Exploiting Milling By-Products in Bread-Making: The Case of Sprouted Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
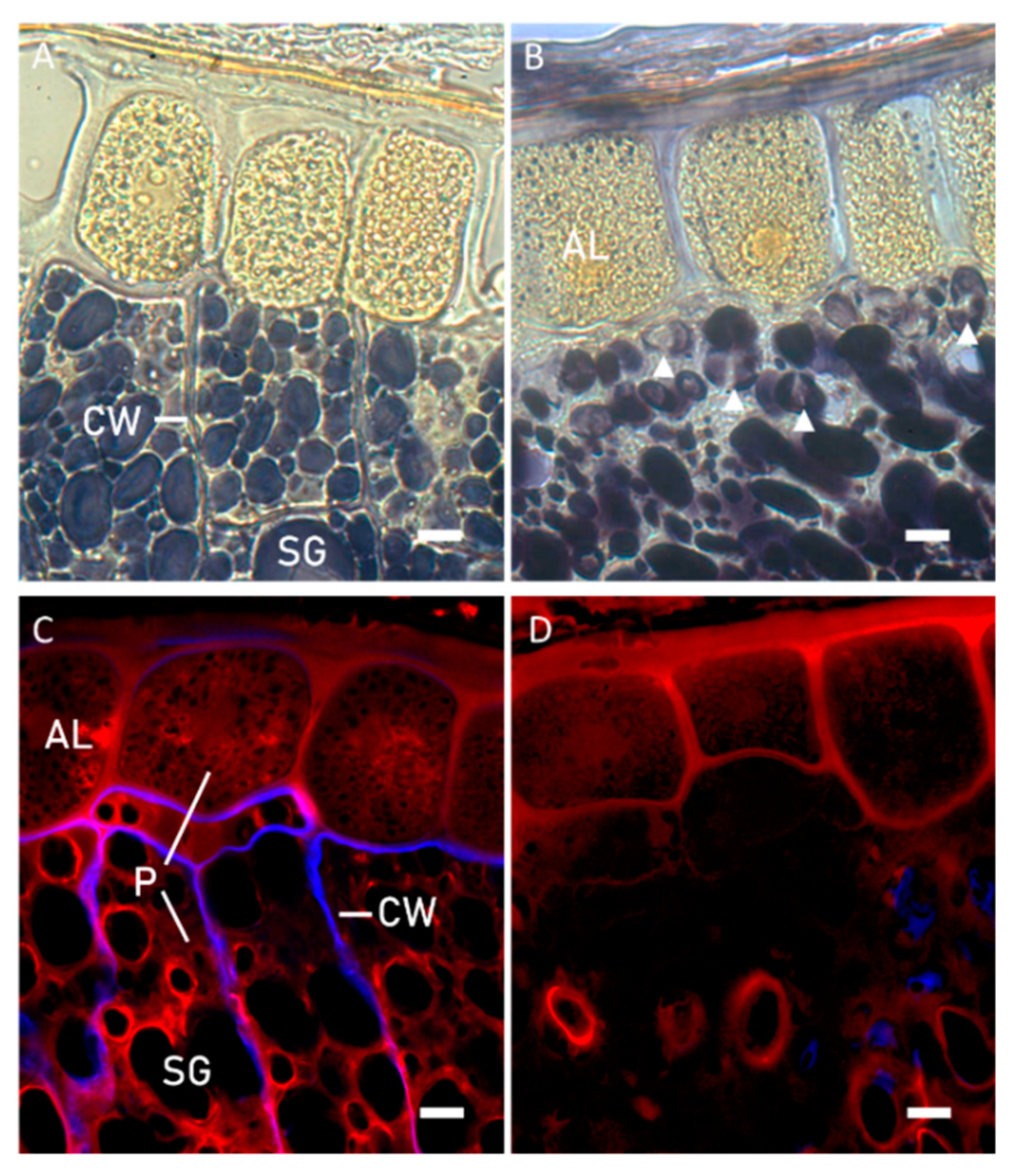
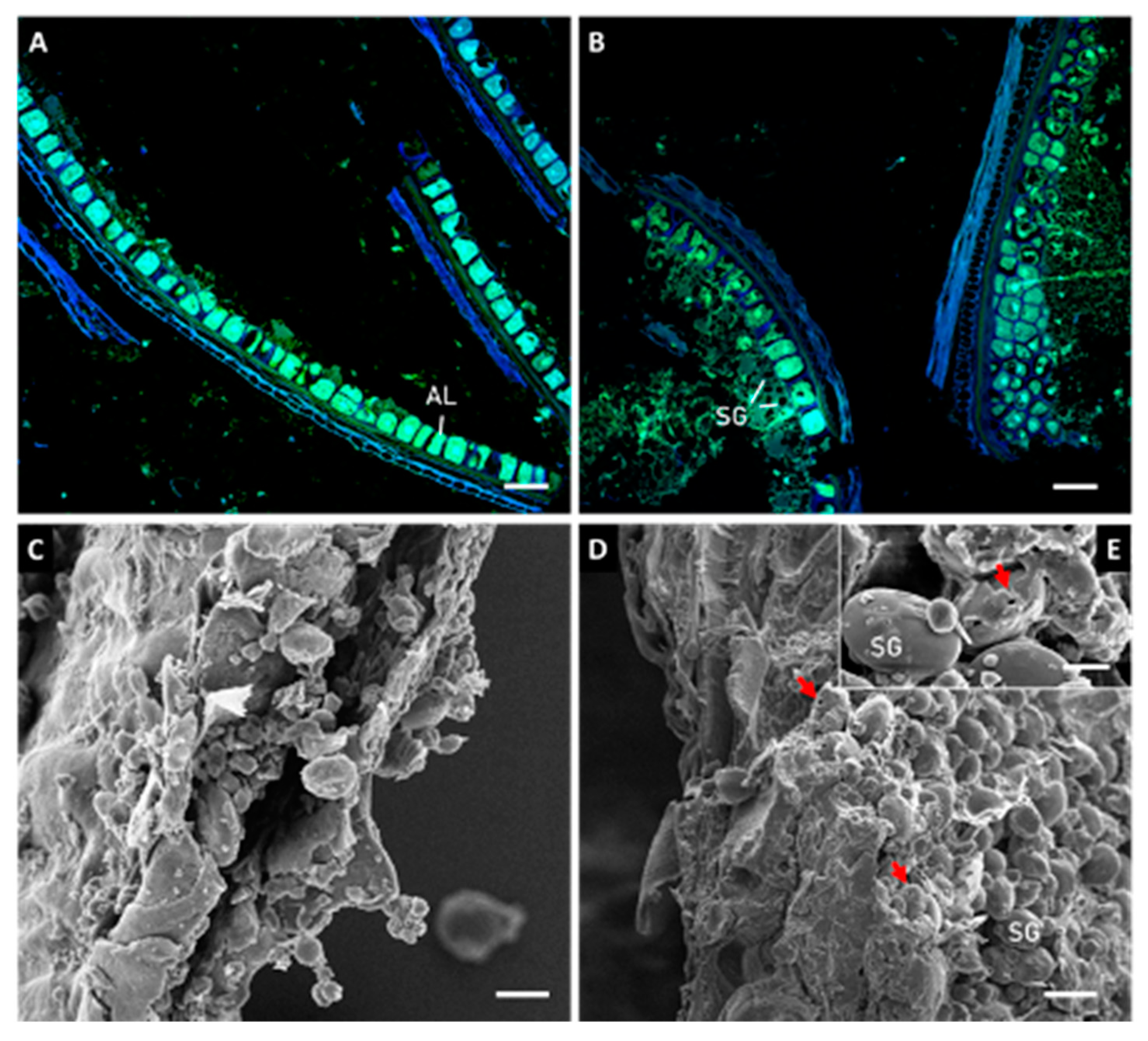
2.2.1. Microstructural Evaluation
2.2.2. Chemical Composition
2.2.3. Enzymatic Activities
2.2.4. Hydration Properties
2.2.5. Rheological Properties
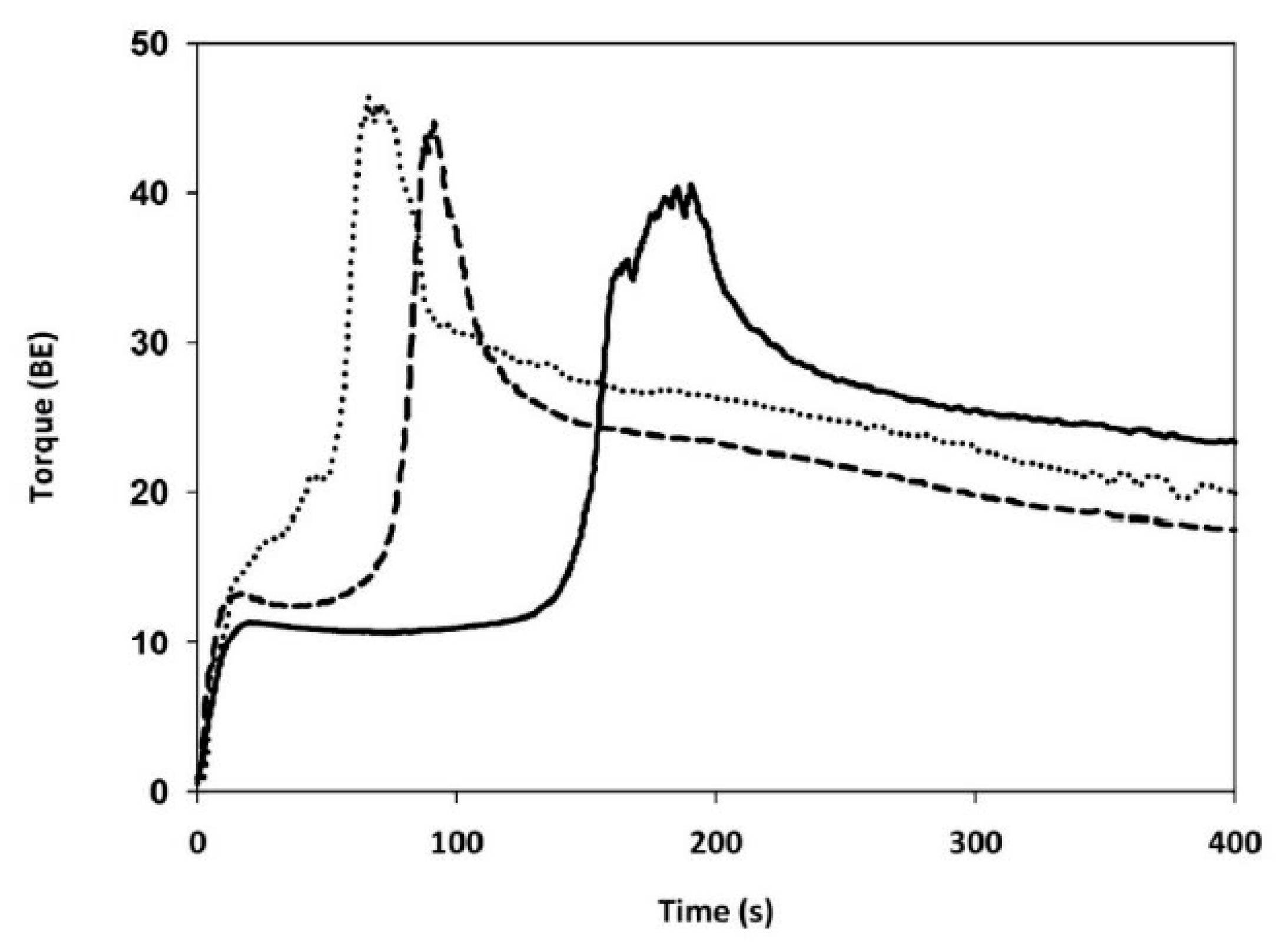
Gluten Aggregation Properties
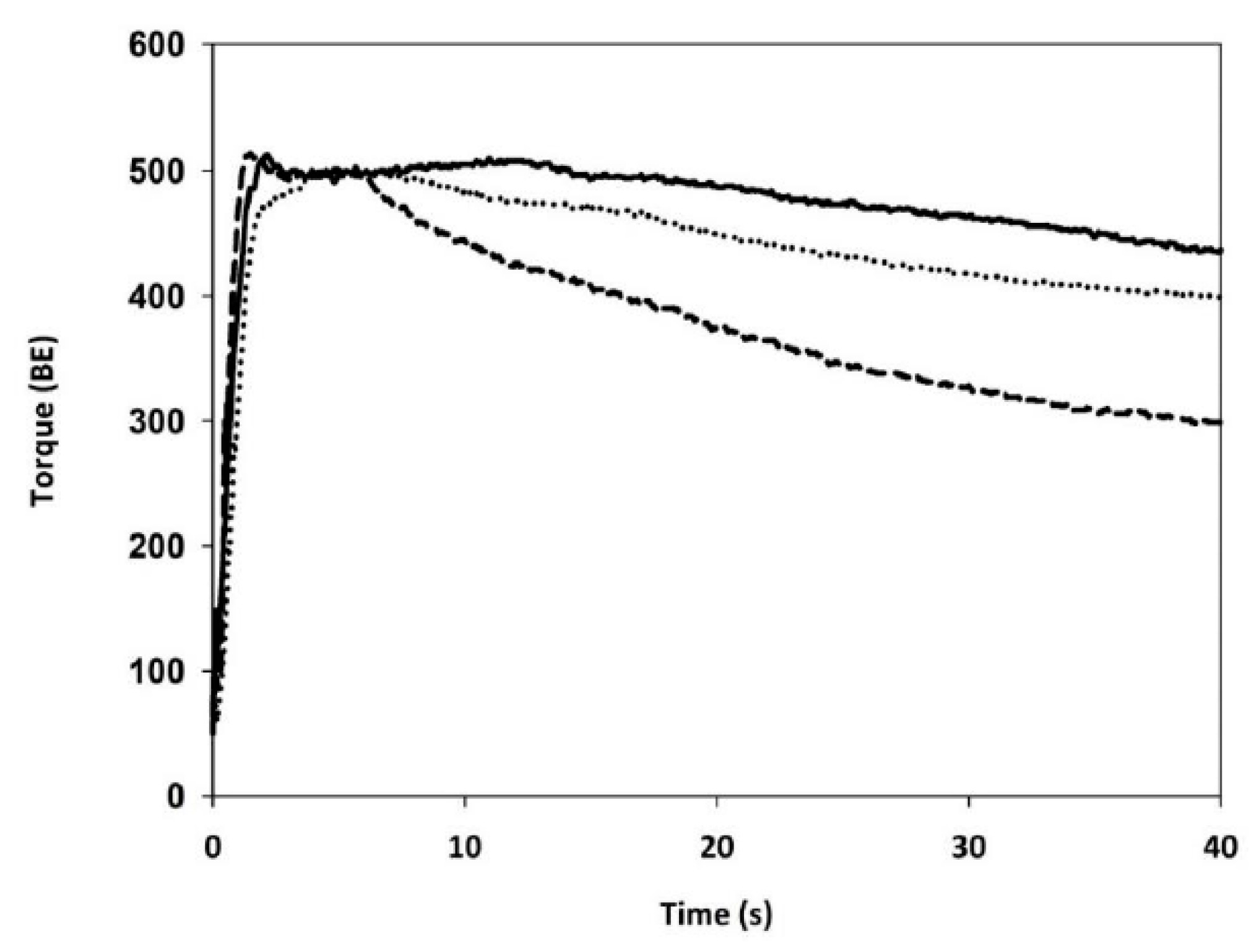
Mixing Properties
Extensibility Properties
Leavening Properties
2.2.6. Baking Test
2.2.7. Bread Properties
