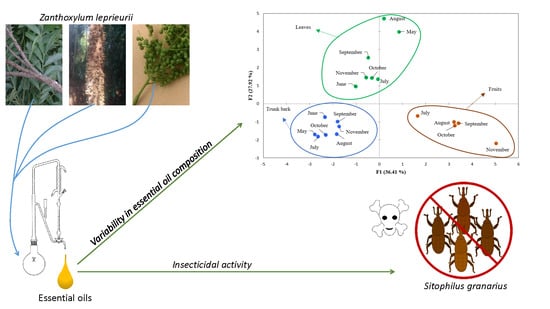
Seasonal Effect on the Chemical Composition, Insecticidal Properties and Other Biological Activities of Zanthoxylum leprieurii Guill. & Perr. Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Hydrodistillation Procedure
2.2. GC/MS Chemical Analysis of Essential Oils
2.3. Biological Activities
2.3.1. Antioxidant Assay
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Capacity
Ferric-Reducing Power Assay
2.3.2. Anti-Inflammatory Activity
Inhibition Lipoxygenase Assay
Inhibition of Albumin Denaturation Assay
2.3.3. Insecticidal Activity
Determination of Mortality Values
Repulsive Assay
2.3.4. Anti-Plasmodial Activity
2.4. Statistical Analysis
2.4.1. Data Analysis
2.4.2. Biological Activities Analysis
3. Results and Discussion
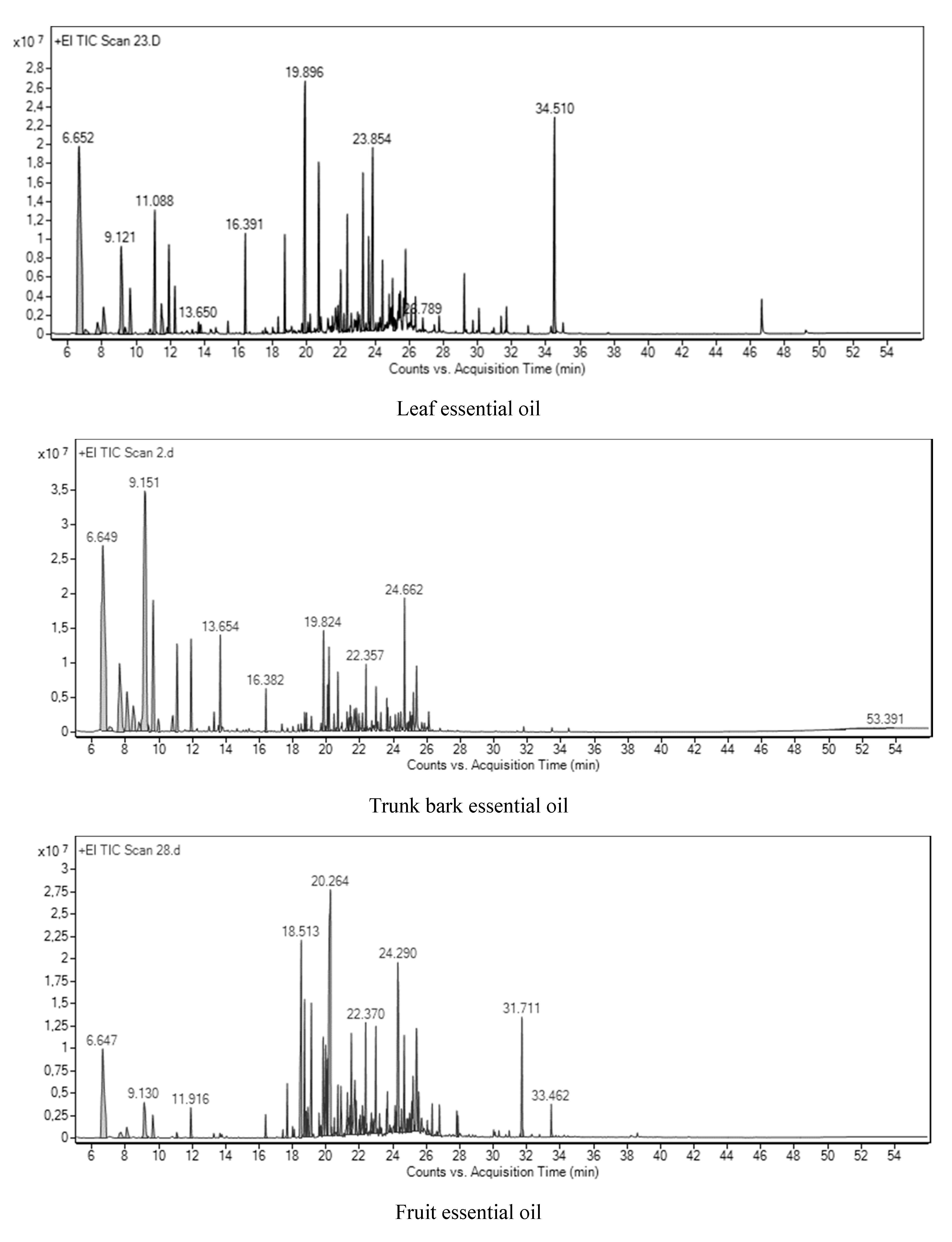
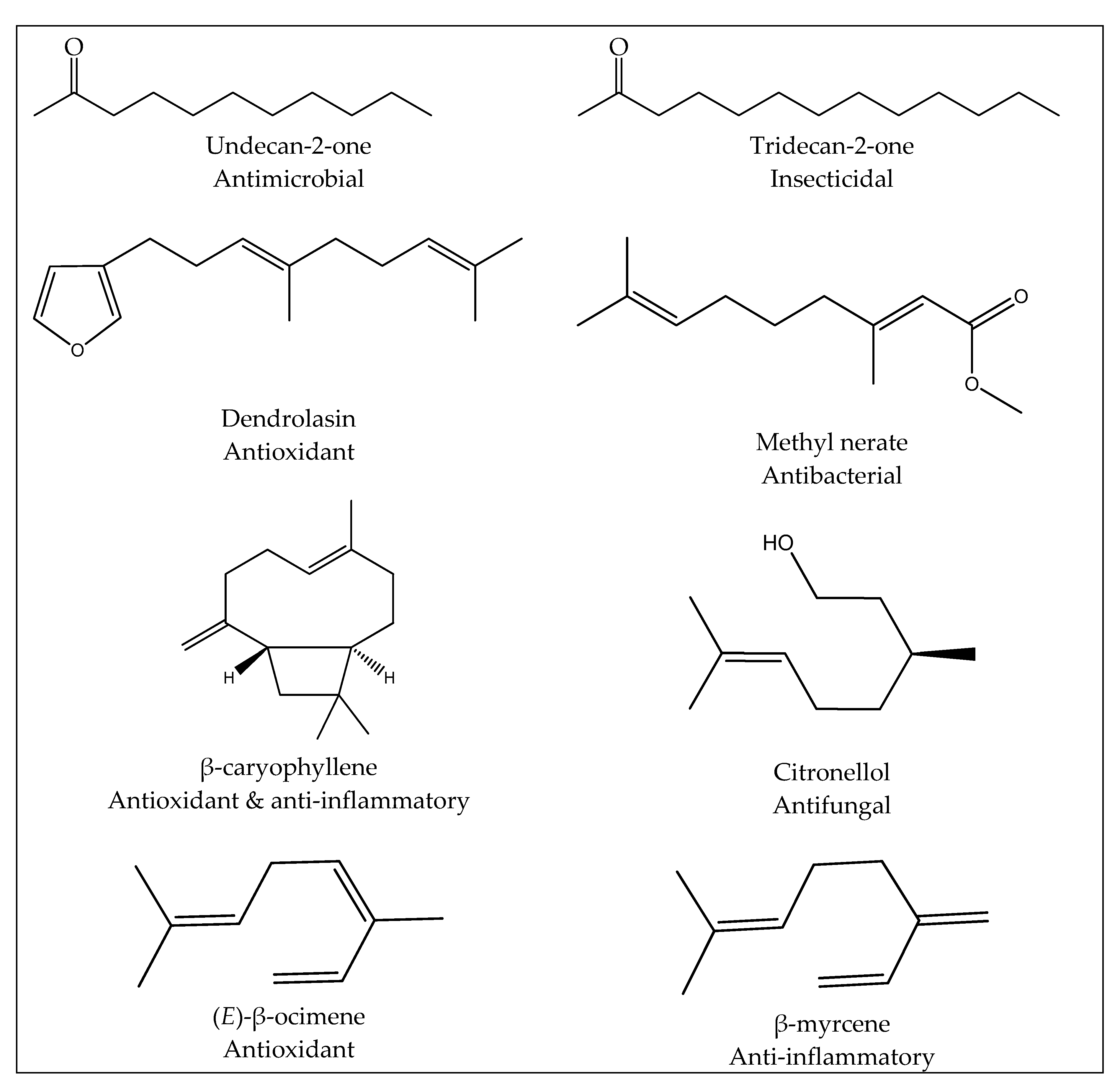
3.1. Chemical Composition of Essential oils and Yields
3.1.1. Leaf Essential Oils
3.1.2. Trunk Bark Essential Oils
3.1.3. Fruit Essential Oils
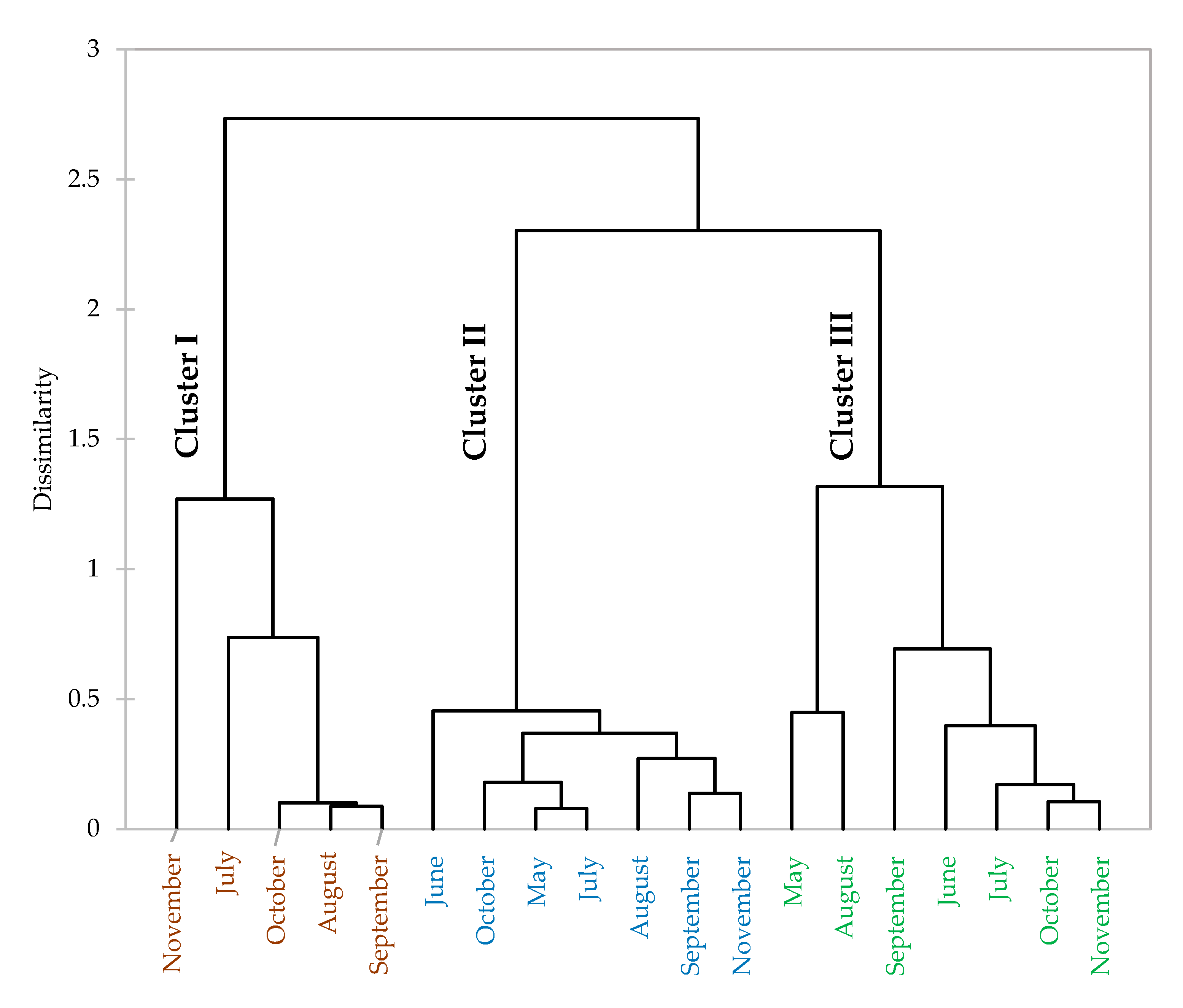
3.2. Seasonal Effect on Essential Oil Composition
3.3. Essential oil Biological Activities
3.3.1. Antioxidant Activity
DPPH Free Radical Scavenging Assay
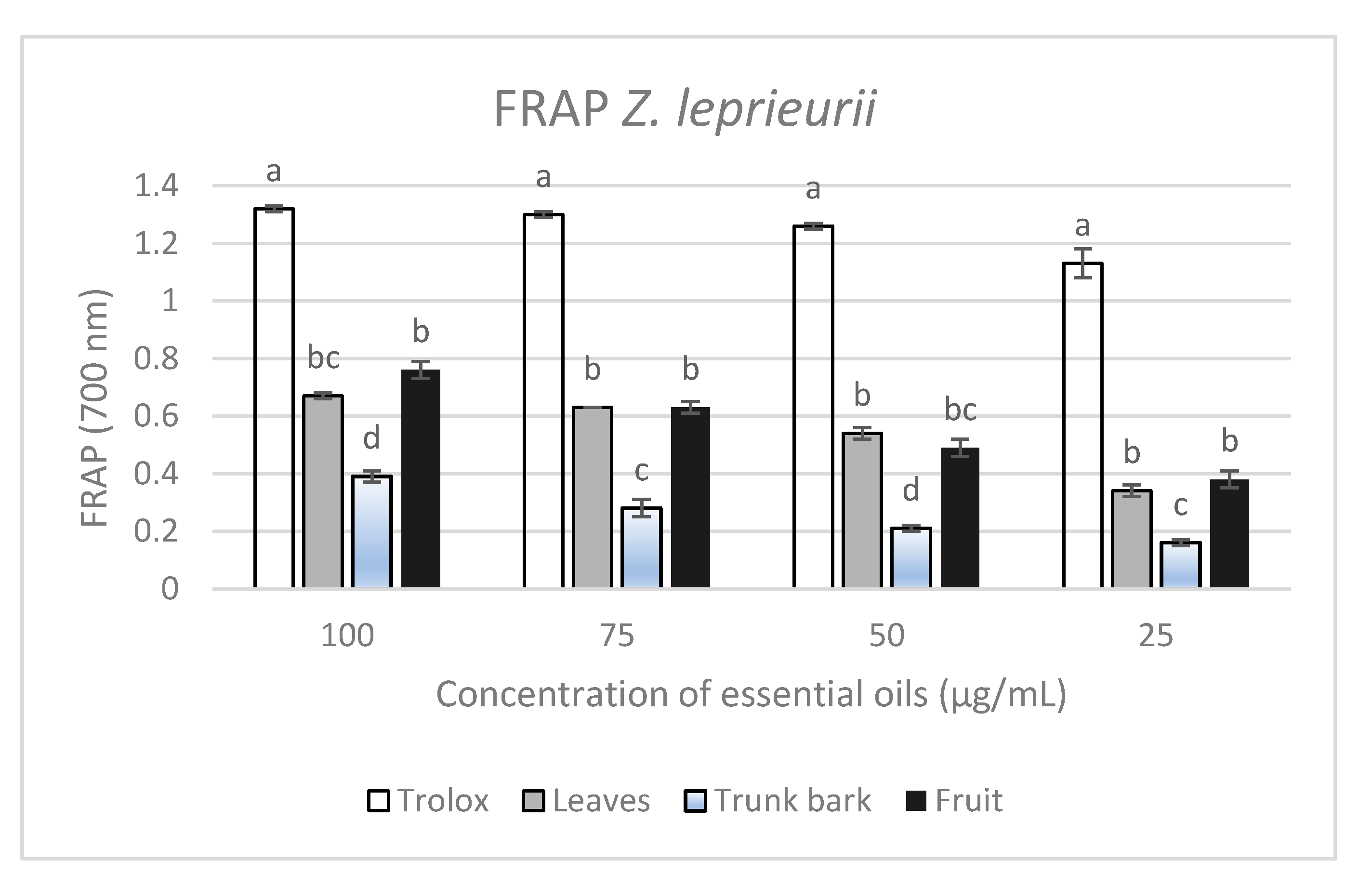
Ferric-Reducing Antioxidant Power
3.3.2. Anti-Inflammatory Activity
Lipoxygenase Denaturation Inhibition Activity
Inhibition of Albumin Denaturation
3.3.3. Insecticidal Activity
3.3.4. Anti-Plasmodial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonçalves, M.A.M. Micromorphology and in vitro Antibacterial Evaluation of Zanthoxylum and Hymenocardia Species. Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2018. [Google Scholar]
- Dongmo, P.M.J.; Tchoumbougnang, F.; Sonwa, E.T.; Kenfack, S.M.; Zollo, P.H.A.; Menut, C. Antioxidant and anti-inflammatory potential of essential oils of some Zanthoxylum (Rutaceae) of Cameroon. Int. J. Essent. Oil 2008, 2, 82–88. [Google Scholar]
- Sriwichai, T.; Sookwong, P.; Siddiqui, M.W.; Sommano, S.R. Aromatic profiling of Zanthoxylum myriacanthum (makwhaen) essential oils from dried fruits using different initial drying techniques. Ind. Crops Prod. 2019, 133, 284–291. [Google Scholar] [CrossRef]
- Bunalema, L.; Obakiro, S.; Tabuti, J.R.; Waako, P. Knowledge on plants used traditionally in the treatment of tuberculosis in Uganda. J. Ethnopharmacol. 2014, 151, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Burkil, H.M. Royal Botanic Gardens; Kew Year. In The Useful Plants of West Tropical Africa; Royal Botanic Gardens: London, UK, 1994; Volume 2. [Google Scholar]
- Tine, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. A Method for LC-MS/MS profiling of coumarins in Zanthoxylum xanthoxyloides (Lam.) B. Zepernich and Timler extracts and essential oils. Molecules 2017, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Mester, I. Chemistry and Chemical Taxonomy of the Rutales; Waterman, P.G., Grundon, M.F., Eds.; Academic Press: London, UK, 1983; pp. 31–96. [Google Scholar]
- Kpomah, E.D.; Uwakwe, A.A.; Abbey, B.W. Aphrodisiac studies of diherbal mixture of Zanthoxylum leprieurii Guill. & Perr. And Piper guineense Schumach. & Thonn. on male wistar rats. GJRMI 2012, 1, 381. [Google Scholar]
- Agyare, C.; Kisseih, E.; Kyere, I.Y.; Ossei, P.P.S. Medicinal plants used in wound care: Assessment of wound healing and antimicrobial properties of Zanthoxylum Leprieurii. IBSPR 2014, 2, 81–89. [Google Scholar]
