Nutrient and Chemical Contaminant Levels in Five Marine Fish Species from Angola—The EAF-Nansen Programme
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analytical Methods
2.3. Data Management and Presentation of Analytical Data
2.4. Calculation of Potential Contribution to Recommended Nutrient Intakes
2.5. Comparison with Food Products of Terrestrial Animal Origin
2.6. Potential Consumer Exposure to Metals
3. Results and Discussion
3.1. Proximate Composition
3.2. Mineral Composition
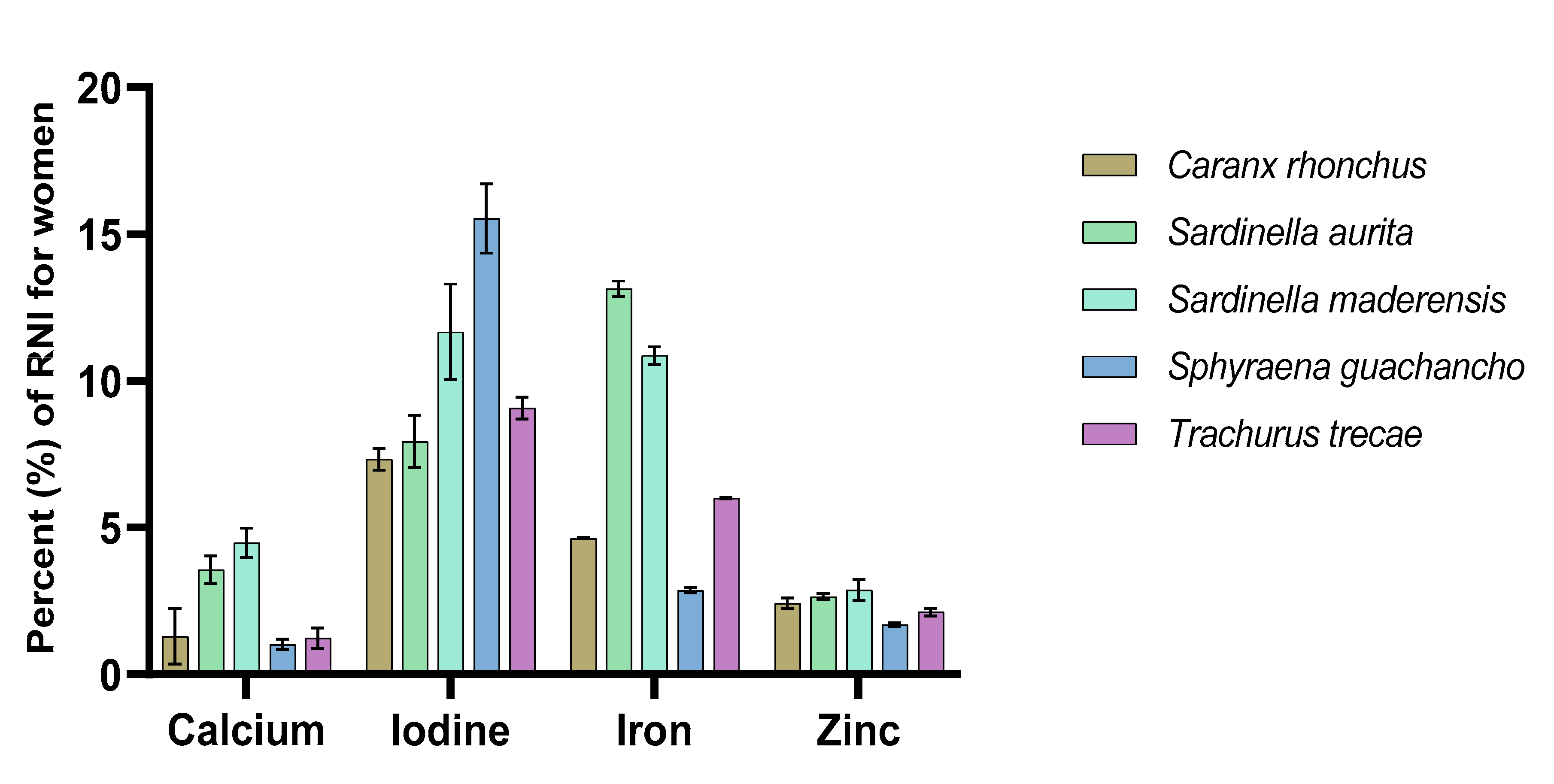
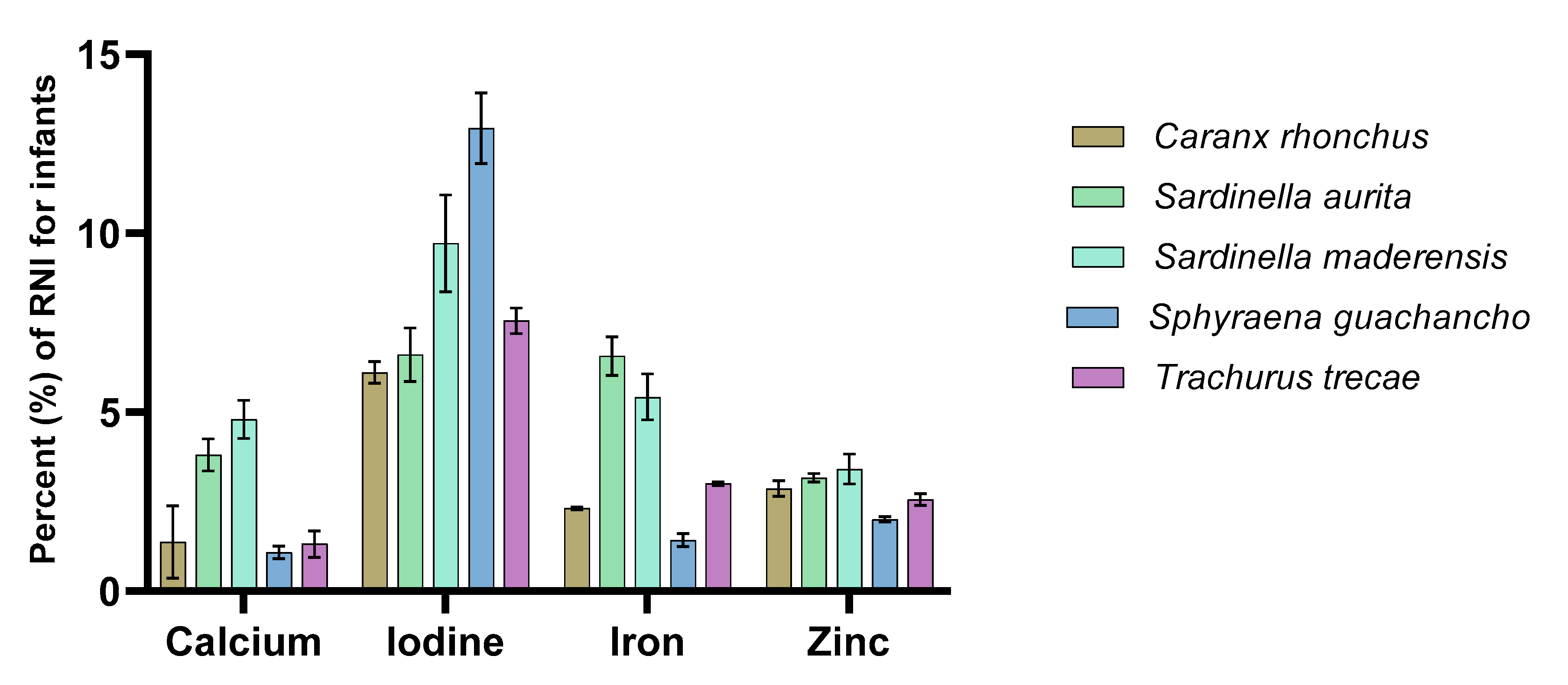
3.3. Contribution to Recommended Nutrient Intakes
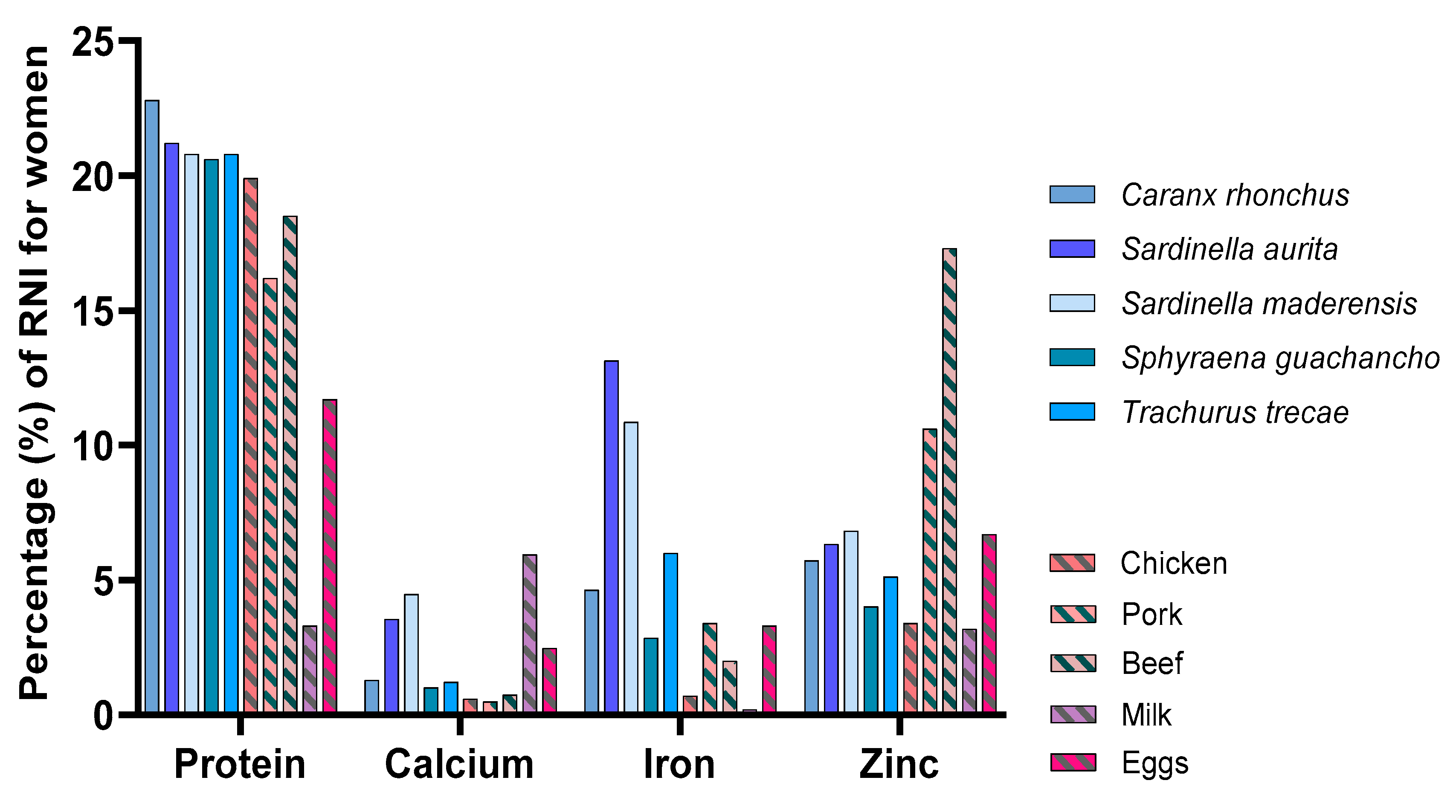
3.4. Comparison to Food Products of Terrestrial Animal Origin
3.5. Fish for Food and Nutrition Security in Angola
3.6. Concentration of Chemical Contaminants
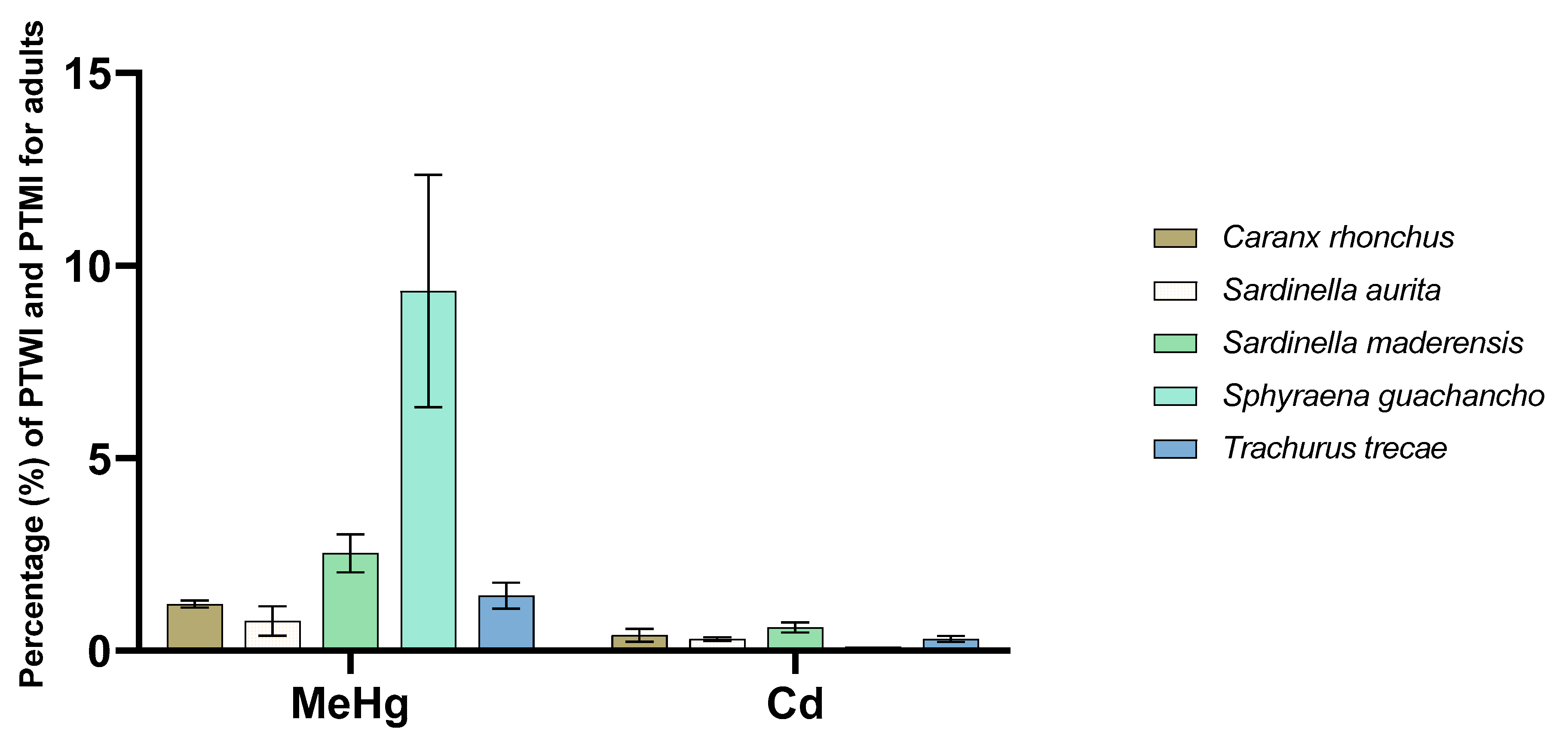
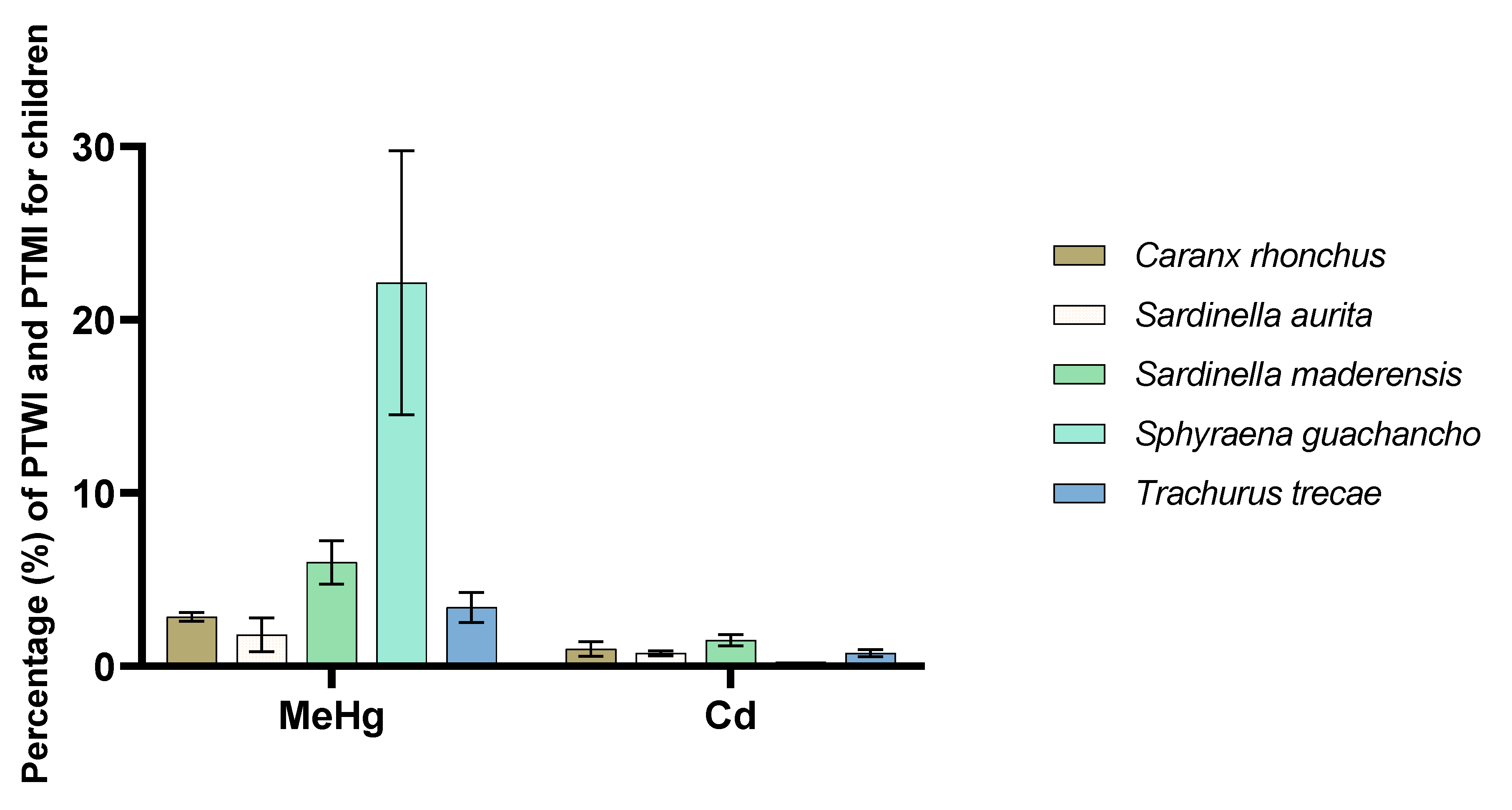
