossRef]
- Hossain, K.; Ulven, C.; Glover, K.; Ghavami, F.; Simsek, S.; Alamri, M.S.; Kumar, A.; Mergoum, M. Interdependence of cultivar and environment on fibre composition in wheat bran. Aust. J. Crop Sci. 2013, 7, 525–531. [Google Scholar]
- Luthria, D.L.; Lu, Y.; John, K.M. Bioactive phytochemicals in wheat: Extraction, analysis, processing, and functional properties. J. Funct. Foods 2015, 18, 910–925. [Google Scholar] [CrossRef]
- Lu, Y.; Luthria, D.; Fuerst, E.P.; Kiszonas, A.M.; Yu, L.; Morris, C.F. Effect of processing on phenolic composition of dough and bread fractions made from refined and whole wheat flour of three wheat varieties. J. Agric. Food Chem. 2014, 62, 10431–10436. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in wholegrain cereals for the prevention oftype-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Gunenc, A.; Tavakoli, H.; Seetharaman, K.; Mayer, P.M.; Fairbanks, D.; Hosseinian, F. Stability and antioxidant activity of alkyresorcinols in breads enriched with hard and soft wheat brans. Food Res. Int. 2013, 51, 571–578. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.-C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Odell, B.L.; Deboland, A.R.; Koirtyohann, S.R. Distribution of phytate and nutritionally important elements among morphological components of cereal grains. J. Agric. Food Chem. 1972, 20, 718–732. [Google Scholar] [CrossRef]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, H.; Özkaya, B.; Duman, B.; Turksoy, S. Effect of Dephytinization by Fermentation and Hydrothermal Autoclaving Treatments on the Antioxidant Activity, Dietary Fiber, and Phenolic Content of Oat Bran. J. Agric. Food Chem. 2017, 65, 5713–5719. [Google Scholar]
- Cunniff, P. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Sheu, S.C.; Chen, A.O. Lipoxygenase as blanching index for frozen vegetable soybeans. J. Food Sci. 1991, 56, 448–451. [Google Scholar] [CrossRef]
- Vyncke, W. Evaluation of direct thiobarbituric acid extraction method for determining oxidative rancidity in mackerel (scomber-scombrus-l). Fette Seifen Anstrichm. 1975, 77, 239–240. [Google Scholar] [CrossRef]
- Sharma, S.; Dar, B.N.; Nayik, G.A.; Kaur, G. Total Phenolic Content and Antioxidant Activity of Cereal Bran Enriched Ready to Eat Breakfast Cereal Porridge. Curr. Nutr. Food Sci. 2016, 12, 142–149. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis - automation and comparison with manual methods. Am. J. Enol Viticult. 1977, 28, 49–55. [Google Scholar]
- Martin-Diana, A.B.; Izquierdo, N.; Alnertos, I.; Sanchez, M.S.; Herrero, A.; Sanz, M.A.; Rico, D. Valorization of Carob’s Germ and Seed Peel as Natural Antioxidant Ingredients in Gluten-Free Crackers. J. Food Process. Preserv. 2017, 41, 2. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gokmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.X.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Miller, N.J.; Riceevans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayalakshmi, M.; Ruckmani, K. Ferric reducing anti-oxidant power assay in plant extract. Bangladesh. J. Pharmacol. 2016, 11, 570–572. [Google Scholar]