- Bunalema, L.; Fotso, G.W.; Waako, P.; Tabuti, J.; Yeboah, S.O. Potential of Zanthoxylum leprieurii as a source of active compounds against drug resistant Mycobacterium tuberculosis. BMC Complement. Altern. Med. 2017, 17, 89. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.; Wansi, J.D.; Evans, A.R.; Sarker, S.D. Kaurane diterpenes from the fruits of Zanthoxylum leprieurii (Rutaceae). Rec. Nat. Prod. 2017, 11, 304–309. [Google Scholar]
- Zondegoumba, E.N.T.; Dibahteu, W.L.; de Araujo, A.; Vidari, G.; Liu, Y.; Luo, S.; Li, S.; Junior, F.J.B.M.; Scotti, L.; Tullius, M. Cytotoxic and Schistosomidal Activities of Extract, Fractions and Isolated Compounds from Zanthoxylum Leprieurii (Rutaceae). IJSBAR 2019, 44, 209–222. [Google Scholar]
- Ngoumfo, R.M.; Jouda, J.B.; Mouafo, F.T.; Komguem, J.; Mbazoa, C.D.; Shiao, T.C.; Choudhary, M.I.; Laatsch, H.; Legault, J.; Pichette, A.; et al. In vitro cytotoxic activity of isolated acridones alkaloids from Zanthoxylum leprieurii Guill. et Perr. Bioorg. Med. Chem. 2010, 18, 3601–3605. [Google Scholar] [CrossRef]
- Adesina, S.K. The Nigerian Zanthoxylum: Chemical and biological values. AJTCAM 2005, 2, 282–301. [Google Scholar] [CrossRef]
- Misra, L.N.; Wouatsa, N.V.; Kumar, S.; Kumar, R.V.; Tchoumbougnang, F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J. Ethnopharmacol. 2013, 148, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Tatsadjieu, L.N.; Ngang, J.E.; Ngassoum, M.B.; Etoa, F.X. Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloıdes and Zanthoxylum leprieurii from Cameroon. Fitoterapia 2003, 74, 469–472. [Google Scholar] [CrossRef]
- Gardini, F.; Belletti, N.; Ndagijimana, M.; Guerzoni, M.E.; Tchoumbougnang, F.; Zollo, P.H.A.; Micci, C.; Lanciotti, R.; Kamdem, S.L.S. Composition of four essential oils obtained from plants from Cameroon, and their bactericidal and bacteriostatic activity against Listeria monocytogenes, Salmonella enteritidis and Staphylococcus aureus. Afr. J. Microbiol. Res. 2009, 3, 264–271. [Google Scholar]
- Tanoh, E.A.; Nea, F.; Yapi, T.A.; Boué, G.B.; Jean-Brice, B.; Tomi, F.; Tonzibo, Z.F. Essential Oil of Zanthoxylum lepreurii Guill. & Perr. Rich in Undecan-2-One and Tridecan-2-One. J. Essent. Oil-Bear. Plants 2018, 21, 1397–1402. [Google Scholar]
- Oyedeji, A.O.; Lawal, O.A.; Adeniyi, B.A.; Alaka, S.A.; Tetede, E. Essential oil composition of three Zanthoxylum species. J. Essent. Oil Res. 2008, 20, 69–71. [Google Scholar] [CrossRef]
- Fogang, H.P.; Tapondjou, L.A.; Womeni, H.M.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Lupidi, G.; Maggi, F.; Papa, F.; et al. Characterization and biological activity of essential oils from fruits of Zanthoxylum xanthoxyloides Lam. and Z. leprieurii Guill. & Perr., two culinary plants from Cameroon. Flavour Fragr. J. 2012, 27, 171–179. [Google Scholar]
- Eiter, L.C.; Fadamiro, H.; Setzer, W.N. Seasonal variation in the leaf essential oil composition of Zanthoxylum clava-herculis growing in Huntsville, Alabama. Nat. Prod. Commun. 2010, 5, 457–460. [Google Scholar] [CrossRef]
- Kim, J.H. Seasonal variations in the content and composition of essential oil from Zanthoxylum Piperitum. J. Ecol. Field Biol. 2012, 35, 195–201. [Google Scholar] [CrossRef]
- Bhatt, V.; Sharma, S.; Kumar, N.; Sharma, U.; Singh, B. Chemical Composition of Essential Oil among Seven Populations of Zanthoxylum armatum from Himachal Pradesh: Chemotypic and Seasonal Variation. Nat. Prod. Commun. 2017, 12, 1643–1646. [Google Scholar]
