Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Raw Materials
2.2. Preparation of Faba Bean Protein Ingredients
2.2.1. Faba Bean Protein-Rich Flour
2.2.2. Faba Bean Protein Isolate
2.3. Compositional Analysis
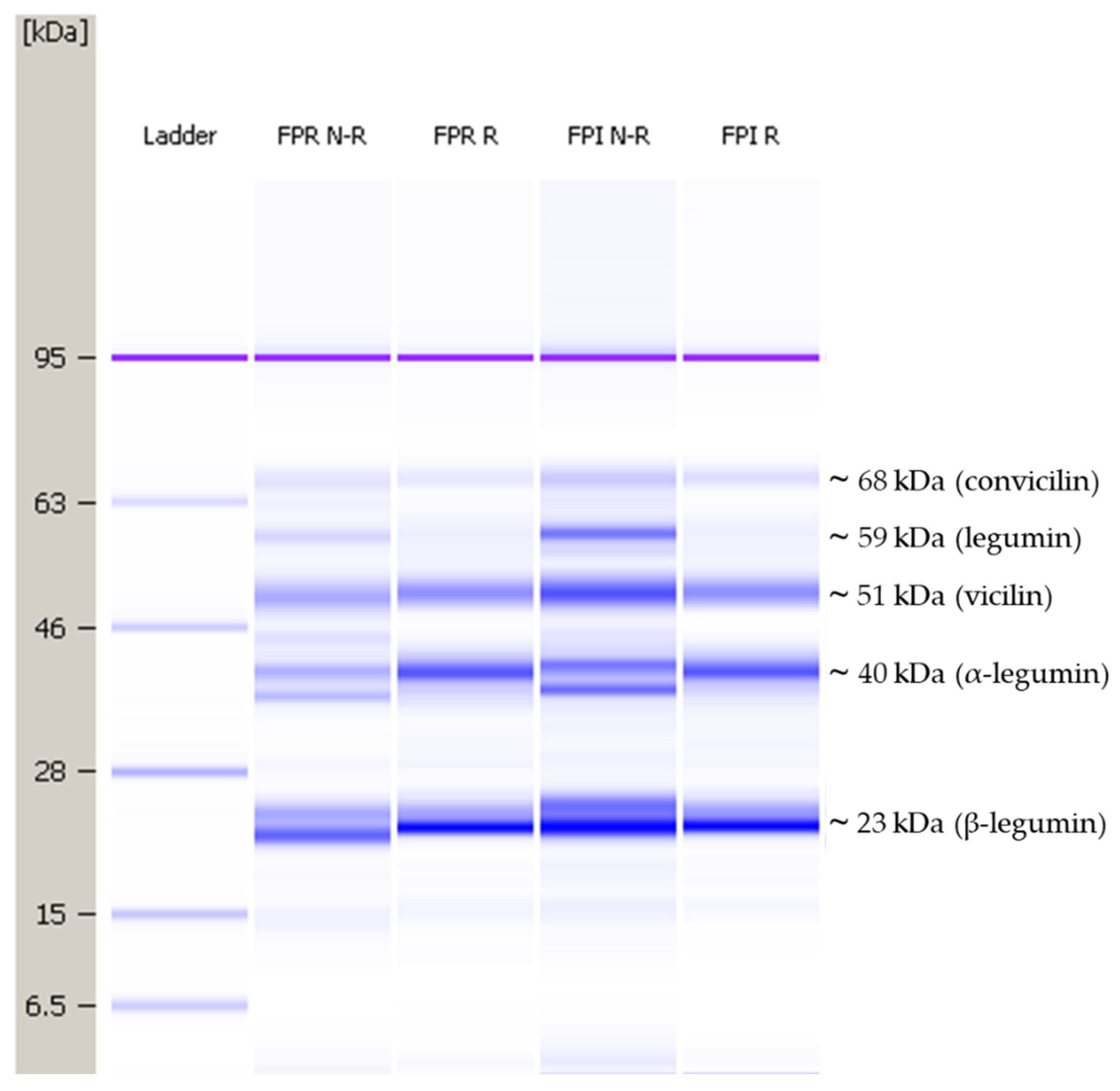
2.4. Protein Profile Analysis
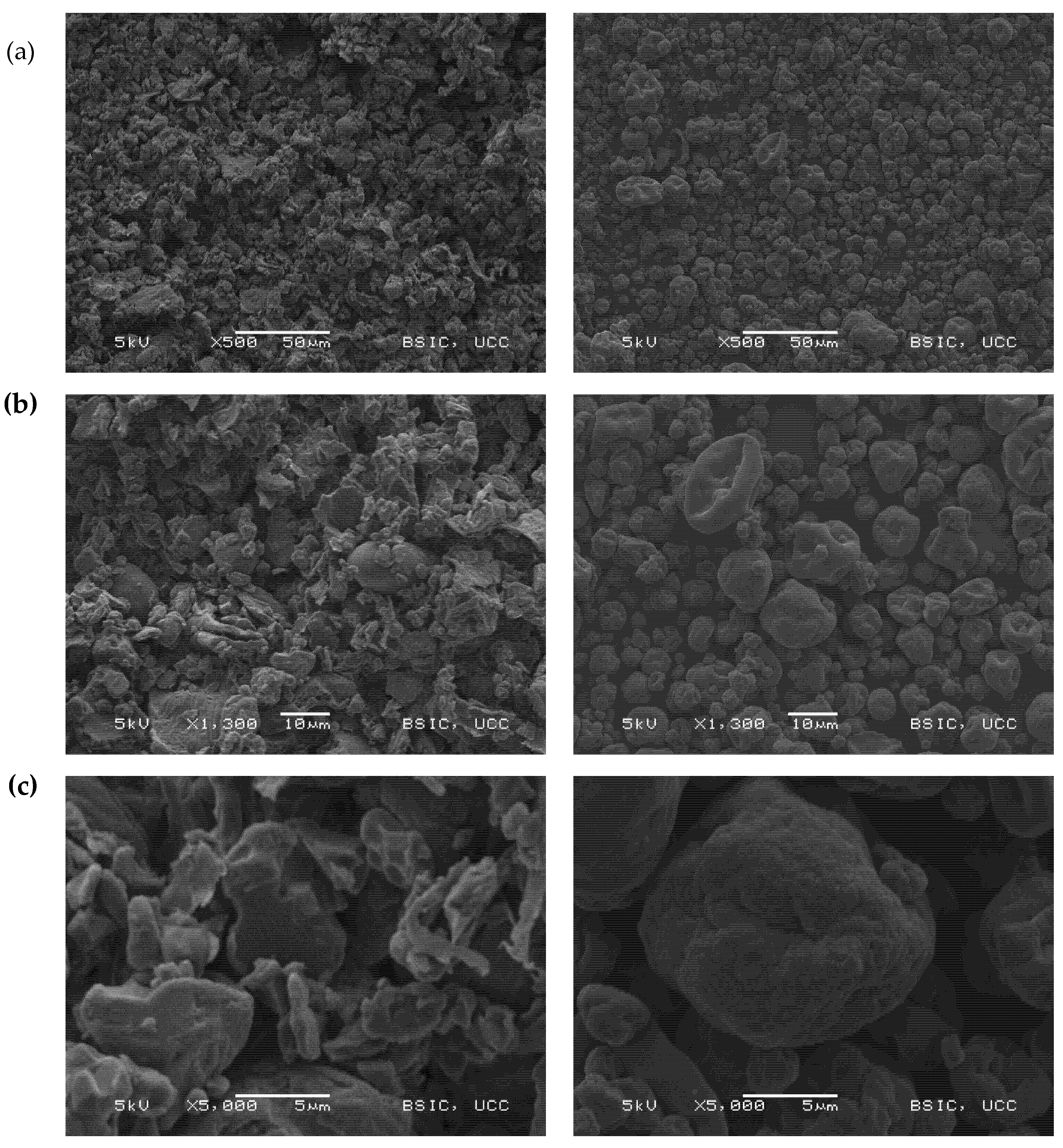
2.5. Scanning Electron Microscopy
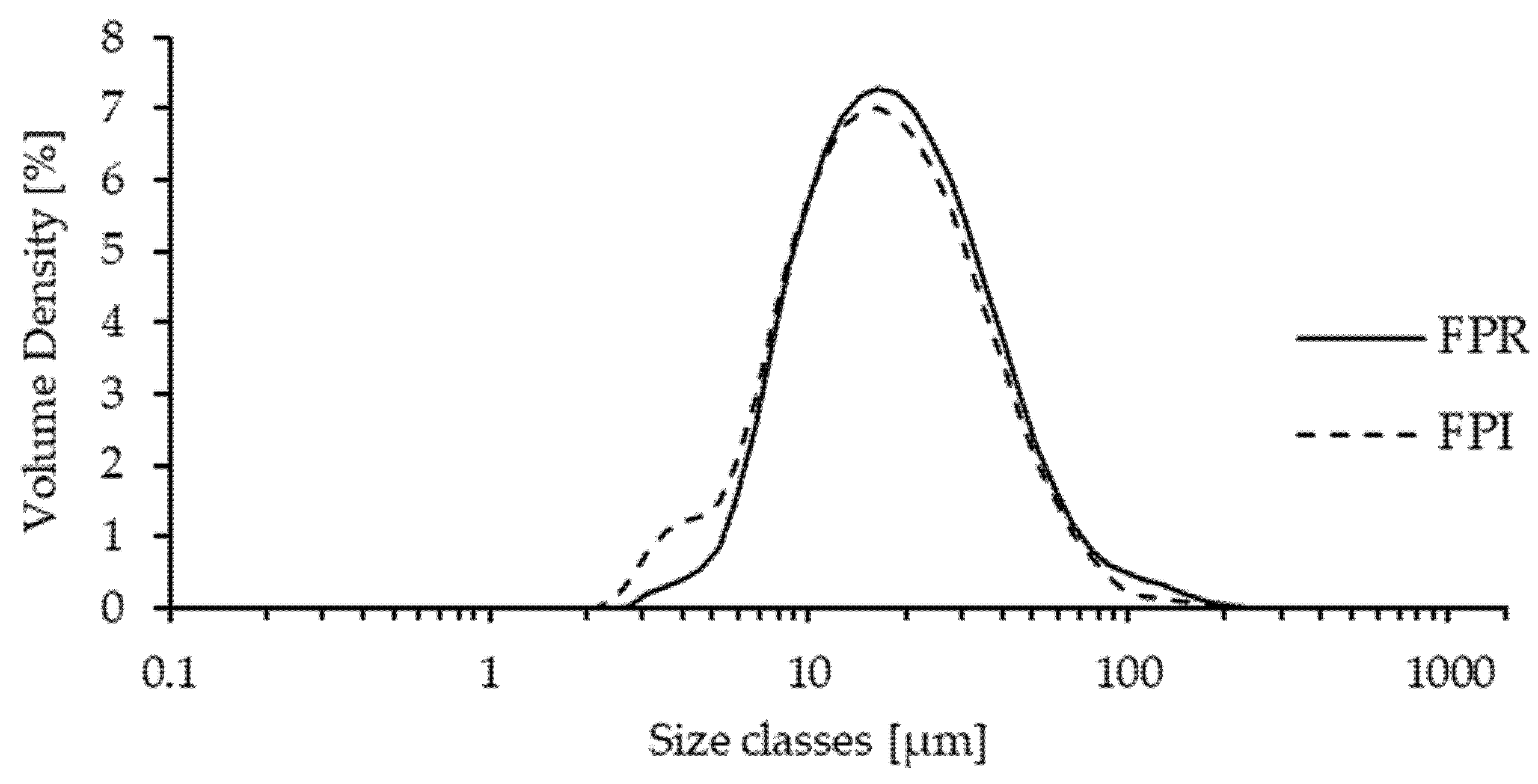
2.6. Particle Size Distribution
2.7. Surface Hydrophobicity
2.8. Protein Solubility
2.9. Zeta Potential
2.10. Fat Absorption Capacity
2.11. Foaming Properties
2.12. Minimum Gelling Concentration
2.13. Rheological Analysis of Heat Gelation Properties
2.14. In Vitro Protein Digestibility
2.15. Trypsin Inhibitor Activity (TIA) Assay
2.16. Vicine and Convicine Analysis
2.17. Quantification of Fermentable Oligo-, Di- and Monosaccharides, and Polyols (FODMAPs)
2.18. Life Cycle Assessment
2.19. Statistical Data Analysis
3. Results and Discussion
3.1. Compositional Analysis
3.2. Structural and Surface Properties
3.2.1. Protein Profile
3.2.2. Scanning Electron Microscopy
3.2.3. Particle Size Distribution of Dispersions
3.2.4. Surface Hydrophobicity
3.3. Techno-Functional Properties
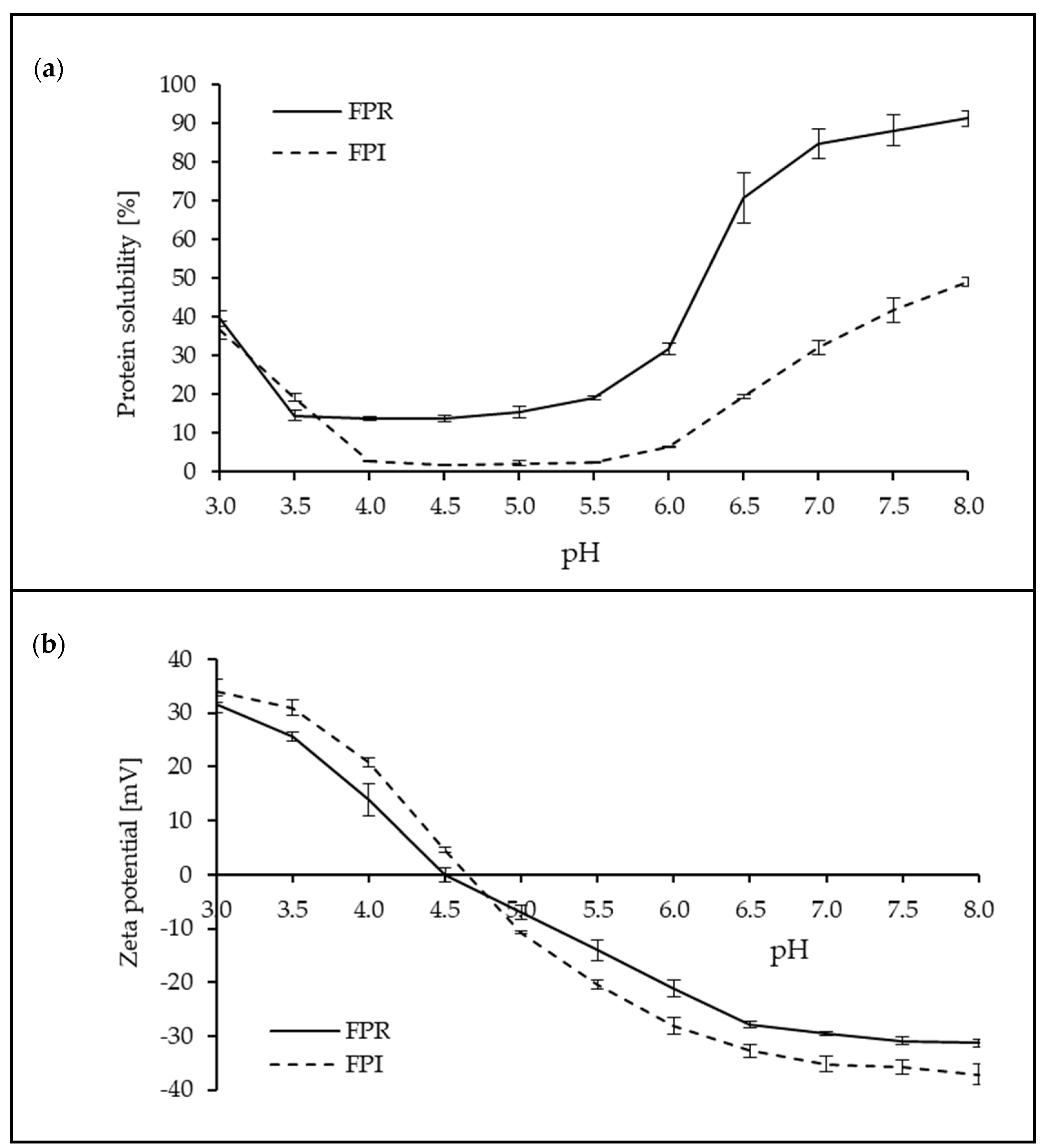
3.3.1. Protein Solubility and Zeta Potential
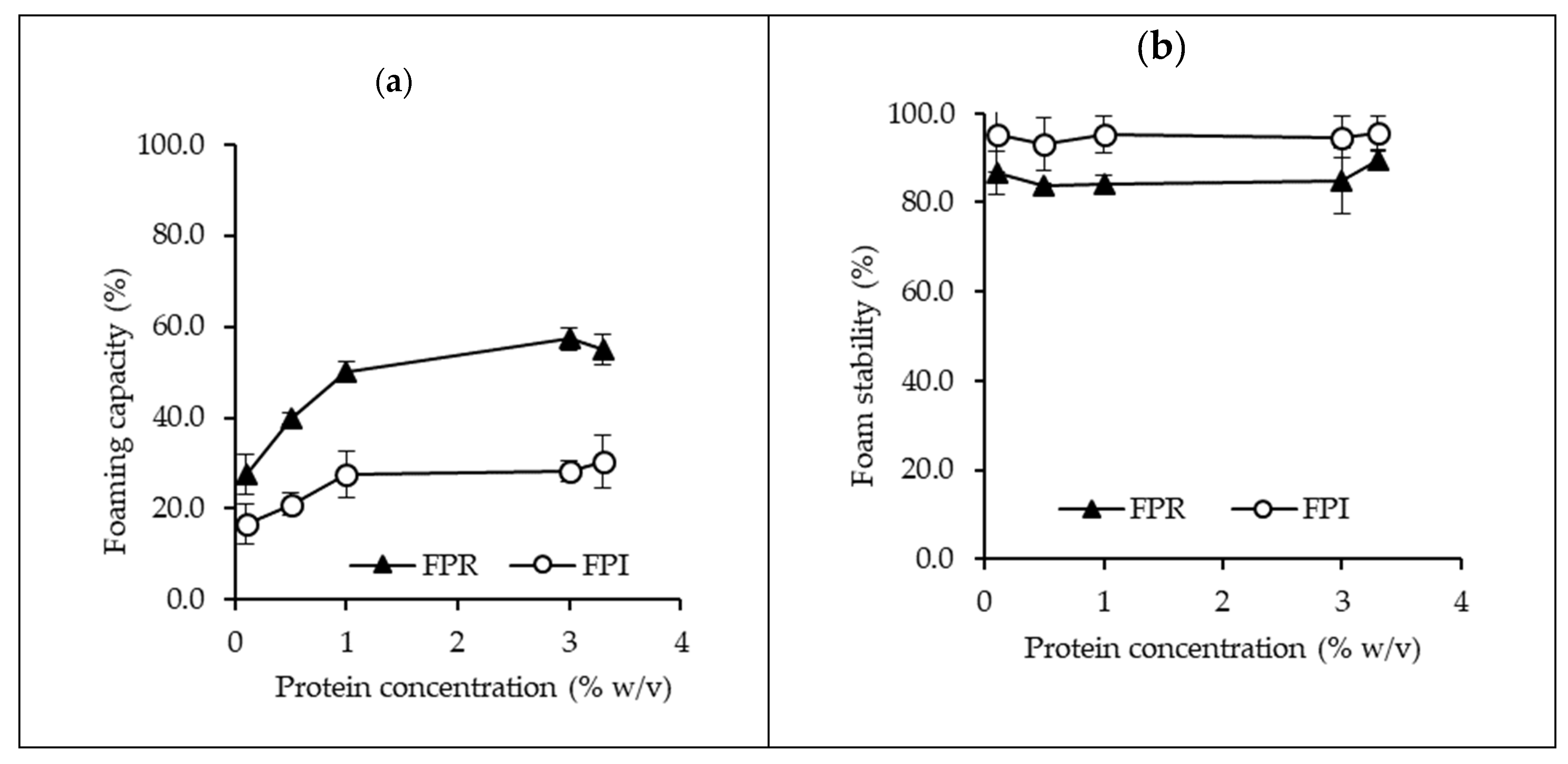
3.3.2. Foaming Properties
3.3.3. Fat Absorption Capacity
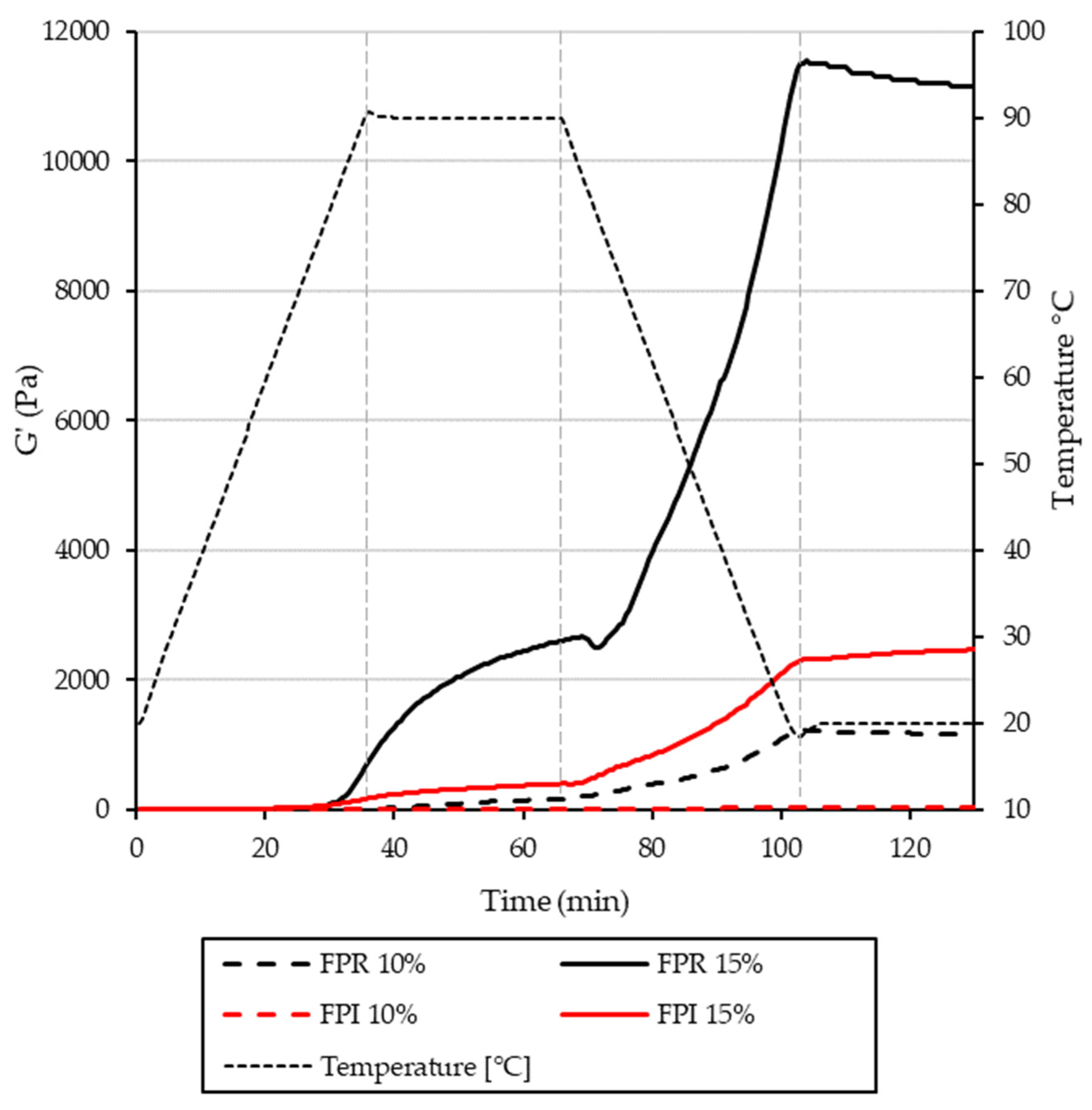
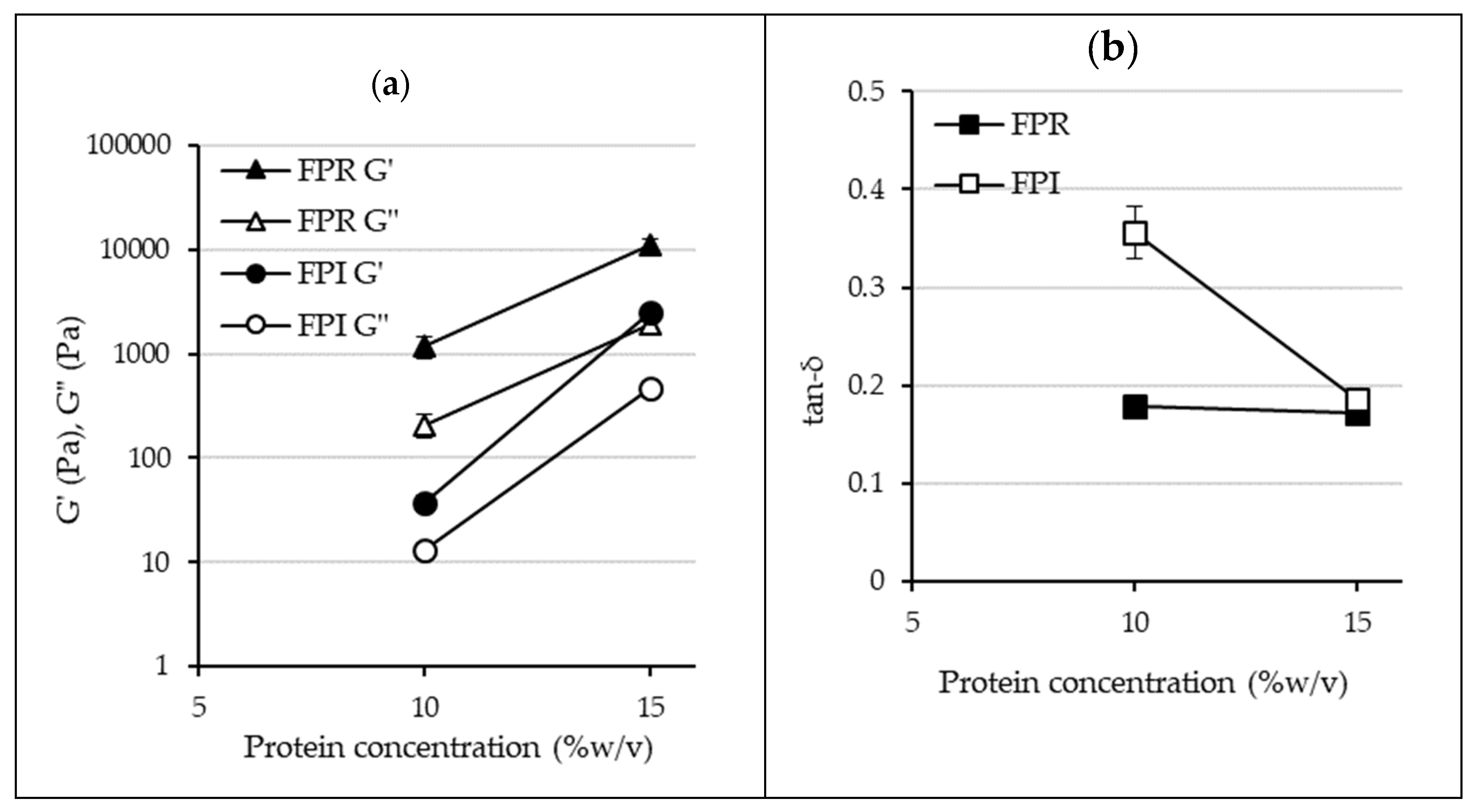
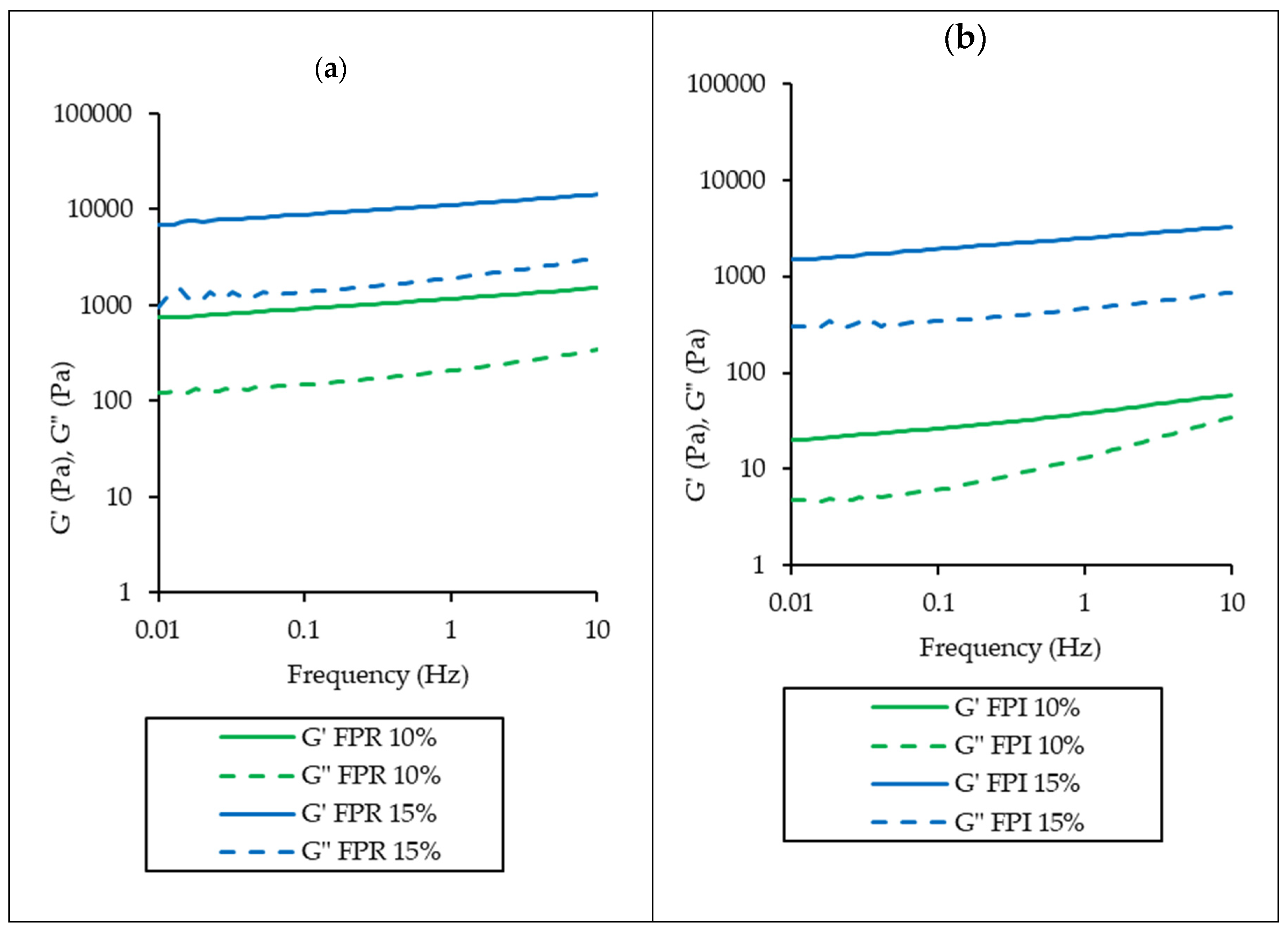
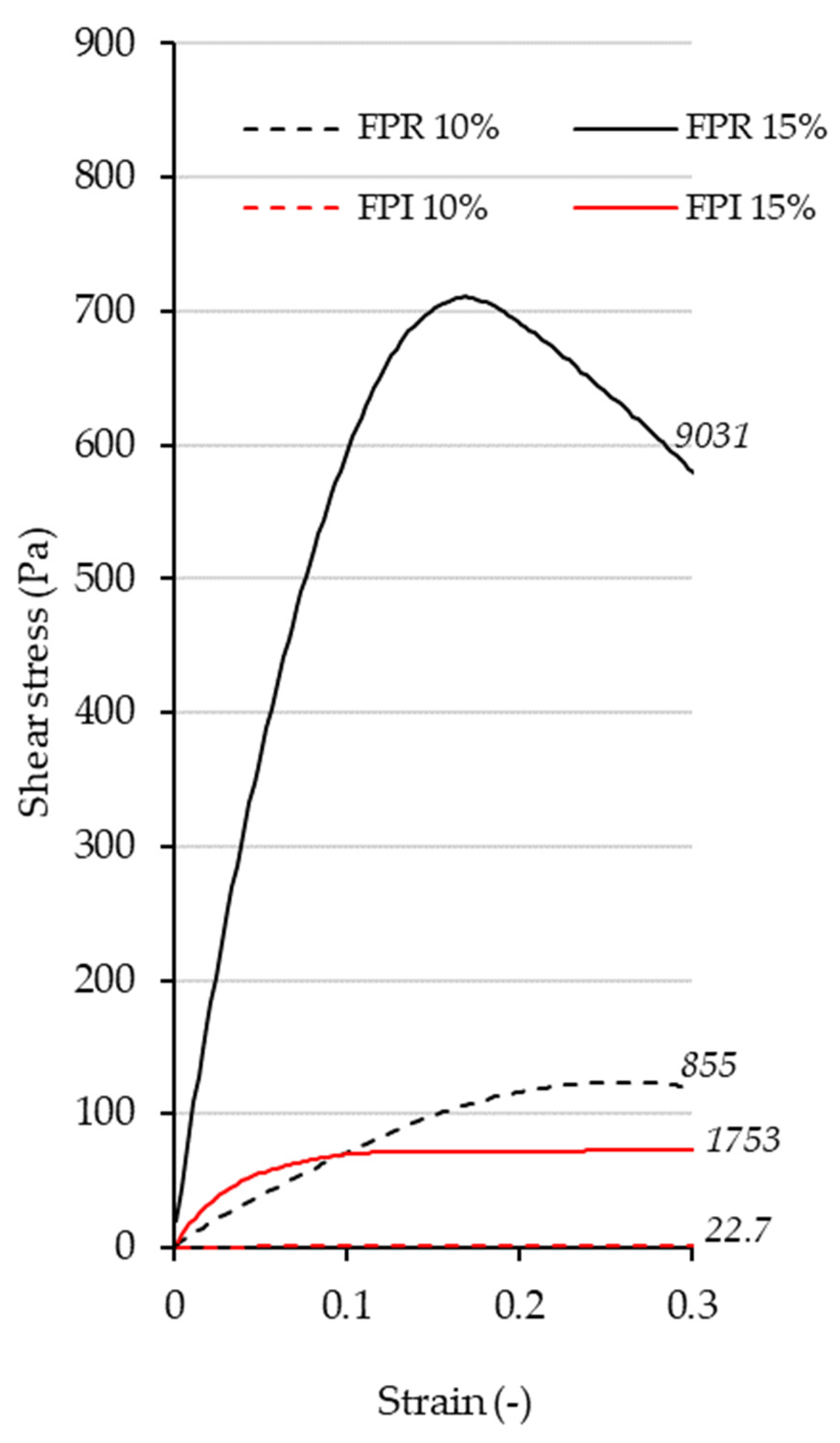
3.3.4. Gelation Properties
3.4. Nutritional Properties
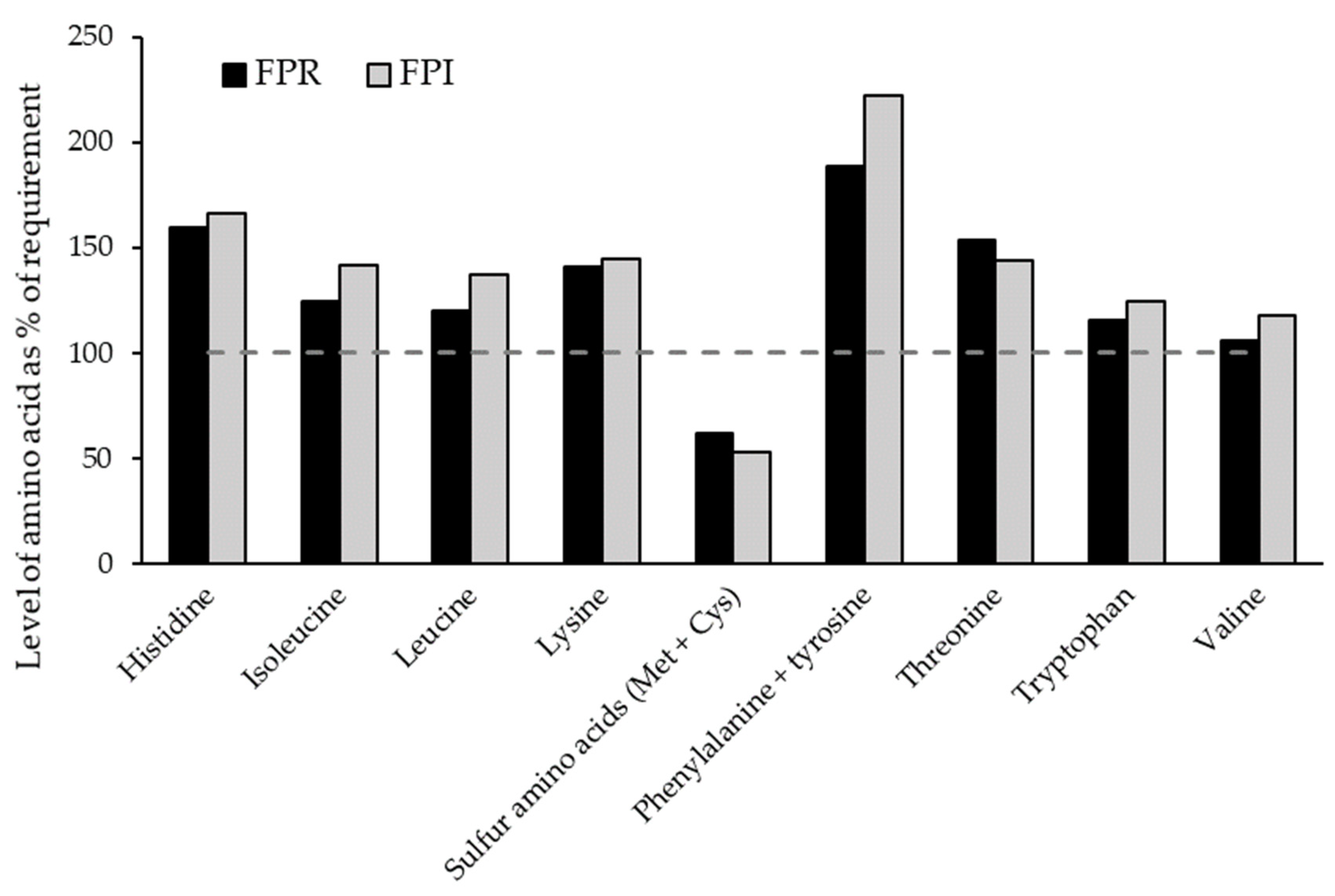
3.4.1. Amino Acid Profile
3.4.2. In Vitro Protein Digestibility and Trypsin Inhibitor Activity
3.4.3. Vicine and Convicine Analysis
3.4.4. FODMAP Analysis
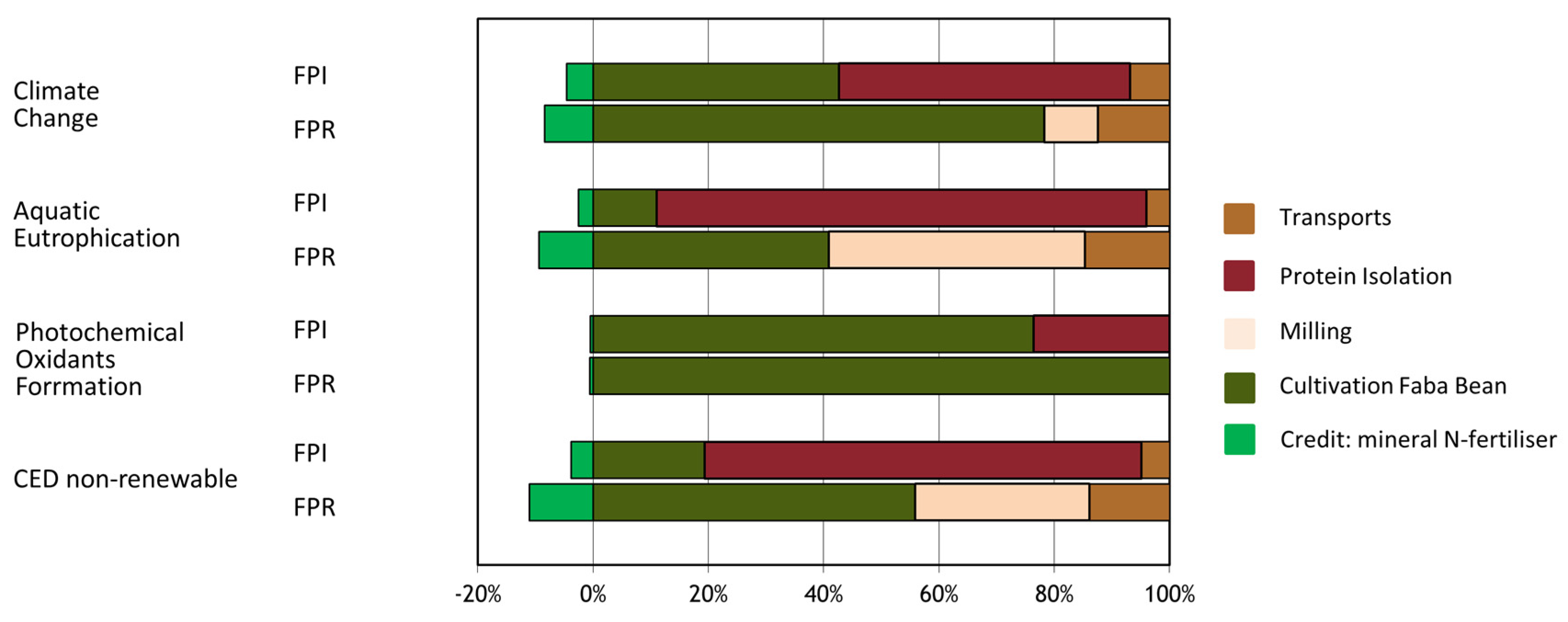
3.5. Life Cycle Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- UN Department of Economic and Social Affairs. World Population Prospects 2019 Highlights; UN Department of Economic and Social Affairs: New York, NY, USA, 2019; p. 1. [Google Scholar]