- Gularte, M.A.; Rosell, C.M. Physicochemical properties and enzymatic hydrolysis of different starches in the presence of hydrocolloids. Carbohydr. Polym. 2011, 85, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Granfeldt, Y.; Liljeberg, H.; Drews, A.; Newman, R.; Bjorck, I. Glucose and insulin responses to barley products—Influence of food structure and amylose-amylopectin ratio. Am. J. Clin. Nutr. 1994, 59, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.; De Lamo, B.; Harasym, J.; Ronda, F. Microwave radiation and protein addition modulate hydration, pasting and gel rheological characteristics of rice and potato starches. Carbohydr. Polym. 2018, 201, 374–381. [Google Scholar] [CrossRef]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa) flour, pigeonpea (Cajanus cajan) flour and their blends. Lwt Food Sci. Technol. 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Collar, C.; Angioloni, A. Pseudocereals and teff in complex breadmaking matrices: Impact on lipid dynamics. J. Cereal Sci. 2014, 59, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, B.A.; Avanza, M.V.; Chaves, M.G.; Ronda, F. Gelation, thermal and pasting properties of pigeon pea (Cajanus cajan L.), dolichos bean (Dolichos lablab L.) and jack bean (Canavalia ensiformis) flours. J. Food Eng. 2013, 119, 65–71. [Google Scholar] [CrossRef]
- AACC International. Approved Method of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010; Method 76-21.01. [Google Scholar]
- Abebe, W.; Ronda, F. Rheological and textural properties of tef [Eragrostis tef (Zucc.) Trotter] grain flour gels. J. Cereal Sci. 2014, 60, 122–130. [Google Scholar] [CrossRef]
- Carabante, K.M.; Prinyawiwatkul, W. Data analyses of a multiple-samples sensory ranking test and its duplicated test: A review. J. Sens. Stud. 2018, 33, e12435. [Google Scholar] [CrossRef]
- Sharanappa, T.; Chetana, R.; Suresh Kumar, G. Evaluation of genotypic wheat bran varieties for nutraceutical compounds. J. Food Sci. Technol. 2016, 53, 4316–4324. [Google Scholar] [CrossRef] [Green Version]
- D’hoe, K.; Conterno, L.; Fava, F.; Falony, G.; Vieira-Silva, S.; Vermeiren, J.; Tuohy, K.; Raes, J. Prebiotic wheat bran fractions induce specific microbiota changes. Front. Microbiol. 2018, 9, 31. [Google Scholar] [CrossRef]
- Chalamacharla, R.B.; Harsha, K.; Sheik, K.B.; Viswanatha, C.K. Wheat Bran-Composition and Nutritional Quality: A Review. Adv. Biotechnol. Microbiol. 2018, 9, 555754. [Google Scholar]
- Onipe, O.O.; Jideani, A.I.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Arthey, D.; Dennis, C. Vegetable Processing; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Gili, R.D.; Pend, M.C.; Torrez Irigoyen, M.R.; Giner, S.A.; Ribotta, P.D. Effect of Wheat Germ Heat Treatment by Fluidised Bed on the Kineticsof Lipase Inactivation. Food Bioprocess Technol. 2018, 11, 1002–1011. [Google Scholar] [CrossRef]
