Corn-Starch-Based Materials Incorporated with Cinnamon Oil Emulsion: Physico-Chemical Characterization and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chia Mucilage Extraction
2.2. Oil-in-Water (O/W) Emulsion Preparation
2.3. Characterization of Emulsions
2.3.1. Creaming Index
2.3.2. Thermal Stability
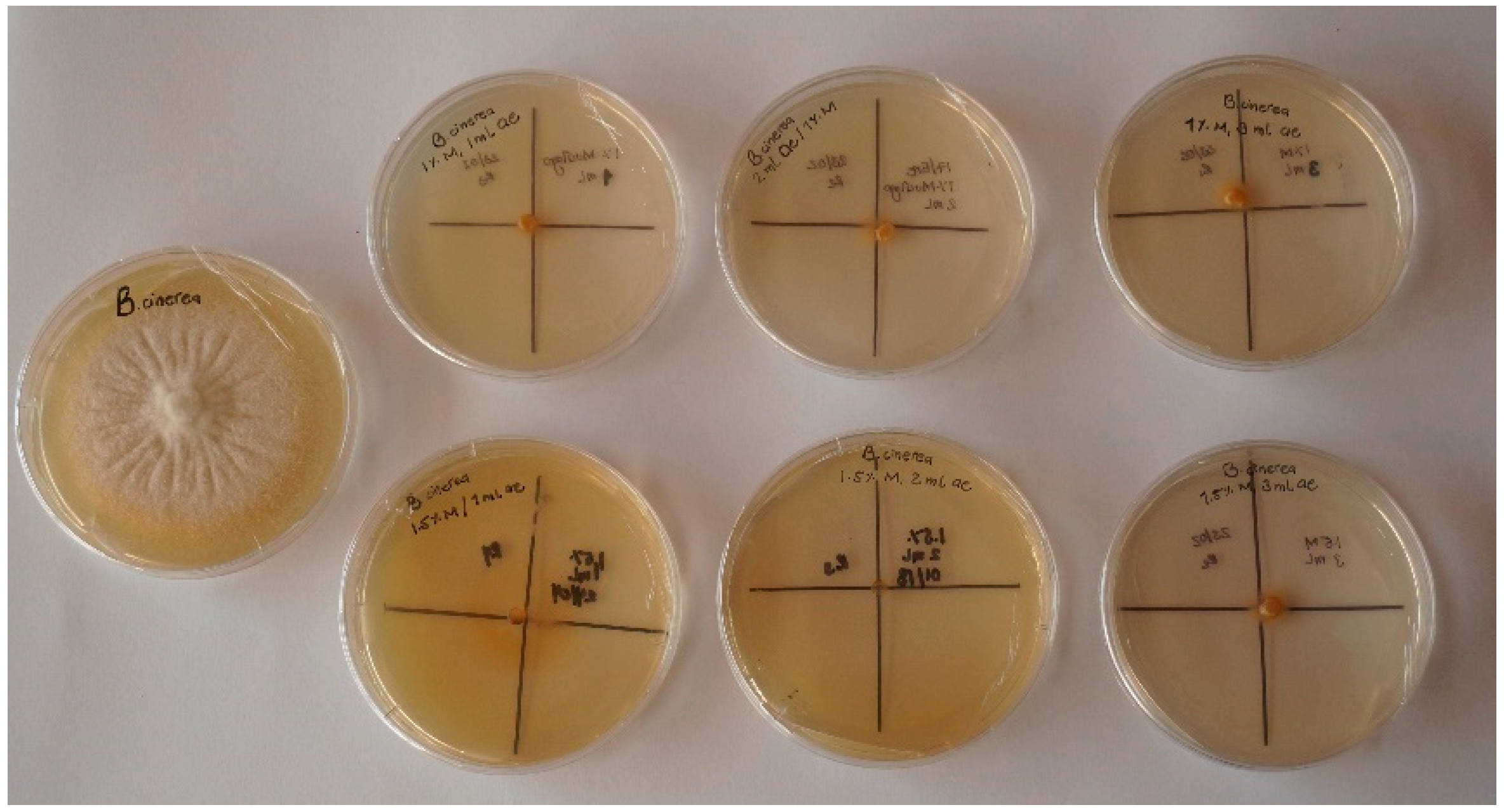