3.7. Potential Consumer Exposure
3.8. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| UHT | Ultra-high-temperature processing |
| Ca | calcium |
| Fe | iron |
| I | iodine |
| K | potassium |
| Mg | magnesium |
| Na | sodium |
| P | phosphorus |
| SD | standard deviation |
| Se | selenium |
| Zn | zinc |
| As | arsenic |
| Cd | cadmium |
| Hg | mercury |
| Pb | lead |
| SD | standard deviation |
| w.w. | wet weight |
References
- Shannon, V. A plan comes together. Large Mar. Ecosyst. 2006, 14, 3–10. [Google Scholar]
- FAO 2014–2019. Fishery and Aquaculture Country Profiles. Available online: http://www.fao.org/fishery/facp/AGO/en (accessed on 12 January 2020).
- Sowman, M.; Cardoso, P. Small-scale fisheries and food security strategies in countries in the Benguela Current Large Marine Ecosystem (BCLME) region: Angola, Namibia and South Africa. Mar. Policy 2010, 34, 1163–1170. [Google Scholar] [CrossRef]
- WorldFish; Food and Agriculture Organization of the United Nations; Duke University. Illuminating Hidden Harvests: The Contribution of Small-Scale Fisheries to Sustainable Development: Program Brief; WorldFish: Penang, Malaysia; Food and Agriculture Organization of the United Nations: Rome, Italy; Duke University: Durham, NC, USA, 2018. [Google Scholar]
- FAO. FAO yearbook. In Fishery and Aquaculture Statistics 2017; FAO: Rome, Italy, 2019. [Google Scholar]
- Humbwavali, J.B.; Giugliani, C.; Nunes, L.N.; Dalcastagnê, S.V.; Duncan, B.B. Malnutrition and its associated factors: A cross-sectional study with children under 2 years in a suburban area in Angola. BMC Public Health 2019, 19, 220. [Google Scholar] [CrossRef]
- McDonald, C.; Hyder, Z.; Albino Cossa, H. Angola Nutrition Gap Analysis. In Health, Nutrition and Population (HNP) Discussion Paper; World Bank: Washington, DC, USA, 2011; Available online: https://openknowledge.worldbank.org/handle/10986/13582 (accessed on 8 December 2019).
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Health Observatory Data Repository: Prevalence of Anemia in Women. Available online: http://apps.who.int/gho/data/node.main.ANAEMIAWOMEN?lang=en (accessed on 17 December 2019).
- UNICEF. Under-Five Mortality. Available online: https://data.unicef.org/topic/child-survival/under-five-mortality/ (accessed on 14 February 2020).
- European Commission. Country Profile on Nutrition: Angola. Available online: https://ec.europa.eu/international-partnerships/system/files/2017-country-profile-on-nutrition-angola_en.pdf (accessed on 17 December 2019).
- Global Nutrition Report. Angola Nutrition Profile. Available online: https://globalnutritionreport.org/media/profiles/v1.9.7/pdfs/angola.pdf (accessed on 8 January 2020).