- Sozer, N.; Holopainen-Mantila, U.; Poutanen, K. Traditional and New Food Uses of Pulses. Cereal Chem. J. 2017, 94, 66–73. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Gueguen, J. Legume seed protein extraction, processing, and end product characteristics. Qual. Plant. Plant Foods Hum. Nutr. 1983, 32, 267–303. [Google Scholar] [CrossRef]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U.; et al. Eliminating vicine and convicine, the main anti-nutritional factors restricting faba bean usage. Trends Food Sci. Technol. 2019, 91, 549–556. [Google Scholar] [CrossRef]
- Bhatty, R.S.; Christison, G.I. Composition and nutritional quality of pea (Pisum sativum L.), faba bean (Vicia faba L. spp. minor) and lentil (Lens culinaris Medik.) meals, protein concentrates and isolates. Plant Foods Hum. Nutr. 1984, 34, 41–51. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J. Gastroenterol. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef] [Green Version]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Arntfield, S.D.; Maskus, H.D. Peas and other legume proteins. In Handbook of Food Proteins; Woodhead: Oxford, UK, 2011; pp. 233–266. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; van der Goot, A.J.; Boom, R.M. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Functional analysis of mildly refined fractions from yellow pea. Food Hydrocoll. 2015, 44, 12–22. [Google Scholar] [CrossRef]
- Felix, M.; Lopez-Osorio, A.; Romero, A.; Guerrero, A. Faba bean protein flour obtained by densification: A sustainable method to develop protein concentrates with food applications. LWT 2018, 93, 563–569. [Google Scholar] [CrossRef]
- Andersen, K.E.; Sørensen, H.; Sørensen, J.C.; Sørensen, A.D. A Process for the Manufacture of a Product from a Plant Material. Patent WO2012116703, 1 March 2012. [Google Scholar]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Jeske, S.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; Wriessnegger, C.L.; O’Mahony, J.A.; Zannini, E.; Arendt, E.K. Membrane filtration and isoelectric precipitation technological approaches for the preparation of novel, functional and sustainable protein isolate from lentils. Eur. Food Res. Technol. 2019. [Google Scholar] [CrossRef]
- Hayakawa, S.; Nakai, S. Relationships of Hydrophobicity and Net Charge to the Solubility of Milk and Soy Proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- O’Mahony, J.; McSweeney, P.L.H.; Lucey, J.A. Rheological properties of rennet-induced skim milk gels made from milk protein concentrate solutions with different ratios of αs-: β-casein. Milchwissenschaft 2009, 64, 135–138. [Google Scholar]
