Acute Oral Toxicity and Genotoxicity of Polysaccharide Fraction from Young Barley Leaves (Hordeum vulgare L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BLE0
2.2. Chemical Composition of BLE0
2.3. Bacterial Reverse Mutation Test
2.4. In Vitro Mammalian Chromosomal Aberration Assay
2.5. Acute Oral Toxicity Test
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of BLE0
3.2. Bacterial Reverse Mutation Test
3.3. In Vitro Mammalian Chromosomal Aberration Assay
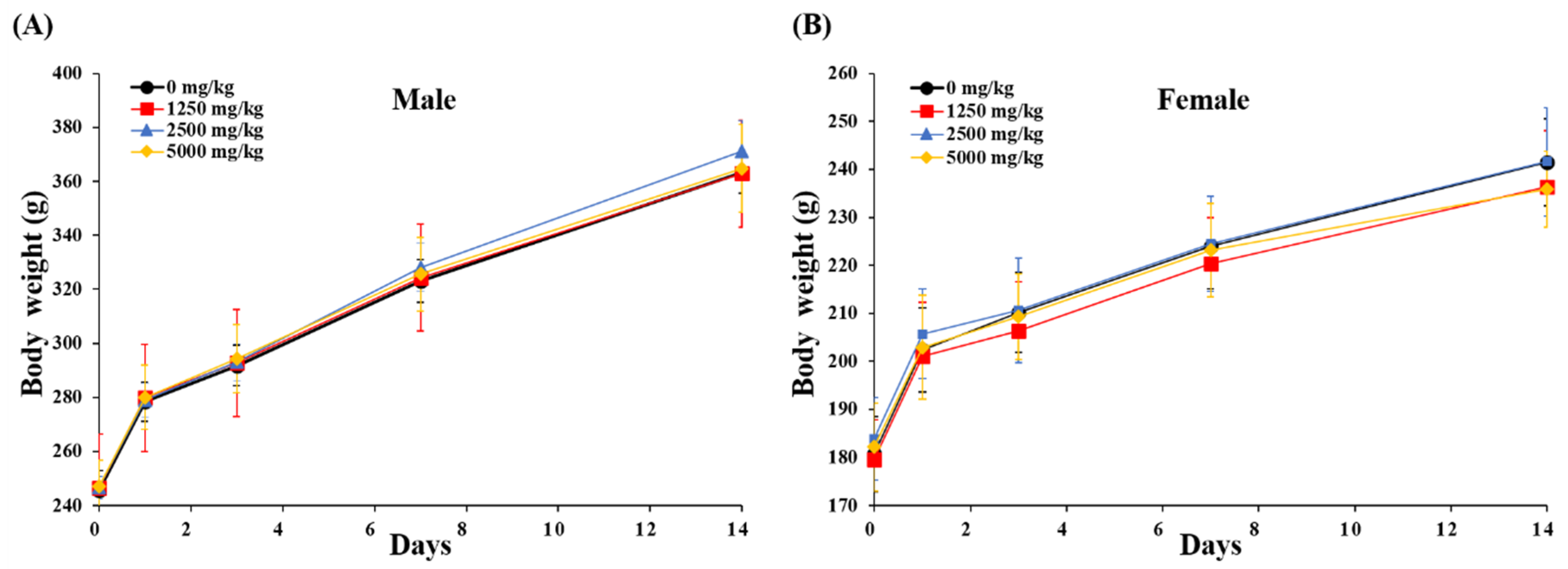
3.4. Acute Oral Toxicity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamaura, K.; Nakayama, N.; Shimada, M.; Bi, Y.; Fukata, H.; Ueno, K. Antidepressant-like effects of young green barley leaf (Hordeum vulgare L.) in the mouse forced swimming test. Pharmacogn. Res. 2012, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Krskova, Z.; Goncalves, R.F.; Valentao, P.; Pereira, J.A.; Dusek, J.; Martin, J.; Andrade, P.B. Free water-soluble phenolics profiling in barley (Hordeum vulgare L.). J. Agric. Food Chem. 2009, 57, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Mitchell, K.A. The mis-identification of the major antioxidant flavonoids in young barley (Hordeum vulgare) leaves. Z. Naturforschung C J. Biosci. 2003, 58, 53–56. [Google Scholar] [CrossRef]
- Park, S.E.; Seo, S.H.; Kim, E.J.; Lee, K.M.; Son, H.S. Quality characteristics of string cheese prepared with barley sprouts. J. Korean Soc. Food Sci. Nutr. 2017, 48, 841–847. [Google Scholar]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Krasteva, I.; Manov, V.; Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. Biomed. Res. Int. 2013, 2013, 757126. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, A.; Goswami, R.; Basu, S. Hypoglycemic activity of the antioxidant saponarin, characterized as alpha-glucosidase inhibitor present in Tinospora cordifolia. J. Enzym. Inhib. Med. Chem. 2009, 24, 684–690. [Google Scholar] [CrossRef]
- Seo, W.D.; Yuk, H.J.; Curtis-Long, M.J.; Jang, K.C.; Lee, J.H.; Han, S.I.; Kang, H.W.; Nam, M.H.; Lee, S.J.; Lee, J.H.; et al. Effect of the growth stage and cultivar on policosanol profiles of barley sprouts and their adenosine 5’-monophosphate-activated protein kinase activation. J. Agric. Food. Chem. 2013, 61, 1117–1123. [Google Scholar] [CrossRef]
- Benedet, J.A.; Umeda, H.; Shibamoto, T. Antioxidant activity of flavonoids isolated from young green barley leaves toward biological lipid samples. J. Agric. Food Chem. 2007, 55, 5499–5504. [Google Scholar] [CrossRef]