2.3.3. In Vitro Antifungal Activity of Emulsions
2.4. Preparation of Thermo-Plasticized Starch-Emulsion Plates
2.5. Characterization of Plates
2.5.1. TGA
2.5.2. Mechanical Analysis
2.5.3. In Vitro Antifungal Activity
3. Results
3.1. Emulsions
3.2. Characterization of Starch/Emulsion Materials
3.2.1. Mechanical Analysis
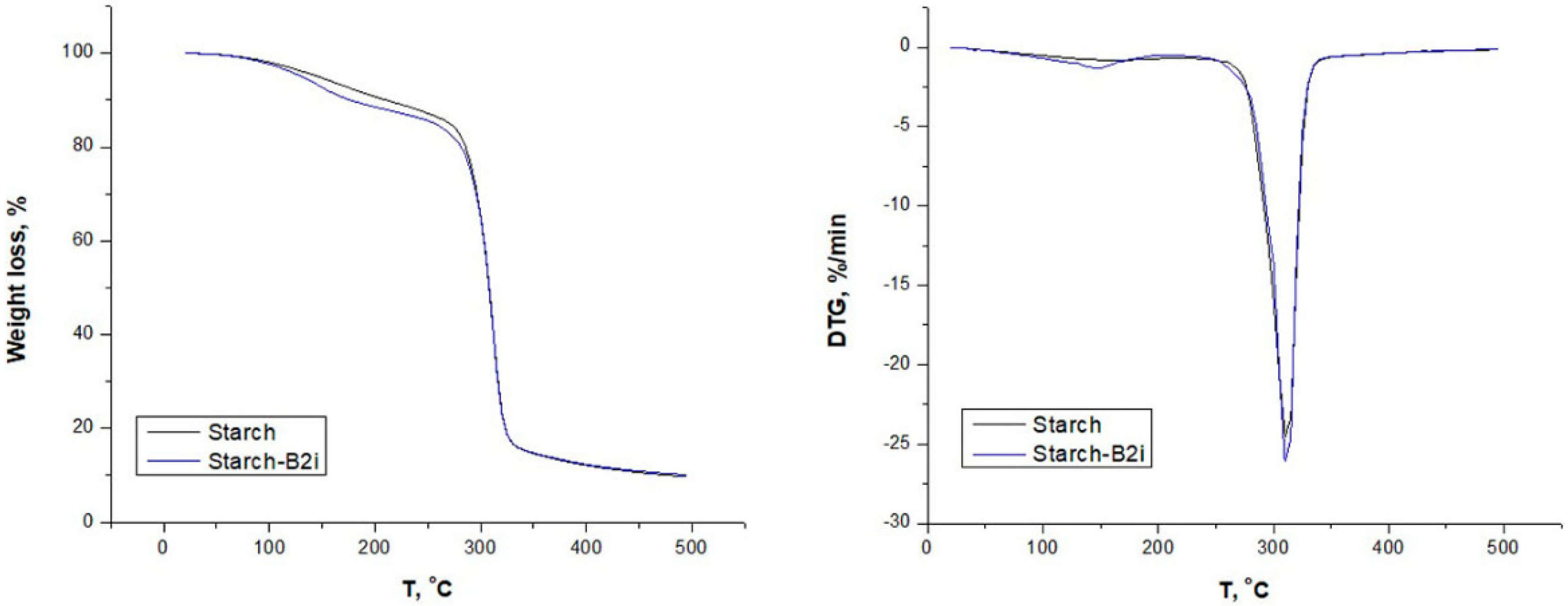