- Joehnke, M.S.; Lametsch, R.; Sorensen, J.C. Improved in vitro digestibility of rapeseed napin proteins in mixtures with bovine beta-lactoglobulin. Food Res. Int. 2019, 123, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Joehnke, M.S.; Rehder, A.; Sorensen, S.; Bjergegaard, C.; Sorensen, J.C.; Markedal, K.E. In Vitro Digestibility of Rapeseed and Bovine Whey Protein Mixtures. J. Agric. Food Chem. 2018, 66, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Petersen, I.L.; Hansen, H.C.B.; Ravn, H.W.; Sørensen, J.C.; Sørensen, H. Metabolic effects in rapeseed (Brassica napus L.) seedlings after root exposure to glyphosate. Pestic. Biochem. Physiol. 2007, 89, 220–229. [Google Scholar] [CrossRef]
- Bjergegaard, C.; Simonsen, H.; Sørensen, H. Determination of heterocyclic compounds by micellar electrokinetic capillary chromatography. J. Chromatogr. A 1994, 680, 561–569. [Google Scholar] [CrossRef]
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. Characterization of the FODMAP-profile in Cereal-product Ingredients. J. Cereal Sci. under review. [CrossRef]
- Detzel, A.; Kauertz, B.; Grahl, B.; Heinisch, J. Prüfung und Aktualisierung der Ökobilanzen für Getränkeverpackungen; Umweltbundesamt Publication Series 19/2016; Umweltbundesamt: Dessau-Roßlau, Gemany, 2016. [Google Scholar]
- Schutyser, M.A.I.; van der Goot, A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Technol. 2011, 22, 154–164. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef]
- Gunawardena, C.K.; Zijlstra, R.T.; Beltranena, E. Characterization of the nutritional value of air-classified protein and starch fractions of field pea and zero-tannin faba bean in grower pigs. J. Anim. Sci. 2010, 88, 660–670. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Stone, A.K.; Yovchev, A.G.; Peter, R.; Vandenberg, A.; Nickerson, M.T. Effect of genotype and environment on the surface characteristics and functionality of air-classified faba bean protein concentrates. Eur. Food Res. Technol. 2016, 242, 1903–1911. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Vissers, A.M.; Boom, R.M.; Schutyser, M.A.I. Dry fractionation for production of functional pea protein concentrates. Food Res. Int. 2013, 53, 232–239. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Berghout, J.A.M.; van der Goot, A.J.; Boom, R.M.; Schutyser, M.A.I. Preparation of functional lupine protein fractions by dry separation. LWT Food Sci. Technol. 2014, 59, 680–688. [Google Scholar] [CrossRef]