- Takano, A.; Kamiya, T.; Tomozawa, H.; Ueno, S.; Tsubata, M.; Ikeguchi, M.; Takagaki, K.; Okushima, A.; Miyata, Y.; Tamaru, S.; et al. Insoluble fiber in young barley leaf suppresses the increment of postprandial blood glucose level by increasing the digesta viscosity. Evid. Based Complement. Altern. Med. 2013, 2013, 137871. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Liu, J.; Willfor, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Kim, H.; Kwak, B.S.; Hong, H.D.; Suh, H.J.; Shin, K.S. Structural features of immunostimulatory polysaccharide purified from pectinase hydrolysate of barley leaf. Int. J. Biol. Macromol. 2016, 87, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hong, H.D.; Shin, K.S. Structure elucidation of an immunostimulatory arabinoxylan-type polysaccharide prepared from young barley leaves (Hordeum vulgare L.). Carbohydr. Polym. 2017, 157, 282–293. [Google Scholar] [CrossRef]
- Kim, H.; Yu, K.W.; Hong, H.D.; Shin, K.S. Effect of arabinoxylan-and rhamnogalacturonan I-rich polysaccharides isolated from young barley leaf on intestinal immunostimulatory activity. J. Funct. Foods 2017, 35, 384–390. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Ha, H.; Kim, R.; Cho, C.W.; Song, Y.R.; Hong, H.D.; Kim, T. Protective effects of a polysaccharide BLE0 isolated from barley leaf on bone loss in ovariectomized mice. Int. J. Biol. Macromol. 2019, 123, 314–321. [Google Scholar] [CrossRef]
- Han, H.S.; Shin, J.S.; Song, Y.R.; Rhee, Y.K.; Cho, C.W.; Ryu, J.H.; Inn, K.S.; Hong, H.D.; Lee, K.T. Immunostimulatory effects of polysaccharides isolated from young barley leaves (Hordeum vulgare L.) with dual activation of Th1 and Th2 in splenic T cells and cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2020, 147, 954–964. [Google Scholar] [CrossRef]
- Vettorazzi, A.; Lopez de Cerain, A.; Sanz-Serrano, J.; Gil, A.G.; Azqueta, A. European Regulatory Framework and Safety Assessment of Food-Related Bioactive Compounds. Nutrients 2020, 12, 613. [Google Scholar] [CrossRef]
- Chan, K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 2003, 52, 1361–1371. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, T.; Cho, W.K.; Yang, H.J.; Kwak, D.H.; Ha, H.; Song, K.H.; Ma, J.Y. In vitro and in vivo genotoxicity assessment of Aristolochia manshuriensis Kom. Evid. Based Complement. Altern. Med. 2012, 2012, 412736. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Park, H.; Ma, J.Y. In vitro and in vivo safety evaluation of Acer tegmentosum. J. Ethnopharmacol. 2013, 148, 99–105. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- OECD. Test No. 471: Bacterial Reverse Mutation Test. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 1997. [Google Scholar]
- National Institute of Food and Drug Safety Evaluation, Korea. Ministry of Food and Drug Safety Notification No. 2016-141 ‘Regulation on Approval of Functional Ingredient for Health Functional Food’; Guidebook on Ministry of Food and Drug Safety Reference Standards: Seoul, Korea, 2016. [Google Scholar]