3.2.2. Thermal Analysis
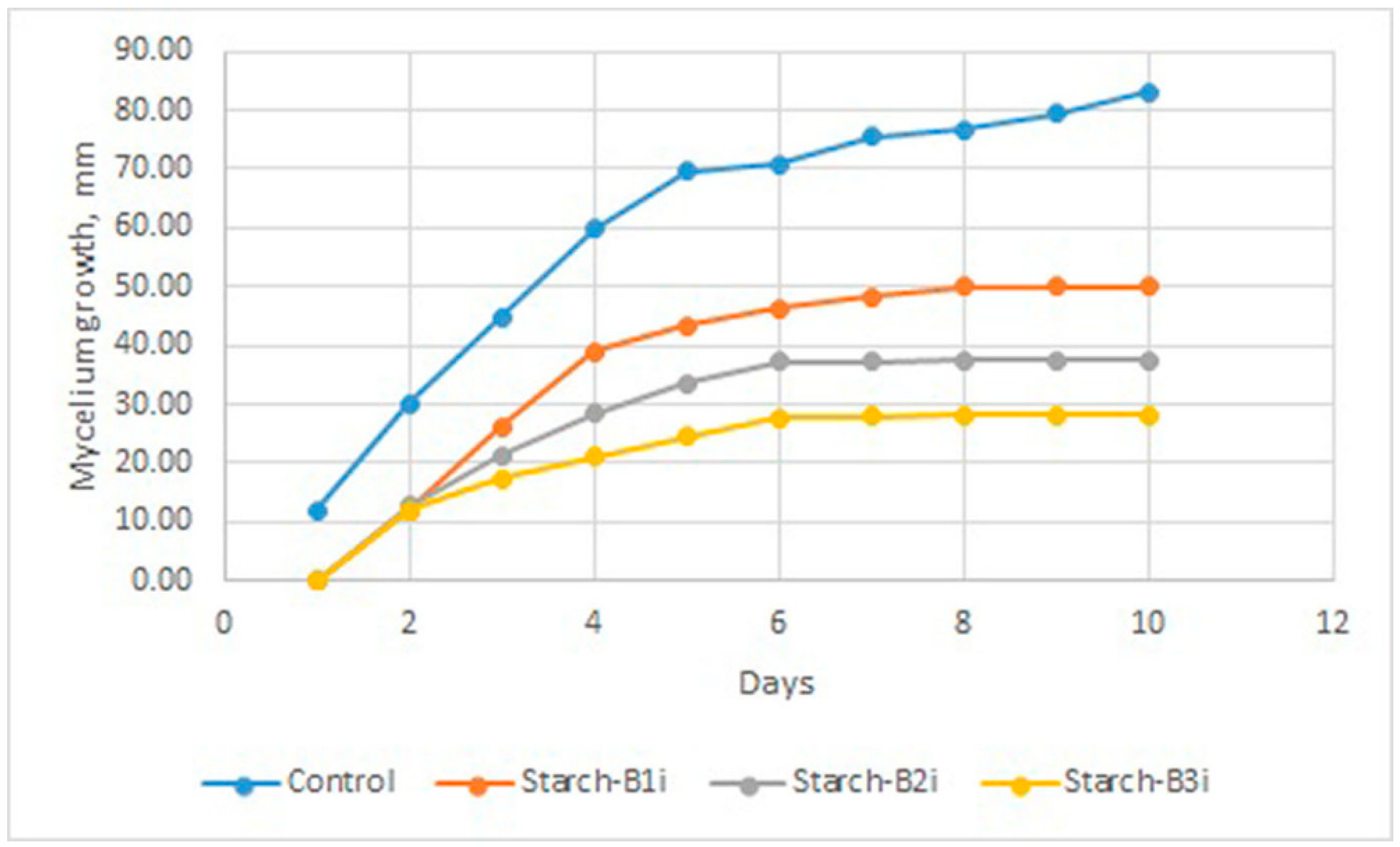
3.2.3. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fruits and Vegetable Industry in Chile—Growth, Trends, and Forecast (2020–2025). Available online: https://www.mordorintelligence.com/industry-reports/colombia-fruit-vegetable-market (accessed on 18 March 2020).