- Benini, C.; Ringuet, M.; Wathelet, J.P.; Lognay, G.; du Jardin, P.; Fauconnier, M.L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2012, 27, 356–366. [Google Scholar]
- Bettaieb Rebey, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni Selami, I.; Saidani Tounsi, M.; Marzouk, B.; Fauconnier, M.L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst. 2018, 152, 971–978. [Google Scholar] [CrossRef]
- Lins, L.; Dal Maso, S.; Foncoux, B.; Kamili, A.; Laurin, Y.; Genva, M.; Jijakli, M.H.; De Clerck, C.; Fauconnier, M.-L.; Deleu, M. Insights into the relationships between herbicide activities, molecular structure and membrane interaction of cinnamon and citronella essential oils components. Int. J. Mol. Sci. 2019, 20, 4007. [Google Scholar] [CrossRef] [PubMed]
- Tanoh, E.A.; Nea, F.; Kenne Kemene, T.; Genva, M.; Saive, M.; Tonzibo, F.Z.; Fauconnier, M.L. Antioxidant and lipoxygenase inhibitory activities of essential oils from endemic plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi. Molecules 2019, 24, 2445. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Ding, C. Seasonal Variations in the Chemical Composition of Liangshan Olive Leaves and Their Antioxidant and Anticancer Activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, B.S.; Hwang, K.H.; Kim, G.H. Evaluation for anti-inflammatory effects of Siegesbeckia glabrescens extract in vitro. Food Agric. Immunol. 2011, 22, 145–160. [Google Scholar] [CrossRef]
- Nikhila, G.S.; Sangeetha, G. Anti-inflammatory properties of the root tubers of Gloriosa superba and its conservation through micropropagation. J. Med. Plants Res. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Kar, B.; Kumar, R.S.; Karmakar, I.; Dola, N.; Bala, A.; Mazumder, U.K.; Hadar, P.K. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S976–S980. [Google Scholar] [CrossRef]
- Nea, F.; Tanoh, E.A.; Wognin, E.L.; Kemene, T.K.; Genva, M.; Saive, M.; Tonzibo, Z.F.; Fauconnier, M.L. A new chemotype of Lantana rhodesiensis Moldenke essential oil from Côte d’Ivoire: Chemical composition and biological activities. Ind. Crops Prod. 2019, 141, 111766. [Google Scholar] [CrossRef]
- Fleurat-Lessard, F. Gestion intégrée de la protection des stocks de céréales contre les insectes sans traitement insecticide rémanent. Phytoma 2018, 716, 32–40. [Google Scholar]
- Darwish, Y.A.; Omar, Y.M.; Hassan, R.E.; Mahmoud, M.A. Repellent effects of certain plant essential oil, plant extracts and inorganic salts to granary weevil, Sitophilus granarius (L.). Arch. Arch. Phytopathol. Pflanzenschutz 2013, 46, 1949–1957. [Google Scholar] [CrossRef]
- Mc Donald, L.L.; Guyr, H.; Speire, R.R. Preliminary evaluation of new candidate materials as toxicants, repellents, and attractants against stored-product insects. Mark. Res. Rep. 1970, 882, 189. [Google Scholar]
- Ledoux, A.; St-Gelais, A.; Cieckiewicz, E.; Jansen, O.; Bordignon, A.; Illien, B.; Di Giovanni, N.; Marvilliers, A.; Hoareau, F.; Pendeville, H.; et al. Antimalarial activities of alkyl cyclohexenone derivatives isolated from the leaves of Poupartia borbonica. J. Nat. Prod. 2017, 80, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- BEI Reagent Search. Available online: https://www.beiresources.org/Catalog/BEIParasiticProtozoa/MRA-102.aspx (accessed on 27 July 2017).