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic compounds and the antioxidant activity of the bran, flour and whole grain of different wheat varieties. Proc. Chem. 2010, 2, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Randhir, R.; Kwon, Y.; Shetty, K. Effect of thermal processing on phenolics antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innov. Sci. Emerg. Technol. 2008, 9, 355–364. [Google Scholar] [CrossRef]
- Peleg, H.; Naim, M.; Rouseff, R.L.; Zehavi, U. Distribution of bound and free phenolic acid in oranges (Citrus sinensis) and grapefruits (Citrus paradisi). J. Sci. Food Agric. 1991, 57, 417–426. [Google Scholar] [CrossRef]
- Zhu, K.X.; Huang, S.; Peng, W.; Qian, H.F.; Zhou, H.M. Effect of ultrafine grinding on hydration and antioxidant properties of wheat bran dietary fiber. Food Res. Int. 2010, 43, 943–948. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.M.M.; Noort, M.W.J.; Rouau, X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res. Int. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Spaggiari, M.; Calani, L.; Folloni, S.; Ranieri, R.; Dall’Asta, C.; Galaverna, G. The impact of processing on the phenolic acids, free betaine and choline in Triticum spp. L. whole rains and milling by-products. Food Chem. 2020, 311, 125940. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.F.; Vodnar, D.N. Thermal processing for the release of phenolic compounds from wheat and oat bran. Biomolecules 2020, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.D.; Zhou, J.; Xiao, J.N. Extraction of Ferulic acid from cereals and determination by High performance liquid chromatography. Food Sci. Technol. 2007, 2, 218–220. [Google Scholar]
- Hostetler, G.L.; Riedl, K.M.; Schwartz, S.J. Effects of food formulation and thermal processing on flavones in celery and chamomile. Food Chem. 2013, 141, 1406–1411. [Google Scholar] [CrossRef] [Green Version]
- Gordillo-Bastidas, E.; Díaz-Rizzolo, D.A.; Roura, E.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa wild), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6, 1–10. [Google Scholar]
- Wang, S.J.; Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2013, 4, 1564–1580. [Google Scholar] [CrossRef]
- Arrigoni, E.; Caprez, A.; Amado, R.; Neukom, H. Chemical composition and physical properties of modified dietary fibre sources. Food Hydrocoll. 1986, 1, 57–64. [Google Scholar] [CrossRef]
- Bucsella, B.; Molnar, D.; Harasztos, A.H.; Tomoskozi, S. Comparison of the rheological and end-product properties of an industrial aleurone-rich wheat flour, whole grain wheat and rye flour. J. Cereal Sci. 2016, 69, 40–48. [Google Scholar] [CrossRef]
- Yalcin, E.; Sakiyan, O.; Sumnu, G.; Celik, S.; Koksel, H. Functional Properties of Microwave-Treated Wheat Gluten. Eur. Food Res. Technol. 2008, 227, 1411–1417. [Google Scholar] [CrossRef]
- Radha, C.; Ramesh Kumar, P.; Prakash, V. Enzymatic Modification as a Tool to Improve the Functional Properties of Heat-Processed Soy Flou. J. Sci. Food Agric. 2007, 1243, 1237–1243. [Google Scholar]
- Jogihalli, P.; Singh, L.; Sharanagat, V.S. Effect of Microwave Roasting Parameters on Functional and Antioxidant Properties of Chickpea (Cicer arietinum). LWT Food Sci. Technol. 2017, 79, 223–233. [Google Scholar] [CrossRef]