- Santagata, G.; Valerio, F.; Cimmino, A.; Dal Poggetto, G.; Masi, M.; Di Biase, M.; Malinconico, M.; Lavermicocca, P.; Evidente, A. Chemico-physical and antifungal properties of poly(butylene succinate)/cavoxin blend: Study of a novel bioactive polymeric based system. Eur. Polym. J. 2017, 94, 230–247. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Soy protein—Poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Nguyen Van Long, N.; Joly, C.; Dantigny, P. Active packaging with antifungal activities. Int. J. Food Microbiol. 2016, 220, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Manso, S.; Becerril, R.; Nerín, C.; Gómez-Lus, R. Influence of pH and temperature variations on vapor phase action of an antifungal food packaging against five mold strains. Food Control 2015, 47, 20–26. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and carvacrol migration from PHBV films and antibacterial action in different food matrices. Food Chem. 2019, 277, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gazdag, Z.; Szente, L.; Meggyes, M.; Horváth, G.; Lemli, B.; Kunsági-Máté, S.; Kuzma, M.; Kőszegi, T. Antioxidant and antimicrobial properties of randomly methylated β cyclodextrin—Captured essential oils. Food Chem. 2019, 278, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Galvão, J.A.; Mazine, M.R.; Gloria, E.M.; Oetterer, M. Control of Staphylococcus aureus biofilms by the application of single and combined treatments based in plant essential oils. Int. J. Food Microbiol. 2018, 286, 128–138. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Lopez, O.; Garcia, M.A.; Villar, M.A.; Gentili, A.; Rodriguez, M.S.; Albertengo, L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT Food Sci. Technol. 2014, 57, 106–115. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Ambrosio-Martín, J.; Lagaron, J.M. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef]
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crops Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Melgarejo-Flores, B.G.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Miranda, M.R.A.; Ayala-Zavala, J.F. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Innov. Food Sci. Emerg. Technol. 2009, 10, 97–102. [Google Scholar] [CrossRef]
- Quinzio, C.; Ayunta, C.; López de Mishima, B.; Iturriaga, L. Stability and rheology properties of oil-in-water emulsions prepared with mucilage extracted from Opuntia ficus-indica (L). Miller. Food Hydrocoll. 2018, 84, 154–165. [Google Scholar] [CrossRef]
- Capitani, M.I.; Nolasco, S.M.; Tomás, M.C. Stability of oil-in-water (O/W) emulsions with chia (Salvia hispanica L.) mucilage. Food Hydrocoll. 2016, 61, 537–546. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Yao, Y.; Wen, Y.; Hu, W.; Zhang, J.; Liu, X.; Bell, A.E.; Tikkanen-Kaukanen, C. Chemical components and emulsification properties of mucilage from Dioscorea opposita Thunb. Food Chem. 2017, 228, 315–322. [Google Scholar] [CrossRef]
- Campos, B.E.; Dias Ruivo, T.; da Silva Scapim, M.R.; Madrona, G.S.; de C. Bergamasco, R. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Wu, Y.; Eskin, N.A.M.; Cui, W.; Pokharel, B. Emulsifying properties of water soluble yellow mustard mucilage: A comparative study with gum Arabic and citrus pectin. Food Hydrocoll. 2015, 47, 191–196. [Google Scholar] [CrossRef]
- Velázquez-Gutiérrez, S.K.; Figueira, A.C.; Rodríguez-Huezo, M.E.; Román-Guerrero, A.; Carrillo-Navas, H.; Pérez-Alonso, C. Sorption isotherms, thermodynamic properties and glass transition temperature of mucilage extracted from chia seeds (Salvia hispanica L.). Carbohydr. Polym. 2015, 121, 411–419. [Google Scholar] [CrossRef]
- Guiotto, E.N.; Capitani, M.I.; Nolasco, S.M.; Tomás, M.C. Stability of Oil-in-Water Emulsions with Sunflower (Helianthus annuus L.) and Chia (Salvia hispanica L.) By-Products. J. Am. Oil Chem. Soc. 2016, 93, 133–143. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Rheological and microstructural properties of the chia seed polysaccharide. Int. J. Biol. Macromol. 2015, 81, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Sukatta, U.; Haruthaithanasan, V.; Chantarapanont, W.; Dilokkunanant, U.; Suppakul, P. Antifungal Activity of Clove and Cinnamon Oil and Their Synergistic Against Postharvest Decay Fungi of Grape in vitro. Kasetsart J.-Nat. Sci. 2008, 42, 169–174. [Google Scholar]