- Bordignon, A.; Frédérich, M.; Ledoux, A.; Campos, P.E.; Clerc, P.; Hermann, T.; Quetin-Leclercq, J.; Cieckiewicz, E. In vitro antiplasmodial and cytotoxic activities of sesquiterpene lactones from Vernonia fimbrillifera Less. (Asteraceae). Nat. Prod. Res. 2018, 32, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Tchabong, F.; Sameza, S.R.; Tchameni, M.L.; Mounbain, N.S.; Mouelle, F.; Jazet, S.A.; Menut, D.P.M.; Tchoumbougnang, C. Chemical composition, free radical scavenging and antifungal activity of Zanthoxylum leprieurii essential oils against Epidermophyton floccosum and Microsporum gypseum, two most prevalent cutaneous Mycosis. J. Pharm. 2018, 8, 13–19. [Google Scholar]
- Shams, M.; Esfahan, S.Z.; Esfahan, E.Z.; Dashtaki, H.N.; Dursun, A.; Yildirim, E. Effects of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.). INDJSRT 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Azzaz Nabil, A.E.; El-Khateeb Ayman, Y.; Farag Ayman, A. Chemical composition and biological activity of the essential oil of Cyperus articulatus. Int. J. Acad. Res. 2014, 6, 265–269. [Google Scholar]
- Choucry, M.A. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J. Pharm. Phytother. 2017, 9, 1–5. [Google Scholar]
- Azizan, N.; Mohd Said, S.; Zainal Abidin, Z.; Jantan, I. Composition and antibacterial activity of the essential oils of Orthosiphon stamineus Benth and Ficus deltoidea Jack against Pathogenic Oral Bacteria. Molecules 2017, 22, 2135. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Rodríguez-Moyá, M.; Li, M.; Pichersky, E.; San, K.Y.; Gonzalez, R. Synthesis of methyl ketones by metabolically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2012, 39, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Pareja, M.; Qvarfordt, E.; Webster, B.; Mayon, P.; Pickett, J.; Birkett, M.; Glinwood, R. Herbivory by a phloem-feeding insect inhibits floral volatile production. PLoS ONE 2012, 7, e31971. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Wang, Z.W.; Chiang, C.J.; Chao, Y.P. Metabolic engineering of Escherichia coli for production of butyric acid. J. Agric. Food Chem. 2014, 62, 4342–4348. [Google Scholar] [CrossRef] [PubMed]
- Lamaty, G.; Menut, C.; Bessiere, J.M.; Aknin, M. Aromatic plants of tropical Central Africa. II. A comparative study of the volatile constituents of Zanthoxylum leprieurii (Guill. et Perr.) Engl. and Zanthoxylum tessmannii Engl. leaves and fruit pericarps from the Cameroon. Flavour Fragr. J. 1989, 4, 203–205. [Google Scholar] [CrossRef]
- Pereira, F.D.; Mendes, J.M.; Lima, I.O.; Mota, K.S.; Oliveira, W.A.; Lima, E.D. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef]
- Watson, A.D. Lipidomics: A global approach to lipid analysis in biological systems. J. Lipid Res. 2006, 47, 2101–2111. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Yasari, M.; Mirzargar, S.S.; Hoseini, S.M. Rainbow trout (Oncorhynchus mykiss) anesthesia with myrcene: Efficacy and physiological responses in comparison with eugenol. Fish Physiol. Biochem. 2018, 44, 919–926. [Google Scholar] [CrossRef]
- Geng, S.; Cui, Z.; Huang, X.; Chen, Y.; Xu, D.; Xiong, P. Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind. Crops Prod. 2011, 33, 248–252. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova. 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Lima, H.R.P.; Kaplan, M.A.C.; Cruz, A.V.D.M. Influência dos fatores abióticos na produção e variabilidade de terpenóides em plantas. Flor. Am. 2012, 10, 71–77. [Google Scholar]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P.; Saavedra-Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A.; Garciglia, R.S. Antioxidant activity of the essential oil and its major terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2017, 13, S875–S880. [Google Scholar]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Bisht, R.; Kandari Negi, S. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC. essential oil. IJPT 2012, 11, 68–72. [Google Scholar]
- Gogoi, R.; Loying, R.; Sarma, N.; Munda, S.; Pandey, S.K.; Lal, M. A comparative study on antioxidant, anti-inflammatory, genotoxicity, anti-microbial activities and chemical composition of fruit and leaf essential oils of Litsea cubeba Pers from North-east India. Ind. Crops Prod. 2018, 125, 131–139. [Google Scholar] [CrossRef]
- Ouedraogo, R.A.; Koala, M.; Dabire, C.; Hema, A.; Bazie, V.B.E.J.T.; Outtara, L.P.; Nebie, R.H. Teneur en phénols totaux et activité antioxydante des extraits des trois principales variétés d’oignons (Allium cepa L.) cultivées dans la région du Centre-Nord du Burkina Faso. IJBCS 2015, 9, 281–291. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; et Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Ghasemi, P.A.; Izadi, A.; Malek, P.F.; Hamedi, B. Chemical composition, antioxidant and antibacterial activities of essential oils from Ferulago angulata. Pharm. Biol. 2016, 54, 2515–2520. [Google Scholar] [CrossRef]