- Chan, C.Y.; Tran, N.; Pethiyagoda, S.; Crissman, C.C.; Sulser, T.B.; Phillips, M.J. Prospects and challenges of fish for food security in Africa. Glob. Food Secur. 2019, 20, 17–25. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish Sci. Aquac. 2017, 26, 243–253. [Google Scholar] [CrossRef]
- Hicks, C.C.; Cohen, P.J.; Graham, N.A.J.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 2019, 574, 95–98. [Google Scholar] [CrossRef]
- Kawarazuka, N.; Bene, C. The potential role of small fish species in improving micronutrient deficiencies in developing countries: Building evidence. Public Health Nutr. 2011, 14, 1927–1938. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Bradl, H.B. (Ed.) Heavy Metals in the Environment: Origin, Interaction and Remediation; Academic Press: London, UK, 2005; Volume 6. [Google Scholar]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Bio. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharm. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Scaling Up Nutrition. Nutrition and the Sustainable Development Goals. Available online: https://scalingupnutrition.org/nutrition/nutrition-and-the-sustainable-development-goals/ (accessed on 11 February 2020).
- Ene-Obong, H.; Schönfeldt, H.C.; Campaore, E.; Kimani, A.; Mwaisaka, R.; Vincent, A.; El Ati, J.; Kouebou, P.; Presser, K.; Finglas, P.; et al. Importance and use of reliable food composition data generation by nutrition/dietetic professionals towards solving Africa’s nutrition problem: Constraints and the role of FAO/INFOODS/AFROFOODS and other stakeholders in future initiatives. Proc. Nutr. Soc. 2019, 78, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, H.; Southgate, D. Food Composition Data—Production, Management and Use, 2nd ed.; Burlingame, B.A., Charrondiere, U.R., Eds.; FAO: Rome, Italy, 2003. [Google Scholar]
- SAFOODS. South Africa Food Data Systems: Brief History. Available online: http://safoods.mrc.ac.za/about.html (accessed on 22 January 2020).
- Stadlmayr, B.; Charrondiere, U.; Enujiugha, V.; Bayili, G.; Fagbohoun, E.; Samb, B.; Addy, P.; Barikmo, I.; Ouattara, F.; Oshaug, A.; et al. West African Food Composition Table/Table De Composition Des Aliments D’afrique De L’ouest; FAO: Rome, Italy, 2012. [Google Scholar]
- Vincent, A.; Grande, F.; Compaoré, E.; Amponsah Annor, G.; Addy, P.A.; Aburime, L.C.; Ahmed, D.; Bih Loh, A.M.; Dahdouh Cabia, S.; Deflache, N.; et al. FAO/INFOODS Food Composition Table for Western Africa (2019) User Guide & Condensed Food Composition Table/Table de Composition des Aliments FAO/INFOODS Pour L’afrique de L’ouest (2019) Guide D’utilisation & Table de Composition des Aliments Condensée; FAO: Rome, Italy, 2020. [Google Scholar]
- Stadlmayr, B.; Charrondiere, U.R.; Burlingame, B. Development of a regional food composition table for West Africa. Food Chem. 2013, 140, 443–446. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2011; p. 50. [Google Scholar]
- Check Longley, C.; Thilsted, S.H.; Beveridge, M.; Cole, S.; Nyirenda, D.B.; Heck, S.; Hother, A.-L. The role of fish in the first 1000 days in Zambia. IDS Bull. 2014, September, 27–37. [Google Scholar]
- Reksten, A.M.; Bøkevoll, A.; Frantzen, S.; Lundebye, A.-K.; Kögel, T.; Kolås, K.; Aakre, I.; Kjellevold, M. Sampling protocol for determination of nutrients and contaminants in fish and other seafood—The EAF Nansen Programme. MethodsX. unpublished.
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 3 December 2019).
- Norwegian Standard 9402 (Norsk Standard). Atlantic salmon. In Measurement of Fat and Colour, 1st ed.; (Atlantisk laks. Måling av fett og farge. Første utgave); Standard Norge: Oslo, Norway, 1994. [Google Scholar]
- AOAC. Crude Protein in Meat and Meat Products, Combustion Method, 16th ed.; Method 992.15; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Julshamn, K.; Maage, A.; Norli, H.S.; Grobecker, K.H.; Jorhem, L.; Fecher, P. Determination of arsenic, cadmium, mercury, and lead by inductively coupled plasma/mass spectrometry in foods after pressure digestion: NMKL interlaboratory study. J. AOAC Int. 2007, 90, 844–856. [Google Scholar] [CrossRef]
- Julshamn, K.; Dahl, L.; Eckhoff, K. Determination of iodine in seafood by inductively coupled plasma/mass spectrometry. J. AOAC Int. 2001, 84, 1976–1983. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- WHO; FAO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2004. [Google Scholar]
- Mwangi, M.N.; Phiri, K.S.; Abkari, A.; Gbané, M.; Bourdet-Sicard, R.; Braesco, V.A.; Zimmermann, M.B.; Prentice, A.M. Iron for Africa—Report of an Expert Workshop. Nutrients 2017, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Hulthén, L.; Hallberg, L. The importance of bioavailability of dietary iron in relation to the expected effect from iron fortification. Eur. J. Clin. Nutr. 2008, 62, 761–769. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Sixty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives; FAO: Rome, Italy, 2006. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. In Proceedings of the 73rd Joint FAO/WHO Expert Committee on Food Additives (JECFA) Meeting—Food Additives and Contaminants, Geneva, Switzerland, 8–17 June 2010. [Google Scholar]
- WHO. The WHO Child Growth Standards. Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 4 January 2020).