Color
Specific Volume
Crumb Hardness
2.3. Statistics
3. Results
3.1. Microstructure Evaluation of Kernels and Brans before and after Sprouting
3.2. Effect of Sprouting on Bran Features
3.3. Gluten Aggregation and Mixing Properties
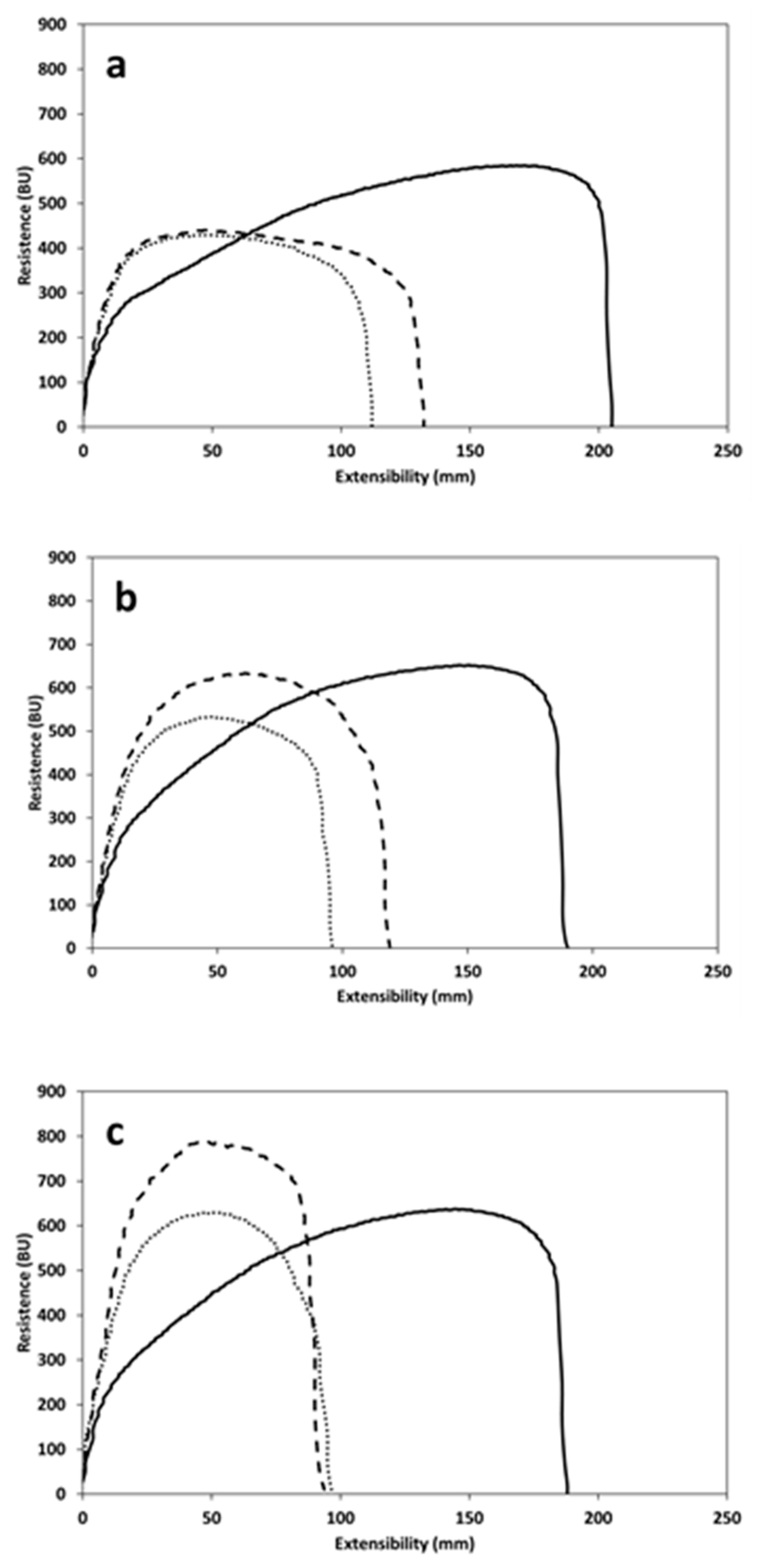
3.4. Dough Extensibility
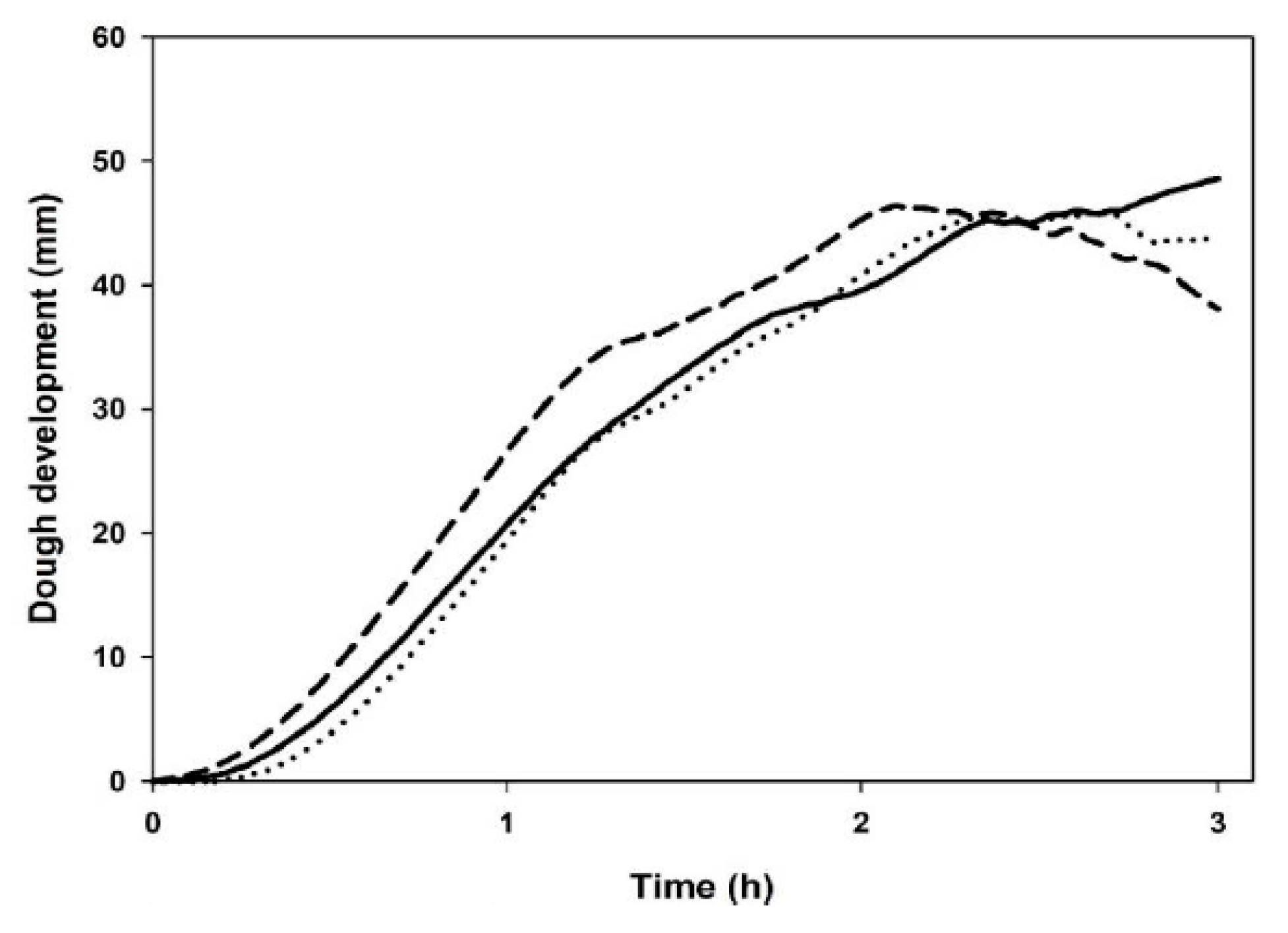
3.5. Leavening Properties
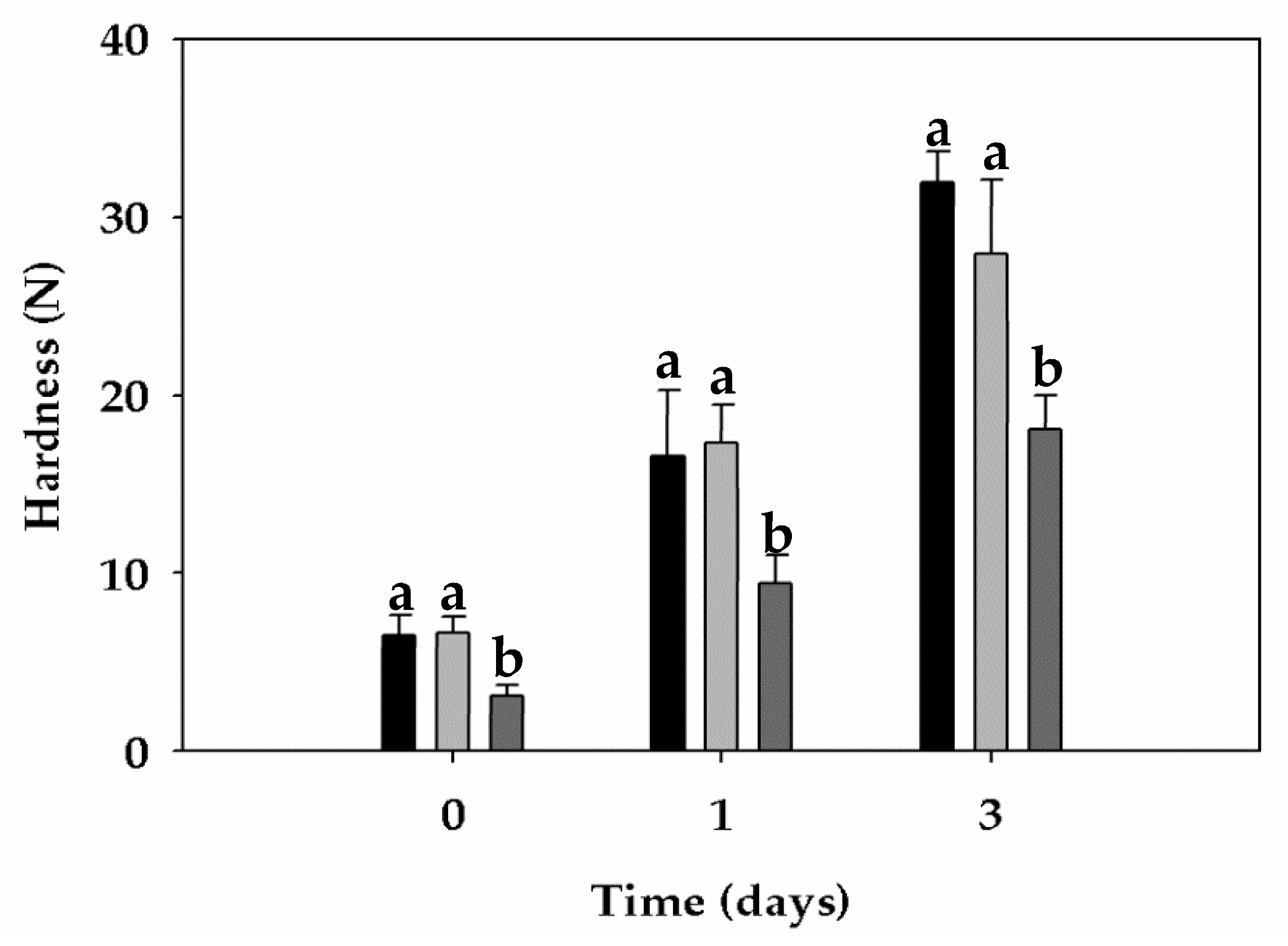
3.6. Bread Properties
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sibakov, J.; Lehtinen, P.; Poutanen, K. Cereal brans as dietary fibre