- Kunwar, G.; Prakash, O.; Chandra, M.; Pant, A.K. Chemical composition, antifungal and antioxidant activities of Perilla frutescens (L.) syn. P. ocimoides L. collected from different regions of Indian Himalaya. AJTM 2013, 8, 88–98. [Google Scholar]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Singh, P.; Sundriyal, R.C. Chemical constituents and biological activities of the genus Zanthoxylum: A review. AJPAC 2011, 5, 412–416. [Google Scholar]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.A.B.S.; Klein-Junior, L.C.; Cruz, S.M.; Cáceres, A.; Quintão, N.L.M.; Delle Monache, F.; Cechinel-Filho, V. Anti-inflammatory and anti-hyperalgesic evaluation of the condiment laurel (Litsea guatemalensis Mez.) and its chemical composition. Food Chem. 2012, 132, 1980–1986. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Shi, C.; Fang, J. A comparative study of sodium houttuyfonate and 2-undecanone for their in vitro and in vivo anti-inflammatory activities and stabilities. Int. J. Mol. Sci. 2014, 15, 22978–22994. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.; Oussou, K.R.; Kanko, C.; Tonzibo, Z.F.; Foua-Bi, K.; Tano, Y. Bioefficacité des Huiles Essentielles de Trois Espèces végétales (Ocimum gratissimum, Ocimum canum et Hyptis suaveolens), de la Famille des Labiées dans la lutte contre Sitophilus zeamais. Eur. J. Sci. Res. 2017, 150, 273–284. [Google Scholar]
- Yallappa, R.; Nandagopal, B.; Thimmappa, S. Botanicals as grain protectants. Psyche J. Entomol. 2012, 2012, 646740. [Google Scholar]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef]
- Mansour, S.A.; El-Sharkawy, A.Z.; Abdel-Hamid, N.A. Toxicity of essential plant oils, in comparison with conventional insecticides, against the desert locust, Schistocerca gregaria (Forskål). Ind. Crops Prod. 2015, 63, 92–99. [Google Scholar] [CrossRef]
- Prieto, J.A.; Patiño, O.J.; Delgado, W.A.; Moreno, J.P.; Cuca, L.E. Chemical composition, insecticidal, and antifungal activities of fruit essential oils of three Colombian Zanthoxylum species. Chil. J. Agric. Res. 2011, 71, 73–82. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Matasyoh, J.C.; Ngoumfo, R.M.; Chepkorir, R. Mosquito larvicidal activity of alkaloids from Zanthoxylum lemairei against the malaria vector Anopheles gambiae. Pestic. Biochem. Physiol. 2011, 99, 82–85. [Google Scholar] [CrossRef]
- Liu, X.C.; Liu, Q.Y.; Zhou, L.; Liu, Q.R.; Liu, Z.L. Chemical composition of Zanthoxylum avicennae essential oil and its larvicidal activity on Aedes albopictus Skuse. Trop. J. Pharm. Res. 2014, 13, 399–404. [Google Scholar] [CrossRef][Green Version]
- Koba, K.W.P.; Poutouli, C.; Raynaud, P.; Yaka, P. Propriétés insecticides de l’huile essentielle d’Aeollanthus pubescens Benth sur les chenilles de deux Lépidoptères: Selepa docilsi butler (noctuidae) et Scrobipalpa ergassima mayr. (geleduidae). J. Rech. Sci. Univ. Lomé. 2007, 9, 19–25. [Google Scholar]
- Toudert-Taleb, K.; Hedjal-Chebheb, M.; Hami, H.; Debras, J.F.; Kellouche, A. Composition of essential oils extracted from six aromatic plants of Kabylian origin (Algeria) and evaluation of their bioactivity on Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Bruchidae). Afr. Entomol. 2014, 22, 417–427. [Google Scholar] [CrossRef]
- Tchinda, A.T.; Fuendjiep, V.; Sajjad, A.; Matchawe, C.; Wafo, P.; Khan, S.; Tane, P.; Choudhary, M.I. Bioactive compounds from the fruits of Zanthoxylum leprieurii. PharmacolOnline 2009, 1, 406–415. [Google Scholar]
- Goodman, C.D.; Hoang, A.T.; Diallo, D.; Malterud, K.E.; McFadden, G.I.; Wangensteen, H. Anti-plasmodial Effects of Zanthoxylum zanthoxyloides. Planta Med. 2019, 85, 1073–1079. [Google Scholar] [CrossRef]
- Muganga, R.; Angenot, L.; Tits, M.; Frederich, M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J. Ethnopharmacol. 2010, 128, 52–57. [Google Scholar] [CrossRef]
- Adebayo, J.O.; Krettli, A.U. Potential antimalarials from Nigerian plants: A review. J. Ethnopharmacol. 2011, 133, 289–302. [Google Scholar] [CrossRef]
- DE Luca, Y. Ingrédients naturels de preservation des grains stockes dans les pays en voie de developpement. J. D’agriculture Tradit. et de Bot. Appliquée 1979, 26, 20–52. [Google Scholar] [CrossRef]

| Months | Rainfall (mm) | Relative Humidity (%) | Daylight (h) | Temperature (°C) |
|---|---|---|---|---|
| May | 164.50 | 80.00 | 196.30 | 28.40 |
| June | 205.70 | 85.00 | 101.60 | 26.80 |
| July | 71.30 | 84.00 | 121.00 | 25.80 |
| August | 61.70 | 84.00 | 134.00 | 25.00 |
| September | 102.40 | 81.00 | 124.20 | 25.90 |
| October | 310.80 | 84.00 | 180.40 | 27.10 |
| November | 206.40 | 78.00 | 214.40 | 27.50 |
| Leaves | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | Cas Number | Identification | Ria | Rib | May | June | July | Aug | Sep | Oct | Nov |
| 1 | α-pinene | 80-56-8 | MS, RI, STD | 931 | 929 | 2.48 ± 0.13 | 8.45 ± 0.57 | 0.30 ± 0.11 | 0.60 ± 0.35 | 0.70 ± 0.15 | 0.50 ± 0.27 | 0.30 ± 0.17 |
| 2 | ß-myrcene | 123-35-3 | MS, RI, STD | 987 | 988 | 0.71 ± 0.03 | - | 5.20 ± 0.21 | 0.90 ± 0.15 | 3.40 ± 0.37 | 0.4 ± 0.31 | 0.70 ± 0.09 |
| 3 | p-cymene | 25155-15-1 | MS, RI, STD | 1022 | 1022 | 1.48 ± 0.03 | - | 0.10 ± 0.01 | 0.30 ± 0.03 | - | 0.40 ± 0.57 | - |
| 4 | limonene | 138-86-3 | MS, RI, STD | 1023 | 1027 | - | 0.90 ± 0.29 | 0.40 ± 0.28 | - | - | - | - |
| 5 | (E)-ß-ocimene | 13877-91-3 | MS, RI, STD | 1041 | 1046 | 23.57 ± 047 | 2.60 ± 0.99 | 3.90 ± 0.84 | 2.50 ± 0.04 | 1.30 ± 0.50 | 2.30 ± 1.91 | - |
| 6 | γ-terpinene | 99-85-4 | MS, RI, STD | 1060 | 1057 | 0.28 ± 0.03 | - | - | - | - | - | - |
| 7 | linalool | 78-70-6 | MS, RI, STD | 1094 | 1098 | 6.44 ± 0.13 | 0.50 ± 0.34 | 1.50 ± 0.55 | 8.50 ± 0.09 | 1.70 ± 0.23 | 4.30 ± 0.95 | 3.20 ± 0.09 |
| 8 | alloocimene | 673-84-7 | MS, RI | 1128 | 1129 | 1.22 ± 0.04 | 0.40 ± 0.13 | 0.20 ± 0.10 | - | - | - | - |
| 9 | terpineol | 98-55-5 | MS, RI, STD | 1190 | 1191 | - | - | 0.20 ± 0.03 | - | - | - | - |
| 10 | decanal | 112-31-2 | MS, RI, STD | 1191 | 1204 | - | - | 1.50 ± 0.52 | - | - | - | - |
| 11 | citronellol | 106-22-9 | MS, RI, STD | 1225 | 1227 | - | - | 2.00 ± 0.48 | - | 1.50 ± 0.27 | - | 0.30 ± 0.02 |
| 12 | geraniol | 106-24-1 | MS, RI, STD | 1250 | 1253 | - | - | 1.40 ± 0.53 | - | 1.00 ± 0.74 | - | - |
| 13 | decyl alcohol | 112-30-1 | MS, RI, STD | 1262 | 1263 | - | - | 1.80 ± 0.92 | - | - | - | - |