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Ketata Khitouni, I.; Abdelmouleh, A.; Bouain, A.; Boudhrioua Mihoubi, N. Variations of the chemical composition of five coastal catch fish species of the Gulf of Gabès (Tunisia). Cybium Int. J. Ichtyology 2010, 34, 175–183. [Google Scholar]
- Huss, H.H. Quality and Quality Changes in Fresh Fish: FAO Fisheries Technical Paper—348; FAO: Rome, Italy, 1995. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFSA J. 2014, 12, 3761. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef]
- Murray, J.; Burt, J.R. The Composition of Fish. Available online: http://www.fao.org/wairdocs/tan/x5916e/x5916e01.htm (accessed on 3 November 2019).
- Norwegian Scientific Committee for Food Safety (VKM). A Comprehensive Assessment of Fish and other Seafood in the Norwegian Diet; VKM: Oslo, Norway, 2006; p. 173. Available online: https://vkm.no/download/18.13735ab315cffecbb5156f8e/1502818024998/d94dff429b.pdf (accessed on 7 January 2020).
- Norwegian Scientific Committee for Food Safety (VKM). Benefit-Risk Assessment of Fish and Fish Products in the Norwegian Diet—An Update. In Scientific Opinion of the Scientific Steering Committee; VKM Report 15; VKM: Oslo, Norway, 2014; p. 293. Available online: https://vkm.no/download/18.2994e95b15cc54507161ea1a/1498222018046/0a646edc5e.pdf (accessed on 7 December 2019).
- Neumann, C.G.; Bwibo, N.O.; Murphy, S.P.; Sigman, M.; Whaley, S.; Allen, L.H.; Guthrie, D.; Weiss, R.E.; Demment, M.W. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: Background, study design and baseline findings. J. Nutr. 2003, 133, 3941–3949. [Google Scholar] [CrossRef]
- Dagnelie, P.C.; van Dusseldorp, M.; van Staveren, W.A.; Hautvast, J.G. Effects of macrobiotic diets on linear growth in infants and children until 10 years of age. Eur. J. Clin. Nutr. 1994, 48, 103–111. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to the safety assessment of wild and farmed fish. EFSA J. 2005, 3, 236. [Google Scholar] [CrossRef]
- FAO Aquaculture Development and Coordination Programme (ADCP). Fish Feed Technology; FAO: Rome, Italy, 1980; p. 395. [Google Scholar]
- Yeannes, M.; Almandos, M. Estimation of fish proximate composition starting from water content. J. Food Compos. Anal. 2003, 16, 81–92. [Google Scholar] [CrossRef]
- Mogobe, O.; Mosepele, K.; Masamba, W.R. Essential mineral content of common fish species in Chanoga, Okavango Delta, Botswana. Afr. J. Food Sci. 2015, 9, 480–486. [Google Scholar] [CrossRef][Green Version]
- Bogard, J.R.; Thilsted, S.H.; Marks, G.C.; Wahab, M.A.; Hossain, M.A.R.; Jakobsen, J.; Stangoulis, J. Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J. Food Compos. Anal. 2015, 42, 120–133. [Google Scholar] [CrossRef]
- Roos, N.; Islam, M.M.; Thilsted, S.H. Small indigenous fish species in Bangladesh: Contribution to vitamin A, calcium, and iron intakes. J. Nutr. 2003, 133, 4021–4026. [Google Scholar] [CrossRef]
- Roos, N.; Islam, M.; Thilsted, S.H. Small fish is an important dietary source of vitamin A and calcium in rural Bangladesh. Int. J. Food Sci. Nutr. 2003, 54, 329–339. [Google Scholar] [CrossRef]
- Reksten, A.M.; Somasanduram, T.; Kjellevold, M.; Nordhagen, A.; Bøkevoll, A.; Pincus, L.M.; Rizwan, A.A.M.; Mamun, A.; Thilsted, S.H.; Htut, T.; et al. Nutrient composition of 19 fish species from Sri Lanka and potential contribution to food and nutrition security. J. Food Compos. Anal. accepted. [CrossRef]
- Roos, N.; Thorseng, H.; Chamnan, C.; Larsen, T.; Gondolf, U.H.; Bukhave, K.; Thilsted, S.H. Iron content in common Cambodian fish species: Perspectives for dietary iron intake in poor, rural households. Food Chem. 2007, 104, 1226–1235. [Google Scholar] [CrossRef]
- Malde, M.K.; Bügel, S.; Kristensen, M.; Malde, K.; Graff, I.E.; Pedersen, J.I. Calcium from salmon and cod bone is well absorbed in young healthy men: A double-blinded randomised crossover design. J. Nutr. Metab. 2010, 7, 61. [Google Scholar] [CrossRef]
- Bogard, J.R.; Farook, S.; Marks, G.C.; Waid, J.; Belton, B.; Ali, M.; Toufique, K.; Mamun, A.; Thilsted, S.H. Higher fish but lower micronutrient intakes: Temporal changes in fish consumption from capture fisheries and aquaculture in Bangladesh. PLoS ONE 2017, 12, e0175098. [Google Scholar] [CrossRef]
- Institute of Marine Research (IMR). Seafood Data. Available online: https://sjomatdata.hi.no/#search/ (accessed on 7 January 2020).
- Mohanty, B.P.; Sankar, T.V.; Ganguly, S.; Mahanty, A.; Anandan, R.; Chakraborty, K.; Paul, B.N.; Sarma, D.; Dayal, J.S.; Mathew, S.; et al. Micronutrient Composition of 35 Food Fishes from India and Their Significance in Human Nutrition. Biol. Trace Elem. Res. 2016, 174, 448–458. [Google Scholar] [CrossRef]
- Nordhagen, A.; Rizwan, A.A.M.; Aakre, I.; Reksten, A.M.; Pincus, L.M.; Bøkevoll, A.; Mamun, A.; Thilsted, S.H.; Htut, T.; Somasanduram, T.; et al. Nutrient composition of demersal, pelagic, and mesopelagic fish species sampled off the coast of Bangladesh and their potential contribution to food and nutrition security—The EAF Nansen Programme. Foods. unpublished.