- Nivala, O.; Makinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring colloidal oat and faba bean protein particles via enzymatic modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintela, A.; Macarulla, M.T.; Del Barrio, A.S.; Martinez, J.A. Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Stone, A.K.; Vandenberg, A.; Tyler, R.; Nickerson, M.T. Effect of genotype on the physicochemical and functional attributes of faba bean (Vicia faba L.) protein isolates. Food Sci. Biotechnol. 2016, 25, 1513–1522. [Google Scholar] [CrossRef]
- Singhal, A.; Karaca, A.C.; Tyler, R.; Nickerson, M. Pulse Proteins: From Processing to Structure-Function Relationships. In Grain Legumes; IntechOpen: London, UK, 2016. [Google Scholar]
- Pelgrom, P.J.M.; Wang, J.; Boom, R.M.; Schutyser, M.A.I. Pre- and post-treatment enhance the protein enrichment from milling and air classification of legumes. J. Food Eng. 2015, 155, 53–61. [Google Scholar] [CrossRef]
- Kelly, G.M.; O’Mahony, J.A.; Kelly, A.L.; Huppertz, T.; Kennedy, D.; O’Callaghan, D.J. Influence of protein concentration on surface composition and physico-chemical properties of spray-dried milk protein concentrate powders. Int. Dairy J. 2015, 51, 34–40. [Google Scholar] [CrossRef]
- Crowley, S.V.; Desautel, B.; Gazi, I.; Kelly, A.L.; Huppertz, T.; O’Mahony, J.A. Rehydration characteristics of milk protein concentrate powders. J. Food Eng. 2015, 149, 105–113. [Google Scholar] [CrossRef]
- Schwenke, K.D. Reflections about the functional potential of legume proteins A Review. Nahr. Food 2001, 45, 377. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2016, 34, 126–147. [Google Scholar] [CrossRef]
- Jiang, Z.-Q.; Pulkkinen, M.; Wang, Y.-J.; Lampi, A.-M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Johnston, S.P.; Nickerson, M.T.; Low, N.H. The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. J. Food Sci. Technol. 2015, 52, 4135–4145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, L. Proteins from land plants – Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Eckert, E.; Han, J.; Swallow, K.; Tian, Z.; Jarpa-Parra, M.; Chen, L. Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem. 2019. [Google Scholar] [CrossRef]