| 14 | thymol | 89-83-8 | MS, RI, STD | 1286 | 1291 | - | 2.20 ± 0.96 | 3.10 ± 0.43 | 13.30 ± 0.31 | 4.10 ± 0.22 | - | - |
| 15 | undecan-2-one | 112-12-9 | MS, RI, STD | 1288 | 1293 | 8.51 ± 0.35 | 1.90 ± 0.50 | 1.20 ± 0.03 | 8.40 ± 0.12 | 2.70 ± 0.56 | 3.20 ± 1.06 | 2.10 ± 0.98 |
| 16 | undecan-2-ol | 1653-30-1 | MS, RI, STD | 1298 | 1300 | - | - | 0.20 ± 0.04 | 0.40 ± 0.04 | - | - | - |
| 17 | methyl nerate | 1862-61-9 | MS, RI, STD | 1319 | 1323 | - | - | 2.20 ± 0.74 | - | 0.80 ± 0.44 | - | - |
| 18 | δ-elemene | 20307-84-0 | MS, RI | 1334 | 1339 | - | 1.20 ± 0.69 | 0.20 ± 0.00 | - | - | - | - |
| 19 | α-copaene | 3856-25-5 | MS, RI, STD | 1376 | 1378 | - | 0.60 ± 0.23 | 0.30 ± 0.08 | - | 0.60 ± 0.11 | 0.20 ± 0.07 | 0.40 ± 0.04 |
| 20 | ß-elemene | 515-13-9 | MS, RI | 1388 | 1394 | 3.92 ± 0.06 | - | 2.70 ± 0.83 | 4.20 ± 0.07 | 5.90 ± 0.26 | 2.30 ± 0.10 | 2.90 ± 0.20 |
| 21 | β-caryophyllene | 87-44-5 | MS, RI, STD | 1419 | 1423 | 13.51 ± 0.11 | 18.90 ± 0.48 | 15.60 ± 0.54 | 13.70 ± 0.1 | 19.85 ± 0.82 | 7.00 ± 1.02 | 8.60 ± 0.62 |
| 22 | cadina-4(14),5-diene | 54324-03-7 | MS, RI | 1430 | 1433 | 1.83 ± 0.02 | 0.90 ± 0.26 | 0.70 ± 0.16 | 0.30 ± 0.02 | 2.40 ± 0.81 | 1.70 ± 0.26 | 1.50 ± 0.19 |
| 23 | γ-elemene | 3242-08-8 | MS, RI | 1432 | 1435 | 1.19 ± 0.01 | 4.40 ± 0.45 | 1.10 ± 0.21 | 1.10 ± 0.04 | 3.10 ± 0.06 | 0.8 ± 0.12 | 1.10 ± 0.01 |
| 24 | α-humulene | 6753-98-6 | MS, RI, STD | 1456 | 1457 | 3.92 ± 0.02 | 6.70 ± 0.62 | 4.50 ± 0.98 | 3.60 ± 0.04 | 6.10 ± 0.27 | 4.10 ± 0.58 | 4.20 ± 0.32 |
| 25 | alloaromadendrene | 25246-27-9 | MS, RI | 1457 | 1465 | - | 0.50 ± 0.21 | 0.30 ± 0.06 | - | 0.50 ± 0.11 | 0.40 ± 0.06 | 0.20 ± 0.01 |
| 26 | germacrene D | 23986-74-5 | MS, RI, STD | 1482 | 1485 | 1.96 ± 0.17 | 0.90 ± 0.50 | 0.40 ± 0.10 | - | 1.60 ± 0.40 | 1.40 ± 0.21 | 1.00 ± 0.14 |
| 27 | ß-ionone | 14901-07-6 | MS, RI, STD | 1482 | 1488 | - | - | 0.40 ± 0.12 | - | - | - | 0.20 ± 0.05 |
| 28 | ß-selinene | 17066-67-0 | MS, RI | 1483 | 1490 | 0.32 ± 0.01 | 1.10 ± 0.13 | 0.70 ± 0.14 | 0.80 ± 0.01 | 1.60 ± 0.30 | 0.20 ± 0.21 | 0.50 ± 0.05 |
| 29 | tridecan-2-one | 593-08-8 | MS, RI, STD | 1487 | 1495 | 18.74 ± 0.57 | 9.00 ± 0.02 | 22.50 ± 0.98 | 30.20 ± 0.39 | 15.80 ± 0.84 | 36.80 ± 0.06 | 33.70 ± 0.36 |
| 30 | selina-4(14),7(11)-diene | 515-17-3 | MS, RI | 1495 | 1498 | - | 1.30 ± 0.31 | 0.80 ± 0.15 | 1.50 ± 0.90 | 2.90 ± 0.86 | - | - |
| 31 | tridecan-2-ol | 1653-31-2 | MS, RI, STD | 1495 | 1501 | - | 2.00 ± 0.82 | 1.70 ± 1.17 | - | 2.30 ± 0.29 | 2.40 ± 0.80 | 3.20 ± 0.33 |
| 32 | (3E,6E)-α-farnesene | 502-61-4 | MS, RI | 1499 | 1509 | 3.22 ± 0.29 | 0.60 ± 0.21 | 1.20 ± 0.34 | 0.90 ± 0.07 | 2.50 ± 0.97 | 9.10 ± 0.82 | 4.60 ± 0.55 |
| 33 | γ-cadinene | 39029-4-9 | MS, RI | 1513 | 1517 | 0.42 ± 0.06 | 1.10 ± 0.36 | 0.60 ± 0.08 | 0.20 ± 0.04 | 1.00 ± 0.21 | 1.20 ± 0.26 | 1.30 ± 0.02 |
| 34 | δ-cadinene | 483-76-1 | MS, RI | 1524 | 1526 | 2.25 ± 0.09 | 4.20 ± 1.01 | 2.20 ± 0.09 | 1.00 ± 0.02 | 4.30 ± 0.13 | 3.40 ± 0.49 | 4.30 ± 0.33 |
| 35 | elemol | 639-99-6 | MS, RI, STD | 1547 | 1552 | 0.28 ± 0.02 | 0.60 ± 0.22 | 0.20 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.05 | 0.40 ± 0.03 | 1.20 ± 0.09 |
| 36 | nerolidol | 7212-44-4 | MS, RI, STD | 1557 | 1564 | - | 1.50 ± 0.30 | 1.10 ± 0.39 | 1.00 ± 0.03 | 1.60 ± 0.49 | 4.80 ± 0.22 | 3.40 ± 0.32 |
| 37 | dendrolasin | 23262-34-2 | MS, RI | 1576 | 1580 | 1.79 ± 0.08 | 8.60 ± 0.95 | 9.40 ± 0.90 | 4.00 ± 0.06 | 7.60 ± 0.09 | 10.60 ± 0.44 | 16.40 ± 0.85 |
| 38 | spathulenol | 6750-60-3 | MS, RI | 1578 | 1582 | - | 1.50 ± 0.46 | 1.10 ± 0.03 | - | - | - | 0.40 ± 0.00 |
| 39 | caryophyllene oxide | 1139-30-6 | MS, RI, STD | 1583 | 1588 | - | 6.00 ± 0.83 | 5.70 ± 0.79 | - | - | - | - |
| 40 | ζ-cadinol | 5937-11-1 | MS, RI | 1639 | 1645 | - | 1.10 ± 0.08 | 0.60 ± 0.03 | - | 0.70 ± 0.21 | 0.60 ± 0.15 | 0.80 ± 0.04 |
| 41 | α-cadinol | 481-34-5 | MS, RI | 1650 | 1659 | 0.28 ± 0.03 | 1.30 ± 0.11 | 0.70 ± 0.01 | - | 1.10 ± 0.44 | 0.80 ± 0.23 | 0.90 ± 0.09 |
| 42 | pentadecanal | 2765-11-9 | MS, RI | 1713 | 1715 | - | 2.10 ± 0.28 | - | - | - | - | 1.60 ± 0.79 |
| Monoterpene hydrocarbons (%) | 29.74 | 12.85 | 10.10 | 4.30 | 5.40 | 3.60 | 1.00 | |||||
| Oxygenated monoterpene (%) | 6.44 | 2.70 | 11.50 | 21.80 | 8.20 | 4.30 | 3.50 | |||||
| Sesquiterpene hydrocarbons (%) | 32.54 | 48.10 | 30.90 | 27.30 | 52.35 | 31.80 | 30.60 | |||||
| Oxygenated sesquiterpenes (%) | 2.35 | 22.70 | 18.10 | 5.30 | 12.10 | 17.20 | 25.40 | |||||
| Others (%) | 27.25 | 13.10 | 28.90 | 39.00 | 20.80 | 42.40 | 38.50 | |||||
| Identified compounds (%) | 98.32 | 99.45 | 99.50 | 97.70 | 98.85 | 99.30 | 99.00 | |||||
| Yield (%) | 0.02 | 0.02 | 0.03 | 0.04 | 0.02 | 0.02 | 0.02 | |||||
| Trunk Bark | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | Cas Number | Identification | Ria | Rib | May | June | July | Aug | Sep | Oct | Nov |
| 1 | α-pinene | 80-56-8 | MS, RI, STD | 931 | 929 | 0.52 ± 0.03 | - | - | - | - | - | - |
| 2 | ß-myrcene | 123-35-3 | MS, RI, STD | 987 | 988 | - | - | - | - | 1.00 ± 0.14 | - | - |
| 3 | citronellal | 106-23-0 | MS, RI, STD | 1153 | 1153 | - | - | - | - | 1.50 ± 0.08 | - | - |
| 4 | citronellol | 106-22-9 | MS, RI, STD | 1225 | 1227 | - | - | - | - | 1.60 ± 0.08 | - | - |
| 5 | geraniol | 106-24-1 | MS, RI, STD | 1250 | 1253 | - | - | - | - | 1.50 ± 0.14 | - | - |
| 6 | thymol | 89-83-8 | MS, RI, STD | 1286 | 1291 | - | 5.10 ± 0.36 | 0.50 ± 0.08 | - | 1.30 ± 0.05 | - | 4.10 ± 0.50 |
| 7 | ß-elemene | 515-13-9 | MS, RI | 1388 | 1394 | - | 0.60 ± 0.15 | - | - | 1.30 ± 0.03 | - | - |