- Onimawo, I.A.; Akpojovwo, A.E. Toasting (Dry Heat) and Nutrient Composition, Functional Properties and Antinutritional Factors of Pigeon Pea (Cajanus cajan) Flour. J. Food Process. Preserv. 2006, 30, 742–753. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.M.H. Influence of heat treatment for some wheat milling fractions on fino bread quality. J. Food Sci. Technol. 2019, 56, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, M.F. Fibre and water binding. Proc. Nutr. Soc. 2003, 62, 223–227. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Van Vliet, T. Heat-Induced Gel Formation by Soy Proteins at Neutral pH. J. Agric. Food Chem. 2002, 50, 1569–1573. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Study of hydration properties of wheat bran as a function of particle size. Food Chem. 2015, 179, 296–304. [Google Scholar] [CrossRef]
- Cai, L.; Induck, C.; Park, C.S.; Baki, B.K. Bran Hydration and Physical Treatments Improve the Bread-Baking Quality of Whole Grain Wheat Flour. Cereal Chem. 2015, 92, 557–564. [Google Scholar] [CrossRef]

| Proximal Composition | WB (g/100 g) |
|---|---|
| Protein | 17.31 ± 0.02 |
| Fat | 4.32 ± 0.41 |
| Total dietary fibre (TDF) | 36.81 ± 3.24 |
| Carbohydrates (Including TDF) | 61.93 ± 0.09 |
| Ash | 4.93 ± 0.10 |
| Moisture | 11.50 ± 0.20 |
| Phenols | Control | AUT100 | AUT115 | AUT130 |
|---|---|---|---|---|
| Caffeic acid O-hexoside | 0.85 ± 0.02 a | 1.11 ± 0.15 b | 1.17 ± 0.12 b | 1.06 ± 0.06 b |
| Apigenin-6/8-C-pentoside-8/6-C-hexoside i1 | 1.81 ± 0.05d | 0.29 ± 0.09 a | 0.37 ± 0.00 b | 0.5 ± 0.04 c |
| Apigenin-6/8-C-pentoside-8/6-C-hexoside i2 | 2.25 ± 0.87d | 0.18 ± 0.04 a | 0.25 ± 0.02 b | 1.36 ± 0.25 c |
| Apigenin-6-C-arabinoside-8-C-hexoside | 0.52 ± 0.06 a | 0.93 ± 0.05 c | 0.76 ± 0.81 b | 0.97 ± 0.20 c |
| Dihydroferulic acid | 0.52 ± 0.06 a | 0.76 ± 0.01 b | 1.00 ± 0.48 b | 0.83 ± 0.15 b |
| Ferulic acid | 6.41 ± 0.08 a | 7.21 ± 0.69 a | 22.43 ± 0.46 b | 24.13 ± 0.68 c |
| WAC | OAC | WAI | WSI | SP | FC | FS | EA | ES | LGC | |
|---|---|---|---|---|---|---|---|---|---|---|
| (g/g) | (g/g) | (g/g) | (g/100g) | (g/g) | (mL/g) | (%) | (%) | (%) | (%) | |
| Control | 2.80 ± 0.06 b | 2.62 ± 0.01 ab | 4.22 ± 0.19 b | 13.1 ± 0.9 b | 3.9 ± 0.19 ab | 5.1 ± 1.0 d | 42 ± 7 a | 49 ± 1 a | 49 ± 3 a | 15 ± 1.4 a |
| AUT100 | 2.80 ± 0.02 b | 2.73 ± 0.04 c | 4.21 ± 0.13 b | 11.0 ± 0.2 a | 4.0 ± 0.13 b | 1.7 ± 0.3 b | 72 ± 5 b | n.d. | n.d. | 17 ± 1.4 a |
| AUT115 | 2.64 ± 0.08 a | 2.66 ± 0.02 b | 4.11 ± 0.03 ab | 13.1 ± 0.3 b | 3.8 ± 0.04 ab | 3.6 ± 0.5 c | 50 ± 11 a | 47 ± 3 a | 46 ± 3 a | 16 ± 2.8 a |
| AUT130 | 3.05 ± 0.05 c | 2.58 ± 0.01 a | 3.95 ± 0.14 a | 11.7 ± 0.4 a | 3.7 ± 0.15 a | 0.5 ± 0.0 a | 100 ± 0 c | n.d. | n.d. | 15 ± 1.4 a |
| Control | AUT100 | AUT115 | AUT130 | |
|---|---|---|---|---|
| Pasting properties | ||||
| Pasting Temperature (°C) | 70.3 ± 0.2 a | 73.53 ± 0.2 b | 73.6 ± 0.3 b | 73.9 ± 0.0 b |
| Peak viscosity (Pa·s) | 1.54 ± 0.03 c | 1.44 ± 0.04 b | 1.47 ± 0.01 bc | 1.07 ± 0.00 a |
| Peak time (s) | 676 ± 10 a | 719 ± 60 b | 784 ± 4 c | 799 ± 17 c |