- OECD. Test No. 473: In vitro Mammalian Chromosomal Aberration Test. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar]
- National Institute of Food and Drug Safety Evaluation, Korea. Ministry of Food and Drug Safety Notification No. 2017-71 ‘The standards of Toxicity Study for Medicinal Products’; Guidebook on Ministry of Food and Drug Safety Reference Standards: Seoul, Korea, 2017. [Google Scholar]
- OECD. Test No. 420: Acute Oral Toxicity—Fixed Dose Procedure. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Green, M.H.; Muriel, W.J. Mutagen testing using TRP+ reversion in Escherichia coli. Mutat. Res. 1976, 38, 3–32. [Google Scholar] [CrossRef]
- Ahn, K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. 2017, 50, 111–116. [Google Scholar] [CrossRef]
- Markman, M. Safety issues in using complementary and alternative medicine. J. Clin. Oncol. 2002, 20, 39s–41s. [Google Scholar] [CrossRef]
- Lahlou, M. Screening of natural products for drug discovery. Expert Opin. Drug Discov. 2007, 2, 697–705. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Assessment of Genotoxicity of Herbal Substances/Preparations; Committee on Herbal Medicinal Products (HPMC): London, UK, 2008. [Google Scholar]
- JEMS-MMS. Atlas of Chromosome Aberration by Chemicals; Japanese Environmental Mutagen Society—Mammalian Mutagenicity Study Group: Tokyo, Japan, 1988. [Google Scholar]
- Velusami, C.C.; Boddapati, S.R.; Hongasandra Srinivasa, S.; Richard, E.J.; Joseph, J.A.; Balasubramanian, M.; Agarwal, A. Safety evaluation of turmeric polysaccharide extract: Assessment of mutagenicity and acute oral toxicity. Biomed. Res. Int. 2013, 2013, 158348. [Google Scholar] [CrossRef]
- Kim, K.I.; Kim, J.W.; Hong, B.S.; Shin, D.H.; Cho, H.Y.; Kim, H.K.; Yang, H.C. Antitumor, genotoxicity and anticlastogenic activities of polysaccharide from Curcuma zedoaria. Mol. Cells 2000, 10, 392–398. [Google Scholar]
- Park, Y.C.; Kim, M.H.; Kim, J.W.; Kim, J.B.; Lee, J.G.; Yu, C.Y.; Kim, S.H.; Chung, I.M.; Kim, J.K.; Choi, R.N.; et al. Genotoxicity study of polysaccharide fraction from Astragalus membranaceus’s aerial parts. Toxicol. Res. 2014, 30, 131–138. [Google Scholar] [CrossRef]
- Chang, B.Y.; Cho, H.K.; Jun, K.Y.; Hur, J.M.; Park, H.; Kim, S.Y. Single oral toxicity study on the polysaccharide fraction of Pueraria lobata in rats. Korean J. Pharmacogn. 2010, 41, 210–215. [Google Scholar]

| Column: CarboPac PA1 analytical column (4 × 250 mm, Dionex) Column temperature: 30 °C Injection Volume: 25 µL Flow rate: 1.0 mL/min |
| BLE0 1 | |
|---|---|
| Yield (%) | 4.1 ± 0.3 2 |