| 8 | α-bergamotene | 17699-05-7 | MS, RI | 1415 | 1417 | 4.43 ± 0.10 | 0.50 ± 0.27 | 3.20 ± 0.69 | 1.60 ± 1.12 | 0.70 ± 0.01 | 1.50 ± 0.56 | 4.80 ± 0.60 |
| 9 | β-caryophyllene | 87-44-5 | MS, RI, STD | 1419 | 1423 | 9.51 ± 0.37 | 13.20 ± 0.33 | 8.50 ± 0.35 | 2.10 ± 0.13 | 8.10 ± 0.08 | 4.10 ± 0.79 | 1.80 ± 0.23 |
| 10 | γ-elemene | 3242-08-8 | MS, RI | 1432 | 1435 | - | 0.60 ± 0.21 | 0.10 ± 0.01 | - | - | - | - |
| 11 | geranylacetone | 3796-70-1 | MS, RI | 1455 | 1453 | - | 1.10 ± 0.51 | 0.50 ± 0.19 | - | 0.40 ± 0.02 | 0.30 ± 0.06 | - |
| 12 | α-humulene | 6753-98-6 | MS, RI, STD | 1456 | 1457 | 12.73 ± 1.41 | 4.30 ± 1.09 | 7.70 ± 0.62 | 6.20 ± 0.94 | 7.40 ± 0.11 | 8.10 ± 1.01 | 6.30 ± 0.03 |
| 13 | α-curcumene | 644-30-4 | MS, RI | 1482 | 1484 | 0.83 ± 0.18 | - | 0.70 ± 0.10 | 0.30 ± 0.11 | - | 0.40 ± 0.13 | 1.30 ± 0.08 |
| 14 | ß-selinene | 17066-67-0 | MS, RI | 1483 | 1490 | - | 0.30 ± 0.14 | 0.10 ± 0.01 | - | 0.30 ± 0.01 | - | 0.25 ± 0.33 |
| 15 | tridecan-2-one | 593-08-8 | MS, RI, STD | 1487 | 1495 | 45.26 ± 0.96 | 56.30 ± 0.31 | 51.4 ± 1.15 | 78.80 ± 0.55 | 54.40 ± 0.56 | 70.2 ± 0.95 | 71.36 ± 0.70 |
| 16 | tridecan-2-ol | 1653-31-2 | MS, RI, STD | 1495 | 1501 | 2.23 ± 0.17 | 10.10 ± 0.61 | 6.25 ± 0.17 | 4.30 ± 1.43 | 5.70 ± 0.20 | 6.40 ± 0.04 | 4.20 ± 0.40 |
| 17 | (3E,6E)-α-farnesene | 502-61-4 | MS, RI | 1503 | 1509 | 3.07 ± 0.28 | 1.20 ± 0.03 | 1.70 ± 0.04 | 2.70 ± 0.12 | 2.50 ± 0.09 | 2.30 ± 0.21 | 1.70 ± 0.03 |
| 18 | ß-bisabolene | 495-61-4 | MS, RI | 1505 | 1511 | 1.30 ± 0.05 | - | 1.20 ± 0.24 | 0.60 ± 0.37 | - | 0.50 ± 0.06 | 1.37 ± 0.20 |
| 19 | γ-cadinene | 39029-4-9 | MS, RI | 1513 | 1517 | - | - | 0.20 ± 0.02 | - | 0.60 ± 0.08 | - | 0.2 ± 0.08 |
| 20 | δ-cadinene | 483-76-1 | MS, RI | 1524 | 1526 | - | 0.20 ± 0.04 | 0.30 ± 0.04 | 0.10 ± 0.03 | 1.20 ± 0.05 | - | 0.38 ± 0.23 |
| 21 | elemol | 639-99-6 | MS, RI, STD | 1547 | 1552 | - | 0.30 ± 0.15 | - | - | 2.90 ± 0.07 | - | 0.31 ± 0.30 |
| 22 | nerolidol | 7212-44-4 | MS, RI, STD | 1557 | 1564 | 3.13 ± 0.27 | 0.40 ± 0.16 | 1.80 ± 0.63 | 0.40 ± 0.11 | 0.70 ± 0.03 | 1.20 ± 0.35 | 0.60 ± 0.03 |
| 23 | caryophyllene oxide | 1139-30-6 | MS, RI, STD | 1583 | 1588 | - | 0.20 ± 0.11 | 0.30 ± 0.00 | - | - | - | - |
| 24 | ζ-cadinol | 5937-11-1 | MS, RI | 1639 | 1645 | - | - | - | - | 0.30 ± 0.02 | - | - |
| 25 | α-cadinol | 481-34-5 | MS, RI | 1650 | 1659 | - | - | - | - | 0.50 ± 0.03 | - | - |
| 26 | pentadecan-2-one | 2345-28-0 | MS, RI, STD | 1696 | 1697 | 0.48 ± 0.02 | 0.50 ± 0.23 | 0.70 ± 0.02 | - | - | - | - |
| 27 | (E,E)-farnesol | 106-28-5 | MS, RI | 1722 | 1723 | 12.5 ± 0.85 | 3.00 ± 0.96 | 11.1 ± 0.15 | 1.40 ± 0.90 | 2.20 ± 0.45 | 1.90 ± 0.28 | - |
| 28 | farnesal | 19317-11-4 | MS, RI | 1738 | 1744 | 1.36 ± 0.02 | 0.40 ± 0.15 | 1.20 ± 0.05 | - | 0.30 ± 0.07 | 0.20 ± 0.04 | - |
| 29 | methyl farnesoate | 3675-00-1 | MS, RI | 1779 | 1785 | 1.90 ± 0.10 | 1.00 ± 0.35 | 1.60 ± 0.17 | - | 0.90 ± 0.08 | 0.70 ± 0.14 | - |
| Monoterpene hydrocarbons (%) | 0.52 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |||||
| Oxygenated monoterpene (%) | 0.00 | 5.30 | 0.50 | 0.00 | 5.90 | 0.00 | 4.10 | |||||
| Sesquiterpene hydrocarbons (%) | 31.87 | 21.80 | 24.20 | 13.60 | 22.20 | 16.90 | 18.20 | |||||
| Oxygenated sesquiterpenes (%) | 19.37 | 5.80 | 16.70 | 1.80 | 8.20 | 4.30 | 1.10 | |||||
| Others (%) | 47.46 | 66.40 | 57.65 | 83.10 | 62.10 | 77.10 | 75.60 | |||||
| Identified compounds (%) | 99.22 | 99.30 | 99.05 | 98.50 | 99.40 | 98.30 | 99.00 | |||||
| Yield (%) | 0.88 | 0.91 | 1.18 | 1.20 | 0.86 | 0.89 | 0.86 | |||||
| Fruits | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | Cas Number | Identification | Ria | Rib | July | Aug | Sep | Oct | Nov |
| 1 | α-pinene | 80-56-8 | MS, RI, STD | 931 | 929 | 2.30 ± 0.90 | 0.90 ± 0.12 | 0.80 ± 0.04 | 1.90 ± 0.66 | 0.60 ± 0.13 |
| 2 | ß-myrcene | 123-35-3 | MS, RI, STD | 987 | 988 | 16.40 ± 0.91 | 44.80 ± 0.13 | 46.30 ± 0.10 | 48.27 ± 0.26 | 24.7 ± 0.97 |
| 3 | α-terpinene | 99-86-5 | MS, RI, STD | 1008 | 1017 | - | - | - | - | 0.30 ± 0.06 |
| 4 | p-cymene | 25155-15-1 | MS, RI, STD | 1022 | 1022 | 0.20 ± 0.08 | 0.70 ± 0.09 | 0.40 ± 0.04 | 0.30 ± 0.05 | 0.80 ± 0.04 |
| 5 | limonene | 138-86-3 | MS, RI, STD | 1023 | 1027 | 1.10 ± 0.21 | 2.60 ± 0.29 | 2.00 ± 0.07 | 2.20 ± 0.16 | 6.00 ± 0.42 |
| 6 | (E)-ß-ocimene | 13877-91-3 | MS, RI, STD | 1041 | 1046 | 8.30 ± 0.29 | 2.00 ± 0.12 | 1.50 ± 0.08 | 1.50 ± 0.06 | 2.0 ± 0.15 |
| 7 | γ-terpinene | 99-85-4 | MS, RI, STD | 1060 | 1057 | - | - | - | - | 0.20 ± 0.01 |
| 8 | linalool | 78-70-6 | MS, RI, STD | 1094 | 1098 | - | 2.50 ± 0.27 | 3.40 ± 0.12 | 2.10 ± 0.10 | 4.20 ± 0.6 |
| 9 | perillene | 539-52-6 | MS, RI, STD | 1094 | 1100 | 6.50 ± 0.12 | - | - | - | - |
| 10 | alloocimene | 673-84-7 | MS, RI | 1128 | 1129 | 0.50 ± 0.07 | 0.50 ± 0.05 | 0.50 ± 0.04 | 0.40 ± 0.05 | 0.30 ± 0.02 |
| 11 | isopulegol | 7786-67-6 | MS, RI | 1140 | 1145 | - | - | - | - | 0.60 ± 0.15 |
| 12 | citronellal | 106-23-0 | MS, RI, STD | 1153 | 1153 | 0.20 ± 0.01 | 1.00 ± 0.11 | 1.40 ± 0.13 | 0.90 ± 0.12 | 5.70 ± 0.43 |
| 13 | limonene oxide | 1195-92-2 | MS, RI | 1175 | 1182 | - | - | 0.90 ± 0.01 | - | 0.90 ± 0.24 |
| 14 | terpineol | 98-55-5 | MS, RI, STD | 1190 | 1191 | - | - | - | - | 0.20 ± 0.05 |
| 15 | decanal | 112-31-2 | MS, RI, STD | 1202 | 1204 | 8.30 ± 0.17 | 1.80 ± 0.18 | 2.20 ± 0.02 | 1.60 ± 0.14 | 0.70 ± 0.24 |
| 16 | citronellol | 106-22-9 | MS, RI, STD | 1225 | 1227 | 1.90 ± 0.02 | 6.20 ± 0.55 | 6.60 ± 0.14 | 6.30 ± 0.63 | 28.24 ± 0.10 |
| 17 | geraniol | 106-24-1 | MS, RI, STD | 1250 | 1253 | 1.20 ± 0.03 | 5.50 ± 0.59 | 6.20 ± 0.22 | 6.30 ± 0.51 | 2.33 ± 0.74 |
| 18 | decyl alcohol | 112-30-1 | MS, RI, STD | 1262 | 1263 | 4.50 ± 0.18 | - | - | - | - |