| Trough viscosity (Pa·s) | 1.19 ± 0.03 b | 1.30 ± 0.03 c | 1.41 ± 0.01 d | 1.07 ± 0.00 a |
| Breakdown (Pa·s) | 0.35 ± 0.01 d | 0.14 ± 0.01 c | 0.06 ± 0.02 b | 0.00 ± 0.00 a |
| Final Viscosity (Pa·s) | 2.35 ± 0.05 ab | 2.43 ± 0.03 b | 2.62 ± 0.01 c | 2.30 ± 0.01 a |
| Setback (Pa·s) | 1.15 ± 0.03 a | 1.13 ± 0.00 a | 1.21 ± 0.00 b | 1.24 ± 0.01 b |
| Viscoelastic properties | ||||
| G1′ (Pa) | 867 ± 85 b | 541 ± 107 a | 775 ± 71 b | 732 ± 3 ab |
| a | 0.15 ± 0.01 a | 0.15 ± 0.01 a | 0.15 ± 0.00 a | 0.15 ± 0.00 a |
| G1′′ (Pa) | 179 ± 15 b | 120 ± 22 a | 169 ± 12 b | 164 ± 0 b |
| b | 0.28 ± 0.01 a | 0.32 ± 0.00 b | 0.29 ± 0.01 a | 0.29 ± 0.00 a |
| tan (δ)1 (Pa) | 0.206 ± 0.002 a | 0.222 ± 0.003 b | 0.219 ± 0.005 b | 0.224 ± 0.000 b |
| c | 0.130 ± 0.00 a | 0.168 ± 0.01 b | 0.137 ± 0.02 a | 0.134 ± 0.00 a |
| τ max (Pa) | 3.7 ± 0.4 b | 1.3 ± 0.1 a | 1.2 ± 0.1 a | 1.7 ± 0.4 a |
| Crosspoint (Pa) | 41.4 ± 4.4 b | 27.9 ± 0.0 a | 31.5 ± 1.5 a | 28.6 ± 0.3 a |
| Control | >300 µm | 300–106 µm | <106 µm | AUT100 | AUT115 | AUT130 | |
|---|---|---|---|---|---|---|---|
| L* | 63.48 ± 0.71 c | 64.83 ± 0.70 d | 74.69 ± 0.52 e | 83.55 ± 0.39 f | 61.36 ± 1.5 b | 60.46 ± 1.1 a | 60.14 ± 0.99 a |
| a* | 7.50 ± 0.33 d | 6.92 ± 0.34 c | 4.41 ± 0.14 b | 2.60 ± 0.06 a | 6.97 ± 0.41 c | 6.85 ± 0.5 c | 7.40 ± 0.20 d |
| b* | 17.94 ± 0.62 d | 17.26 ± 0.52 c | 16.10 ± 0.19 b | 13.03 ± 0.18 a | 18.58 ± 0.79 e | 18.83± 0.49 ef | 19.26 ± 0.37 f |
| Δc | 0.85 ± 044 bc | 0.85 ± 0.40b c | 0.53 ± 0.39 ab | 0.34a ± 0.24 a | 1.54 ± 0.66 d | 1.06 ± 0.61 c | 0.96 ± 0.38 c |
| Hue | 1.17 ± 0.01 a | 1.19 ± 0.01 b | 1.30 ± 0.01 e | 1.37 ± 0.01 f | 1.21 ± 0.02 cd | 1.22 ± 0.02 d | 1.2 ± 0.01 c |
| Croma | 19.45 ± 0.67 d | 18.59 ± 0.60 c | 16.70 ± 0.20 b | 13.28 ± 0.18 a | 19.85 ± 0.80 de | 20.50 ± 0.42 e | 20.64 ± 0.34 f |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico, D.; Villaverde, A.; Martinez-Villaluenga, C.; Gutierrez, A.L.; Caballero, P.A.; Ronda, F.; Peñas, E.; Frias, J.; Martin Diana, A.B. Application of Autoclave Treatment for Development of a Natural Wheat Bran Antioxidant Ingredient. Foods 2020, 9, 781. https://doi.org/10.3390/foods9060781
Rico D, Villaverde A, Martinez-Villaluenga C, Gutierrez AL, Caballero PA, Ronda F, Peñas E, Frias J, Martin Diana AB. Application of Autoclave Treatment for Development of a Natural Wheat Bran Antioxidant Ingredient. Foods. 2020; 9(6):781. https://doi.org/10.3390/foods9060781
Chicago/Turabian StyleRico, Daniel, Adriana Villaverde, Cristina Martinez-Villaluenga, Angel L. Gutierrez, Pedro Antonio Caballero, Felicidad Ronda, Elena Peñas, Juana Frias, and Ana Belen Martin Diana. 2020. "Application of Autoclave Treatment for Development of a Natural Wheat Bran Antioxidant Ingredient" Foods 9, no. 6: 781. https://doi.org/10.3390/foods9060781
APA StyleRico, D., Villaverde, A., Martinez-Villaluenga, C., Gutierrez, A. L., Caballero, P. A., Ronda, F., Peñas, E., Frias, J., & Martin Diana, A. B. (2020). Application of Autoclave Treatment for Development of a Natural Wheat Bran Antioxidant Ingredient. Foods, 9(6), 781. https://doi.org/10.3390/foods9060781