ingredients. In Fibre-Rich and Wholegrain Foods; Delcour, J.A., Poutanen, K., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 170–192. ISBN 9780857090386. [Google Scholar]
- Gan, Z.; Galliard, T.; Ellis, P.R.; Angold, R.E.; Vaughan, J.G. Effect of the outer bran layers on the loaf volume of wheat bread. J. Cereal Sci. 1992, 15, 151–163. [Google Scholar] [CrossRef]
- Laurikainen, T.; Härkönen, H.; Autio, K.; Poutanen, K. Effects of enzymes in fibre-enriched baking. J. Sci. Food Agric. 1998, 76, 239–249. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Shogren, M.D.; Finney, K.F.; Bechtel, D.B. Fiber in breadmaking-effects on functional properties. Cereal Chem. 1977, 54, 25–41. [Google Scholar]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A critical review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef]
- Zhang, D.; Moore, W.R. Wheat bran particle size effects on bread baking performance and quality. J. Sci. Food Agric. 1999, 79, 805–809. [Google Scholar] [CrossRef]
- Marti, A.; Barbiroli, A.; Bonomi, F.; Brutti, A.; Iametti, S.; Marengo, M.; Miriani, M.; Pagani, M.A. Effect of high-pressure processing on the features of wheat milling by-products. Cereal Chem. 2014, 91, 318–320. [Google Scholar] [CrossRef]
- Marti, A.; Bottega, G.; Casiraghi, M.C.; Faoro, F.; Iametti, S.; Pagani, M.A. Dietary fibre enzymatic treatment: A way to improve the rheological properties of high-fibre-enriched dough. Int. J. Food Sci. Technol. 2014, 49, 305–307. [Google Scholar] [CrossRef]
- Katina, K.; Juvonen, R.; Laitila, A.; Flander, L.; Nordlund, E.; Kariluoto, S.; Piironen, V.; Poutanen, K. Fermented wheat bran as a functional ingredient in baking. Cereal Chem. 2012, 89, 126–134. [Google Scholar] [CrossRef]