| 19 | geranial | 141-27-5 | MS, RI, STD | 1268 | 1270 | - | 5.30 ± 0.53 | 7.60 ± 0.12 | 5.30 ± 0.31 | 12.50 ± 0.47 |
| 20 | undecan-2-one | 112-12-9 | MS, RI, STD | 1288 | 1293 | 0.20 ±0.03 | - | - | - | - |
| 21 | undecanal | 112-44-7 | MS, RI, STD | 1305 | 1306 | 0.20 ± 0.02 | - | - | - | - |
| 22 | methyl nerate | 1862-61-9 | MS, RI, STD | 1319 | 1323 | 6.70 ± 0.23 | 5.7 ± 0.6 | 4.40 ± 0.15 | 5.30 ± 0.13 | 6.10 ± 0.50 |
| 23 | δ-elemene | 20307-84-0 | MS, RI | 1334 | 1339 | - | - | - | 0.10 ± 0.02 | - |
| 24 | α-cubebene | 17699-14-8 | MS, RI, STD | 1349 | 1351 | - | 0.20 ± 0.01 | 0.10 ± 0.00 | 0.10 ± 0.01 | - |
| 25 | α-copaene | 3856-25-5 | MS, RI, STD | 1376 | 1378 | 0.60 ± 0.01 | 0.20 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.02 | - |
| 26 | ß-elemene | 515-13-9 | MS, RI | 1388 | 1394 | 1.20 ± 0.04 | 2.10 ± 0.21 | 1.50 ± 0.01 | 1.80 ± 0.07 | 0.20 ± 0.1 |
| 27 | β-caryophyllene | 87-44-5 | MS, RI, STD | 1419 | 1423 | 5.80 ± 0.18 | 4.90 ± 0.55 | 4.10 ± 0.03 | 3.50 ± 0.06 | 0.50 ± 0.23 |
| 28 | cadina-4(14),5-diene | 54324-03-7 | MS, RI | 1430 | 1433 | 0.80 ± 0.06 | 1.80 ± 0.20 | 1.60 ± 0.03 | 1.30 ± 0.07 | - |
| 29 | γ-elemene | 3242-08-8 | MS, RI | 1432 | 1435 | 0.40 ± 0.02 | 1.10 ± 0.11 | 0.90 ± 0.01 | 0.90 ± 0.05 | - |
| 30 | α-humulene | 6753-98-6 | MS, RI, STD | 1456 | 1457 | 3.70 ± 0.21 | 1.90 ± 0.21 | 1.60 ± 0.01 | 1.50 ± 0.07 | 0.20 ± 0.09 |
| 31 | alloaromadendrene | 25246-27-9 | MS, RI | 1457 | 1465 | 0.90 ± 0.04 | 0.30 ± 0.03 | 0.10 ± 0.01 | 0.20 ± 0.08 | - |
| 32 | germacrene D | 23986-74-5 | MS, RI, STD | 1482 | 1485 | 0.50 ± 0.04 | 0.80 ± 0.10 | 1.10 ± 0.12 | 1.40 ± 0.18 | 0.20 ± 0.1 |
| 33 | ß-selinene | 17066-67-0 | MS, RI | 1483 | 1490 | - | - | 0.10 ± 0.02 | 0.10 ± 0.04 | - |
| 34 | α-selinene | 473-13-2 | MS, RI | 1488 | 1498 | - | - | 0.40 ± 0.01 | 0.40 ± 0.09 | - |
| 35 | γ-cadinene | 39029-4-9 | MS, RI | 1513 | 1517 | 0.90 ± 0.06 | 1.10 ± 0.08 | 0.70 ± 0.01 | 0.80 ± 0.05 | 0.20 ± 0.07 |
| 36 | δ-cadinene | 483-76-1 | MS, RI | 1524 | 1526 | 2.70 ± 0.14 | 3.30 ± 0.35 | 2.20 ± 0.02 | 2.70 ± 0.05 | 0.60 ± 0.15 |
| 37 | α-calacorene | 21391-99-1 | MS, RI | 1542 | 1547 | 0.50 ± 0.01 | - | - | - | - |
| 38 | elemol | 639-99-6 | MS, RI, STD | 1547 | 1552 | 0.10 ± 0.02 | 0.40 ± 0.04 | 0.20 ± 0.02 | - | - |
| 39 | spathulenol | 6750-60-3 | MS, RI | 1578 | 1582 | 5.20 ± 0.06 | - | - | - | - |
| 40 | caryophyllene oxide | 1139-30-6 | MS, RI, STD | 1583 | 1588 | 9.60 ± 0.29 | - | - | - | - |
| 41 | ζ-cadinol | 5937-11-1 | MS, RI | 1639 | 1645 | 1.20 ± 0.31 | 0.30 ± 0.06 | 0.10 ± 0.01 | 0.40 ± 0.04 | - |
| 42 | α-cadinol | 481-34-5 | MS, RI | 1650 | 1659 | 1.80 ± 0.24 | 0.50 ± 0.05 | - | 0.70 ± 0.08 | - |
| 43 | cadalene | 483-78-3 | MS, RI | 1678 | 1679 | - | - | - | 0.10 ± 0.02 | - |
| Monoterpene hydrocarbons (%) | 28.80 | 51.50 | 51.50 | 54.17 | 34.90 | |||||
| Oxygenated monoterpene (%) | 9.80 | 20.50 | 26.10 | 21.30 | 54.67 | |||||
| Sesquiterpene hydrocarbons (%) | 18.10 | 17.70 | 14.60 | 15.10 | 1.90 | |||||
| Oxygenated sesquiterpenes (%) | 25.10 | 6.90 | 4.70 | 6.90 | 6.10 | |||||
| Others (%) | 17.50 | 1.80 | 2.20 | 1.60 | 0.70 | |||||
| Identified compounds (%) | 99.30 | 98.40 | 99.10 | 99.07 | 98.27 | |||||
| Yield (%) | 1.42 | 1.51 | 1.22 | 1.13 | 1.14 | |||||
| Organs and Standards | Biological Activities IC50 (µL/mL) | |||
|---|---|---|---|---|
| DPPH | LOX Denaturation | BSA Denaturation | Anti-Plasmodial | |
| Leaves | 33.12 ± 0.07 | 26.26 ± 0.04 | 26.08 ± 0.12 | 62.3 ± 3.4 |
| Trunk Bark | 65.68 ± 0.12 | 28.40 ± 0.02 | 35.07 ± 0.15 | 36.29 ± 4.2 |
| Fruits | 103.55 ± 0.35 | 32.42 ± 0.15 | 26.68 ± 0.09 | >100 |
| Trolox | 28.13 ± 0.04 | |||
| Quercetin | 21.57 ± 0.10 | |||
| Diclofenac | 21.90 ± 0.08 | |||
| Artemisinin | 0.004 ± 0.001 | |||
| Tested Substances | Average Repulsion (%) | Class | Effect of Substance Tested |
|---|---|---|---|
| Leaf essential oil | 76.66 | IV | Repulsive |
| Trunk bark essential oil | 88.83 | V | Highly repulsive |
| Fruit essential oil | 61.00 | III | Mildly repulsive |
| Talisma UL | 24.78 | II | Weakly repulsive |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanoh, E.A.; Boué, G.B.; Nea, F.; Genva, M.; Wognin, E.L.; Ledoux, A.; Martin, H.; Tonzibo, Z.F.; Frederich, M.; Fauconnier, M.-L. Seasonal Effect on the Chemical Composition, Insecticidal Properties and Other Biological Activities of Zanthoxylum leprieurii Guill. & Perr. Essential Oils. Foods 2020, 9, 550. https://doi.org/10.3390/foods9050550
Tanoh EA, Boué GB, Nea F, Genva M, Wognin EL, Ledoux A, Martin H, Tonzibo ZF, Frederich M, Fauconnier M-L. Seasonal Effect on the Chemical Composition, Insecticidal Properties and Other Biological Activities of Zanthoxylum leprieurii Guill. & Perr. Essential Oils. Foods. 2020; 9(5):550. https://doi.org/10.3390/foods9050550
Chicago/Turabian StyleTanoh, Evelyne Amenan, Guy Blanchard Boué, Fatimata Nea, Manon Genva, Esse Leon Wognin, Allison Ledoux, Henri Martin, Zanahi Felix Tonzibo, Michel Frederich, and Marie-Laure Fauconnier. 2020. "Seasonal Effect on the Chemical Composition, Insecticidal Properties and Other Biological Activities of Zanthoxylum leprieurii Guill. & Perr. Essential Oils" Foods 9, no. 5: 550. https://doi.org/10.3390/foods9050550
APA StyleTanoh, E. A., Boué, G. B., Nea, F., Genva, M., Wognin, E. L., Ledoux, A., Martin, H., Tonzibo, Z. F., Frederich, M., & Fauconnier, M.-L. (2020). Seasonal Effect on the Chemical Composition, Insecticidal Properties and Other Biological Activities of Zanthoxylum leprieurii Guill. & Perr. Essential Oils. Foods, 9(5), 550. https://doi.org/10.3390/foods9050550