| Chemical composition (%) 3 | |
| Neutral sugar | 79.2 ± 0.1 |
| Uronic acid | 18.0 ± 1.4 |
| 2-Keto-3-Deoxy-Mannoctanoic acid (KDO)-like materials | 2.3 ± 1.2 |
| Protein | 0.4 ± 0.0 |
| Composition of sugar (mol%) 4 | |
| Arabinose | 16.9 ± 0.0 |
| Fucose | 0.8 ± 0.0 |
| Galactose | 18.5 ± 0.1 |
| Glucose | 14.8 ± 0.1 |
| Mannose | nd 5 |
| Rhamnose | 5.5 ± 0.1 |
| Xylose | 29.4 ± 0.2 |
| Galacturonic acid | 11.5 ± 0.2 |
| Glucuronic acid | 2.6 ± 0.0 |
| Trt-Rec Time 1 (h) | S9 mix 2 | Dose (μg/mL) | No. Aberrant Metaphase 3 (%) | PP+ER 3, 4 (%) | RICC 5 (%) |
|---|---|---|---|---|---|
| 6–18 | + | 0 | 0(0.00) | 0(0.00) | 100 |
| 31.25 | 0(0.00) | 0(0.00) | 96 | ||
| 62.5 | 0(0.00) | 0(0.00) | 98 | ||
| 125 | 0(0.00) | 0(0.00) | 89 | ||
| 250 | 0(0.00) | 0(0.00) | 89 | ||
| 500 | 0(0.00) | 0(0.00) | 93 | ||
| 1000 | 0(0.00) | 0(0.00) | 83 | ||
| B[a]P 20 | 51(34.00) ** | 0(0.00) | 52 | ||
| 6–18 | − | 0 | 0(0.00) | 0(0.00) | 100 |
| 31.25 | 0(0.00) | 0(0.00) | 88 | ||
| 62.5 | 0(0.00) | 0(0.00) | 87 | ||
| 125 | 0(0.00) | 0(0.00) | 91 | ||
| 250 | 0(0.00) | 0(0.00) | 90 | ||
| 500 | 0(0.00) | 0(0.00) | 89 | ||
| 1000 | 0(0.00) | 0(0.00) | 77 | ||
| 4NQO 0.4 | 18(12.00) ** | 0(0.00) | 78 | ||
| 24–0 | − | 0 | 0(0.00) | 0(0.00) | 100 |
| 31.25 | 0(0.00) | 0(0.00) | 102 | ||
| 62.5 | 0(0.00) | 0(0.00) | 98 | ||
| 125 | 0(0.00) | 0(0.00) | 99 | ||
| 250 | 0(0.00) | 0(0.00) | 92 | ||
| 500 | 0(0.00) | 0(0.00) | 74 | ||
| 1000 | 0(0.00) | 0(0.00) | 68 | ||
| 4NQO 0.4 | 20(13.33) ** | 0(0.00) | 87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, C.-W.; Song, Y.-R.; Lim, W.-C.; Hwang, Y.-H.; Rhee, Y.K.; Choi, J.W.; Lee, K.-T.; Hong, H.-D. Acute Oral Toxicity and Genotoxicity of Polysaccharide Fraction from Young Barley Leaves (Hordeum vulgare L.). Foods 2020, 9, 809. https://doi.org/10.3390/foods9060809
Cho C-W, Song Y-R, Lim W-C, Hwang Y-H, Rhee YK, Choi JW, Lee K-T, Hong H-D. Acute Oral Toxicity and Genotoxicity of Polysaccharide Fraction from Young Barley Leaves (Hordeum vulgare L.). Foods. 2020; 9(6):809. https://doi.org/10.3390/foods9060809
Chicago/Turabian StyleCho, Chang-Won, Young-Ran Song, Won-Chul Lim, Youn-Hwan Hwang, Young Kyoung Rhee, Jae Woong Choi, Kyung-Tae Lee, and Hee-Do Hong. 2020. "Acute Oral Toxicity and Genotoxicity of Polysaccharide Fraction from Young Barley Leaves (Hordeum vulgare L.)" Foods 9, no. 6: 809. https://doi.org/10.3390/foods9060809
APA StyleCho, C.-W., Song, Y.-R., Lim, W.-C., Hwang, Y.-H., Rhee, Y. K., Choi, J. W., Lee, K.-T., & Hong, H.-D. (2020). Acute Oral Toxicity and Genotoxicity of Polysaccharide Fraction from Young Barley Leaves (Hordeum vulgare L.). Foods, 9(6), 809. https://doi.org/10.3390/foods9060809