- Mvuemba, H.N.; Green, S.E.; Tsopmo, A.; Avis, T.J. Antimicrobial efficacy of cinnamon, ginger, horseradish and nutmeg extracts against spoilage pathogens. Phytoprotection 2009, 90, 65. [Google Scholar] [CrossRef]
- Bang, K.-H.; Lee, D.-W.; Park, H.-M.; Rhee, Y.-H. Inhibition of Fungal Cell Wall Synthesizing Enzymes by trans -Cinnamaldehyde. Biosci. Biotechnol. Biochem. 2000, 64, 1061–1063. [Google Scholar] [CrossRef]
- Abdalla, W. Antibacterial and Antifungal Effect of Cinnamon. Microbiol. Res. J. Int. 2018, 23, 1–8. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Lei, L.; Zhao, G. Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with an emphasis on water resistance. Int. J. Biol. Macromol. 2018, 115, 547–553. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Šuput, D.; Lazić, V.; Pezo, L.; Markov, S.; Vaštag, Ž.; Popović, L.; Radulović, A.; Ostojić, S.; Zlatanović, S.; Popović, S. Characterization of Starch Edible Films with Different Essential Oils Addition. Polish J. Food Nutr. Sci. 2016, 66, 277–286. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Phase transitions in starch based films containing fatty acids. Effect on water sorption and mechanical behaviour. Food Hydrocoll. 2013, 30, 408–418. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Mallardo, S.; De Vito, V.; Malinconico, M.; Volpe, M.G.; Santagata, G.; Di Lorenzo, M.L. Poly(butylene succinate)-based composites containing β-cyclodextrin/d-limonene inclusion complex. Eur. Polym. J. 2016, 79, 82–96. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control 2018, 92, 37–46. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Jiang, L.; Chuan, Y.; Yuan, M.; Chen, H. Characterization of Active Packaging Films Made from Poly(Lactic Acid)/Poly(Trimethylene Carbonate) Incorporated with Oregano Essential Oil. Molecules 2016, 21, 695. [Google Scholar] [CrossRef]
| Sample Code | Mucilage (wt %) | Cinnamon Oil (mL) | CI % | TS % | ||||
|---|---|---|---|---|---|---|---|---|
| 0.d | 30.d | 60.d | 0.d | 30.d | 60.d | |||
| A1i | 1 | 1 | 0 | 0 | 1 | 100 | 100 | 99 |
| A2i | 1 | 2 | 0 | 0 | 1 | 100 | 98 | 97 |
| A3i | 1 | 3 | 0 | 0 | 1 | 95 | 97 | 95 |
| B1i | 1.5 | 1 | 0 | 0 | 0 | 100 | 100 | 99 |
| B2i | 1.5 | 2 | 0 | 0 | 0 | 100 | 100 | 99 |
| B3i | 1.5 | 3 | 0 | 0 | 0 | 100 | 100 | 99 |
| Sample Code | Starch (kg) | Glycerol (g) | Water (g) | Emulsion (g) |
|---|---|---|---|---|
| Starch | 0.5 | 150 | 50 | 0 |
| Starch-B1i | 0.5 | 150 | 0 | 50 |
| Starch-B2i | 0.5 | 150 | 0 | 50 |
| Starch-B3i | 0.5 | 150 | 0 | 50 |
| Sample Code | TS (MPa) | e (%) |
|---|---|---|
| Starch | 2.04 | 50.5 |
| Starch-B1i | 1.65 | 84.4 |
| Starch-B2i | 1.55 | 86.2 |
| Starch-B3i | 1.48 | 90.3 |
| Simple | WL180 (%) | Tonset (°C) | Tdeg (°C) | Char Residue at 500 °C (%) |
|---|---|---|---|---|
| Starch | 7.8 | 280 | 312 | 9.8 |
| Starch-B1i | 11 | 279 | 312 | 10.9 |
| Starch-B2i | 10.3 | 278 | 312 | 11.0 |
| Starch-B3i | 9.1 | 278 | 312 | 11.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Galindo, E.P.; Nesic, A.; Bautista-Baños, S.; Dublan García, O.; Cabrera-Barjas, G. Corn-Starch-Based Materials Incorporated with Cinnamon Oil Emulsion: Physico-Chemical Characterization and Biological Activity. Foods 2020, 9, 475. https://doi.org/10.3390/foods9040475
Díaz-Galindo EP, Nesic A, Bautista-Baños S, Dublan García O, Cabrera-Barjas G. Corn-Starch-Based Materials Incorporated with Cinnamon Oil Emulsion: Physico-Chemical Characterization and Biological Activity. Foods. 2020; 9(4):475. https://doi.org/10.3390/foods9040475
Chicago/Turabian StyleDíaz-Galindo, Edaena Pamela, Aleksandra Nesic, Silvia Bautista-Baños, Octavio Dublan García, and Gustavo Cabrera-Barjas. 2020. "Corn-Starch-Based Materials Incorporated with Cinnamon Oil Emulsion: Physico-Chemical Characterization and Biological Activity" Foods 9, no. 4: 475. https://doi.org/10.3390/foods9040475
APA StyleDíaz-Galindo, E. P., Nesic, A., Bautista-Baños, S., Dublan García, O., & Cabrera-Barjas, G. (2020). Corn-Starch-Based Materials Incorporated with Cinnamon Oil Emulsion: Physico-Chemical Characterization and Biological Activity. Foods, 9(4), 475. https://doi.org/10.3390/foods9040475