- Makri, E.; Papalamprou, E.; Doxastakis, G. Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocoll. 2005, 19, 583–594. [Google Scholar] [CrossRef]
- Escamilla-Silva, E.M.; Guzmán-Maldonado, S.H.; Cano-Medinal, A.; González-Alatorre, G. Simplified process for the production of sesame protein concentrate. Differential scanning calorimetry and nutritional, physicochemical and functional properties. J. Sci. Food Agric. 2003, 83, 972–979. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Clemente, A.; Vioque, J.; Bautista, J.; Millán, F. Protein isolates from chickpea (Cicer arietinum L.): Chemical composition, functional properties and protein characterization. Food Chem. 1999, 64, 237–243. [Google Scholar] [CrossRef]
- Vioque, J.; Alaiz, M.; Giron-Calle, J. Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chem. 2012, 132, 67–72. [Google Scholar] [CrossRef]
- Nicolai, T.; Chassenieux, C. Heat-induced gelation of plant globulins. Curr. Opin. Food Sci. 2019, 27, 18–22. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Nicolai, T. Gelation of food protein-protein mixtures. Adv. Colloid Interface Sci. 2019, 270, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Renkema, J.M.; van Vliet, T. Heat-induced gel formation by soy proteins at neutral pH. J. Agric. Food Chem. 2002, 50, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.A.; Xiao, W.; van Boekel, M.; Minor, M.; Stieger, M. Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd). Food Chem. 2016, 209, 203–210. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A. Heat-induced gelation of pea legumin: Comparison with soybean glycinin. J. Agric. Food Chem. 2004, 52, 5071–5078. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Speroni, F.; Avanza, M.V. Rheological characterization of thermal gelation of cowpea protein isolates: Effect of processing conditions. LWT 2019, 109, 406–414. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Knabben, J.H.M.; van Vliet, T. Gel formation by β-conglycinin and glycinin and their mixtures. Food Hydrocoll. 2001, 15, 407–414. [Google Scholar] [CrossRef]