- Demment, M.W.; Young, M.M.; Sensenig, R.L. Providing Micronutrients through Food-Based Solutions: A Key to Human and National Development. J. Nutr. 2003, 133, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A. Iron and zinc deficiency in children in developing countries. BMJ 2007, 334, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press, Inc.: New York, NY, USA, 2005; p. 908. [Google Scholar]
- Wheal, M.S.; DeCourcy-Ireland, E.; Bogard, J.R.; Thilsted, S.H.; Stangoulis, J.C.R. Measurement of haem and total iron in fish, shrimp and prawn using ICP-MS: Implications for dietary iron intake calculations. Food Chem. 2016, 201, 222–229. [Google Scholar] [CrossRef]
- Rangan, A.; Ho, R.; Blight, G.; Binns, C. Haem iron content of Australian meats and fish. Food Aust. 1997, 49, 508–511. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Young, I.; Parker, H.M.; Rangan, A.; Prvan, T.; Cook, R.L.; Donges, C.E.; Steinbeck, K.S.; O’Dwyer, N.J.; Cheng, H.L.; Franklin, J.L.; et al. Association between Haem and Non-Haem Iron Intake and Serum Ferritin in Healthy Young Women. Nutrients 2018, 10, 81. [Google Scholar] [CrossRef]
- Roos, N.; Wahab, M.A.; Chamnan, C.; Thilsted, S.H. The role of fish in food-based strategies to combat vitamin A and mineral deficiencies in developing countries. J. Nutr. 2007, 137, 1106–1109. [Google Scholar] [CrossRef]
- Navas-Carretero, S.; Pérez-Granados, A.; Sarriá, B.; Carbajal, A.; Pedrosa, M.; Roe, M.; Fairweather-Tait, S.; Vaquero, M.P. Oily Fish Increases Iron Bioavailability of a Phytate Rich Meal in Young Iron Deficient Women. J. Am. Coll. Nutr. 2008, 27, 96–101. [Google Scholar] [CrossRef]
- Nerhus, I.; Wik Markhus, M.; Nilsen, B.M.; Oyen, J.; Maage, A.; Odegard, E.R.; Midtbo, L.K.; Frantzen, S.; Kogel, T.; Graff, I.E.; et al. Iodine content of six fish species, Norwegian dairy products and hen’s egg. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 488/2014 of May 12. 2014 Amending Regulation (EC) No 1881/2006 as Regard Maximum Levels of Cadmium in Foodstuffs, Text with EEA Relevance. O. J. 2014, 138, 75–79. [Google Scholar]
- Francesconi Kevin, A. Arsenic species in seafood: Origin and human health implications. Pure App. Chem. 2010, 82, 373. [Google Scholar] [CrossRef]
- Bonsignore, M.; Salvagio Manta, D.; Oliveri, E.; Sprovieri, M.; Basilone, G.; Bonanno, A.; Falco, F.; Traina, A.; Mazzola, S. Mercury in fishes from Augusta Bay (southern Italy): Risk assessment and health implication. Food Chem. Toxicol. 2013, 56, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.; Howsam, M.; Diop, C.; Cazier, F.; Goossens, J.F.; Diouf, A.; Amara, R. Spatial and seasonal variations of trace elements concentrations in liver and muscle of round Sardinelle (Sardinella aurita) and Senegalese sole (Solea senegalensis) along the Senegalese coast. Chemosphere 2016, 144, 758–766. [Google Scholar] [CrossRef]
- Counter, S.A.; Buchanan, L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharm. 2004, 198, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food. EFSA J. 2009, 980, 1–139. [CrossRef]
- Gomna, A.; Rana, K. Inter-household and intra-household patterns of fish and meat consumption in fishing communities in two states in Nigeria. Br. J. Nr. 2007, 97, 145–152. [Google Scholar] [CrossRef]
| Scientific Name a | Common Name a | Local Name | Habitat a | Weight (g) b | Length (cm) b | Number of Composite Samples |
|---|---|---|---|---|---|---|
| Caranx rhonchus | False scad | Chicharro amarelo | Benthopelagic | 244 (210–314) | 27.5 (26.5–30.5) | 5 |
| Lagocephalus laevigatusc | Smooth puffer | Baiacu | Pelagic | 734 (429–1360) | 38.2 (32.5–44) | 5 |
| Sardinella aurita | Round sardinella | Lombuda | Pelagic | 198 (176–237) | 27.6 (26.5–29) | 5 |
| Sardinella maderensis | Madeiran sardinella | Pallheta | Pelagic | 131 (116–151) | 25.5 (25–27) | 5 |
| Sphyraena guachancho | Guachanche barracuda | Barracuda | Pelagic-neritic | 509 (339–795) | 46.4 (41.5–53) | 5 |