- Withana-Gamage, T.S.; Wanasundara, J.P.; Pietrasik, Z.; Shand, P.J. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): A comparative study with soy (Glycine max) and pea (Pisum sativum L.). J. Sci. Food Agric. 2011, 91, 1022–1031. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.; Moraes, I.C.F.; Pinho, S.C. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef]
- Batista, A.P.; Portugal, C.A.; Sousa, I.; Crespo, J.G.; Raymundo, A. Accessing gelling ability of vegetable proteins using rheological and fluorescence techniques. Int. J. Biol. Macromol. 2005, 36, 135–143. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Amino acid requirements of adults. In Protein and Amino Acid Requirements in Human Nutrition; Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition: Geneva, Switzerland, 2007; pp. 135–159. [Google Scholar]
- Arendt, E.K.; Zannini, E. Wheat and other Triticum grains. In Cereal Grains for the Food and Beverage Industries; Woodhead: Oxford, UK, 2013; pp. 1–67e. [Google Scholar] [CrossRef]
- Njoumi, S.; Josephe Amiot, M.; Rochette, I.; Bellagha, S.; Mouquet-Rivier, C. Soaking and cooking modify the alpha-galacto-oligosaccharide and dietary fibre content in five Mediterranean legumes. Int. J. Food Sci. Nutr. 2019, 70, 551–561. [Google Scholar] [CrossRef] [Green Version]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food composition, defining cutoff values and international application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Joehnke, M.S.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Sørensen, J.C.; Petersen, I.L. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing: FODMAP content, TIA level, and IVPD. Food Chem. under review.
| (g/100 g) 1 | FPR | FPI |
|---|---|---|
| Moisture | 12.2 | 6.11 |
| Protein | 64.1 | 90.1 |
| Fat | 2.43 | 4.36 |
| SFA | 0.40 | 0.77 |
| MUFA | 0.59 | 1.00 |
| PUFA | 1.33 | 2.38 |
| Ash | 4.8 | 5.2 |
| Total carbohydrate | 28.7 | 0.34 |
| Starch | 7.55 ± 0.235 | 2.48 ± 0.048 |
| Sodium | 0.072 | 0.465 |
| Particle Size Distribution (μm) | FPR | FPI |
|---|---|---|
| D[4,3] | 25.4 ± 3.11 a | 22.9 ± 2.63 a |
| D[3,2] | 16.1 ± 0.24 a | 13.9 ± 0.96 b |
| Dv(10) | 8.65 ± 0.12 a | 7.27 ± 0.53 b |
| Dv(50) | 19.3 ± 0.62 a | 17.8 ± 1.22 a |
| Dv(90) | 49.1 ± 7.80 a | 44.5 ± 4.80 a |
| Surface hydrophobicity (-) | 1208 ± 197 a | 2183 ± 370 b |
| Protein conc. | FPR | FPI | |
|---|---|---|---|
| 10% | Tg (G’>G") (°C) | 69.5 | 63.5 |
| Tg (0.5 Pa/°C) (°C) | 84.5 | Not applicable (N/A) 1 | |
| 15% | Tg (G’>G") (°C) | 52.5 | N/A 2 |
| Tg (0.5 Pa/°C) (°C) | 59.5 | 47.5 |
| Amino Acid | FPR | FPI |
|---|---|---|
| Indispensable and conditionally indispensable amino Acids | ||
| Histidine | 2.39 ± 0.29 | 2.49 ± 0.3 |
| Isoleucine | 3.73 ± 0.45 | 4.25 ± 0.52 |
| Leucine | 7.10 ± 0.86 | 8.09 ± 0.98 |
| Lysine | 6.34 ± 0.77 | 6.51 ± 0.79 |
| Methionine | 0.60 ± 0.06 | 0.54 ± 0.04 |
| Cysteine | 0.77 ± 0.06 | 0.62 ± 0.05 |
| Methionine + cysteine | 1.37 ± 0.12 | 1.16 ± 0.09 |
| Phenylalanine | 4.13 ± 0.5 | 4.68 ± 0.57 |
| Tyrosine | 3.05 ± 0.37 | 3.74 ± 0.45 |
| Phenylalanine + tyrosine | 7.18 ± 0.87 | 8.42 ± 1.02 |
| Threonine | 3.54 ± 0.43 | 3.30 ± 0.4 |
| Tryptophan | 0.69 ± 0.14 | 0.74 ± 0.12 |
| Valine | 4.14 ± 0.5 | 4.59 ± 0.56 |
| Dispensable amino acids | ||
| Aspartic acid | 10.30 ± 1.25 | 11.18 ± 1.36 |
| Glutamic acid | 16.25 ± 1.97 | 17.96 ± 2.18 |
| Alanine | 3.85 ± 0.47 | 3.94 ± 0.48 |
| Arginine | 10.48 ± 1.27 | 10.09 ± 1.22 |
| Glycine | 3.81 ± 0.46 | 4.02 ± 0.49 |
| Proline | 4.24 ± 0.52 | 4.45 ± 0.54 |
| Serine | 4.87 ± 0.59 | 5.36 ± 0.65 |
| IVPD (%) | TIA | |||||
|---|---|---|---|---|---|---|
| Pepsin | Pepsin + Pancreatin | |||||
1 h | Short-term 1 + 1 h | Medium-term 1 + 3 h | Long-term 1 + 24 h | TIU/mg sample DM | TIU/mg protein | |
| FDH | 5.4 ± 0.2 b | 22.2 ± 1.0 b | 25.1 ± 0.5 c | 32.9 ± 0.5 b | 1.42 ± 0.20 a | 4.33 ± 0.63 a |
| FPR | 5.6 ± 0.3 b | 23.1 ± 0.9 b | 26.8 ± 0.2 b | 33.9 ± 2.1 b | 2.34 ± 0.61 a | 3.77 ± 0.98 a |
| FPI | 6.4 ± 0.1 a | 26.2 ± 0.9 a | 29.9 ± 0.7 a | 39.2 ± 0.6 a | 0.29 ± 0.05 b | 0.33 ± 0.05 b |
| Ingredient | Vicine (mg/g DM) | Convicine (mg/g DM) | Total (mg/g sample DM) | Total (mg/g protein) |
|---|---|---|---|---|
| FDH | 8.71 ± 0.65 a | 5.20 ± 0.18 a | 13.91 ± 0.67 a | 37.89 ± 1.83 b |
| FPR | 7.97 ± 1.93 a | 4.58 ± 1.23 a | 12.54 ± 2.29 a | 18.64 ± 3.40 a |
| FPI | n.d. | n.d. | n.d. | n.d. |
| FODMAP Category | Carbohydrate | FDH | FPR * | FPI * |
|---|---|---|---|---|
| Mono-/Disaccharides b,c | Glucose | 0.06 ± 0 | 0.13 ± 0 | 0.01 ± 0 |