| Trachurus trecae | Cunene horse mackerel | Carapau do Cunene | Benthopelagic | 200 (173–240) | 26.1 (24.5–29) | 5 |
| Food | Product Description | Edible Conversion Dactor |
|---|---|---|
| Chicken | Light meat with skin, raw | 1 |
| Beef | Meat, moderately fat (ca. 20% fat), raw | 1 |
| Pork | Meat, moderately fat (ca. 20% fat), raw | 1 |
| Milk | Cow, whole, 3.5% fat (pasteurised or UHT) | 1 |
| Eggs | Chicken, raw | 0.87 |
| Species | nb | Moisture | Protein | Lipid |
|---|---|---|---|---|
| % | g/100 g | g/100 g | ||
| Caranx rhonchus | 5 | 73.2 ± 0.3 | 23 ± 0.0 | 2.20 ± 0.3 |
| Sardinella aurita | 5 | 69.8 ± 0.8 | 21 ± 0.5 | 5.40 ± 2.2 |
| Sardinella maderensis | 5 | 74.2 ± 0.7 | 21 ± 0.4 | 2.33 ± 0.5 |
| Sphyraena guachancho | 5 | 75.5 ± 0.6 | 21 ± 0.4 | 2.12 ± 0.8 |
| Trachurus trecae | 5 | 70.8 ± 1.1 | 21 ± 0.4 | 6.98 ± 1.1 |
| Mean Content | 72.7 ± 2.3 | 21 ± 1.0 | 3.81 ± 2.3 |
| Species | nb | Ca | Fe | I | K | Mg | Na | P | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/100 g | mg/100 g | µg/100 g | mg/100 g | mg/100 g | mg/100 g | mg/100 g | µg/100 g | mg/100 g | ||
| Caranx rhonchus | 5 | 25.8 ± 18 | 0.65 ± 0.01 | 22.0 ± 1.1 | 480 ± 7.1 | 37.4 ± 0.5 | 50 ± 0.8 | 294 ± 8.9 | 29.4 ± 1.7 | 0.47 ± 0.04 |
| Sardinella aurita | 5 | 71.2 ± 9.4 | 1.84 ± 0.15 | 23.8 ± 2.7 | 510 ± 10 | 37.6 ± 0.5 | 45 ± 1.6 | 344 ± 8.9 | 37.0 ± 2.2 | 0.52 ± 0.02 |
| Sardinella maderensis | 5 | 89.6 ± 9.9 | 1.52 ± 0.18 | 35.0 ± 4.8 | 528 ± 13 | 39.2 ± 0.8 | 47 ± 3.5 | 340 ± 7.1 | 50.4 ± 0.5 | 0.56 ± 0.07 |
| Sphyraena guachancho | 5 | 20.4 ± 3.6 | 0.40 ± 0.05 | 46.6 ± 3.6 | 478 ± 128 | 38.2 ± 0.8 | 45 ± 2.3 | 310 ± 7.1 | 26.2 ± 1.5 | 0.33 ± 0.01 |
| Trachurus trecae | 5 | 24.6 ± 7.0 | 0.84 ± 0.01 | 27.2 ± 1.3 | 456 ± 11 | 35.0 ± 0.7 | 65 ± 1.1 | 284 ± 5.5 | 38.2 ± 1.8 | 0.42 ± 0.03 |
| Mean Concentration | 46.3 ± 31 | 1.04 ± 0.56 | 31.0 ± 9.6 | 490 ± 60 | 37.5 ± 1.6 | 50 ± 7.8 | 314 ± 26 | 36.2 ± 8.7 | 0.46 ± 0.09 |
| Species | na | As | Cd | Hg | Pb |
|---|---|---|---|---|---|
| mg/kg w.w. | mg/kg w.w. | mg/kg w.w. | mg/kg w.w. | ||
| Caranx rhonchus | 5 | 1.54 ± 0.09 | 0.004 ± 0.002 | 0.022 ± 0.002 | 0.006 ± 0.000 |
| Sardinella aurita | 5 | 1.78 ± 0.20 | 0.003 ± 0.001 | 0.014 ± 0.007 | 0.007 ± 0.000 |
| Sardinella maderensis | 5 | 2.10 ± 0.25 | 0.006 ± 0.001 | 0.046 ± 0.010 | 0.010 ± 0.002 |
| Sphyraena guachancho | 5 | 0.56 ± 0.11 | 0.001 ± 0.000 | 0.170 ± 0.058 | 0.005 ± 0.001 |
| Trachurus trecae | 5 | 2.26 ± 0.05 | 0.003 ± 0.001 | 0.026 ± 0.007 | 0.006 ± 0.001 |
| Mean Concentration | 1.65 ± 0.63 | 0.003 ± 0.002 | 0.056 ± 0.064 | 0.007 ± 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moxness Reksten, A.; Joao Correia Victor, A.M.; Baptista Nascimento Neves, E.; Myhre Christiansen, S.; Ahern, M.; Uzomah, A.; Lundebye, A.-K.; Kolding, J.; Kjellevold, M. Nutrient and Chemical Contaminant Levels in Five Marine Fish Species from Angola—The EAF-Nansen Programme. Foods 2020, 9, 629. https://doi.org/10.3390/foods9050629
Moxness Reksten A, Joao Correia Victor AM, Baptista Nascimento Neves E, Myhre Christiansen S, Ahern M, Uzomah A, Lundebye A-K, Kolding J, Kjellevold M. Nutrient and Chemical Contaminant Levels in Five Marine Fish Species from Angola—The EAF-Nansen Programme. Foods. 2020; 9(5):629. https://doi.org/10.3390/foods9050629
Chicago/Turabian StyleMoxness Reksten, Amalie, Avelina M. Joao Correia Victor, Edia Baptista Nascimento Neves, Sofie Myhre Christiansen, Molly Ahern, Abimbola Uzomah, Anne-Katrine Lundebye, Jeppe Kolding, and Marian Kjellevold. 2020. "Nutrient and Chemical Contaminant Levels in Five Marine Fish Species from Angola—The EAF-Nansen Programme" Foods 9, no. 5: 629. https://doi.org/10.3390/foods9050629
APA StyleMoxness Reksten, A., Joao Correia Victor, A. M., Baptista Nascimento Neves, E., Myhre Christiansen, S., Ahern, M., Uzomah, A., Lundebye, A.-K., Kolding, J., & Kjellevold, M. (2020). Nutrient and Chemical Contaminant Levels in Five Marine Fish Species from Angola—The EAF-Nansen Programme. Foods, 9(5), 629. https://doi.org/10.3390/foods9050629