| Fructose | n.d. | 0.09 ± 0 | 0.02 ± 0 | |
| Excess Fructose d | - | - | - | |
| Polyols b | Xylitol | n.d. | n.d. | n.d. |
| Sorbitol | n.d. | n.d. | n.d. | |
| Mannitol | n.d. | n.d. | n.d. | |
| ∑Polyols | n.d. | n.d. | n.d. | |
| Oligosaccharides | Raffinose/Stachyose b | 1.37 ± 0.01 | 1.42 ± 0.01 | 0.03 ± 0 |
| Verbascose b | 2.56 ± 0.11 | 3.45 ± 0.01 | 0.05 ± 0 | |
| ∑GOS | 3.93 | 4.87 | 0.08 | |
| Total fructan e | n.d. | n.d. | n.d. |
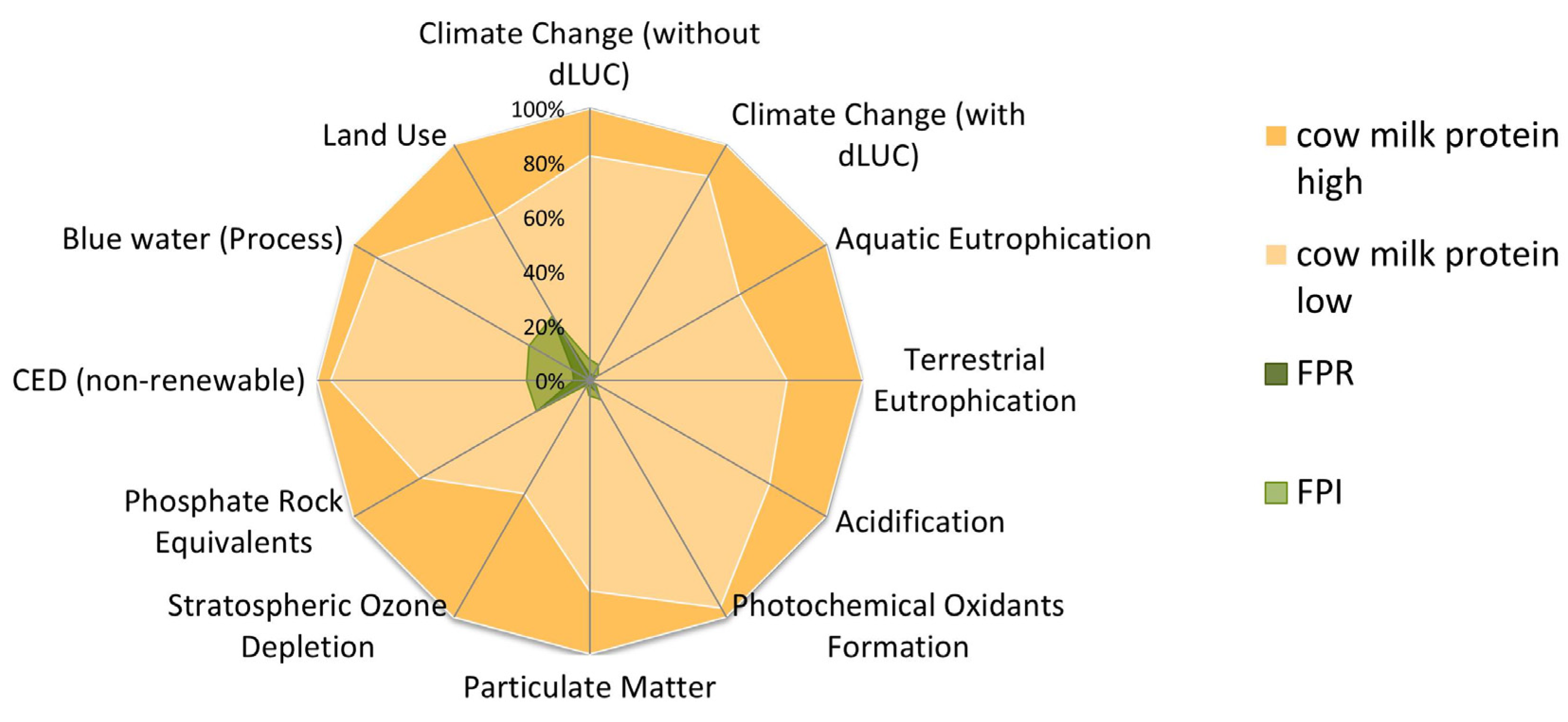
| Environmental Indicator | FPR | FPI |
|---|---|---|
| Environmental impact potentials (life cycle assessment) | ||
| Climate change (kg CO2-e/kg protein) | 1.07 | 3.35 |
| Aquatic eutrophication (g PO4-e/kg protein) | 4.98 | 6.53 |
| Terrestrial eutrophication (g PO4-e/kg protein) | 0.52 | 1.16 |
| Acidification (g SO2-e/kg protein) | 6.95 | 17.3 |
| Photochemical oxidant formation (g O3-e/kg protein) | 1.26 | 2.41 |
| Fine particulate matter (g PM2.5-e/kg protein) | 5.56 | 13.9 |
| Stratospheric ozone depletion (mg CFC11-e/protein) | 12.3 | 13.8 |
| Additional indicators at the inventory level (LCI): | ||
| Phosphorus use (g/kg protein) | 215 | 215 |
| Cumulative energy demand, non-renewable (MJ/kg protein) | 13.7 | 54.9 |
| Blue water (process) (kg/kg protein) | 13.4 | 47.7 |
| Land use (m2/kg protein) | 9.22 | 9.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. https://doi.org/10.3390/foods9030322
Vogelsang-O’Dwyer M, Petersen IL, Joehnke MS, Sørensen JC, Bez J, Detzel A, Busch M, Krueger M, O’Mahony JA, Arendt EK, et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods. 2020; 9(3):322. https://doi.org/10.3390/foods9030322
Chicago/Turabian StyleVogelsang-O’Dwyer, Martin, Iben Lykke Petersen, Marcel Skejovic Joehnke, Jens Christian Sørensen, Juergen Bez, Andreas Detzel, Mirjam Busch, Martina Krueger, James A. O’Mahony, Elke K. Arendt, and et al. 2020. "Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance" Foods 9, no. 3: 322. https://doi.org/10.3390/foods9030322
APA StyleVogelsang-O’Dwyer, M., Petersen, I. L., Joehnke, M. S., Sørensen, J. C., Bez, J., Detzel, A., Busch, M., Krueger, M., O’Mahony, J. A., Arendt, E. K., & Zannini, E. (2020). Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods, 9(3), 322. https://doi.org/10.3390/foods9030322