- Cardone, G.; D’Incecco, P.; Pagani, M.A.; Marti, A. Sprouting improves the bread-making performance of whole wheat flour (Triticum aestivum L.). J. Sci. Food Agric. 2020, in press. [Google Scholar] [CrossRef]
- Poudel, R.; Finnie, S.; Rose, D.J. Effects of wheat kernel germination time and drying temperature on compositional and end-use properties of the resulting whole wheat flour. J. Cereal Sci. 2019, 86, 33–40. [Google Scholar] [CrossRef]
- Marti, A.; Cardone, G.; Nicolodi, A.; Quaglia, L.; Pagani, M.A. Sprouted wheat as an alternative to conventional flour improvers in bread-making. LWT Food Sci. Technol. 2017, 80, 230–236. [Google Scholar] [CrossRef]
- Marti, A.; Cardone, G.; Pagani, M.A.; Casiraghi, M.C. Flour from sprouted wheat as a new ingredient in bread-making. LWT Food Sci. Technol. 2018, 89, 237–243. [Google Scholar] [CrossRef]
- Faltermaier, A.; Zarnkow, M.; Becker, T.; Gastl, M.; Arendt, E.K. Common wheat (Triticum aestivum L.): Evaluating microstructural changes during the malting process by using confocal laser scanning microscopy and scanning electron microscopy. Eur. Food Res. Technol. 2015, 241, 239–252. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 11th ed.; AACC International, Inc.: St. Paul, MN, USA, 2011. [Google Scholar]
- Zygmunt, L.C.; Anderson, E.; Behrens, B.; Bowers, R.; Bussey, M.; Cohen, G.; Colon, M.; Deis, C.; Given, P.S.; Granade, A. High pressure liquid chromatographic determination of mono-and disaccharides in presweetened cereals: Collaborative study. J. Assoc. Off. Anal. Chem. 1982, 65, 256–264. [Google Scholar]
- Official Methods of Analysis of AOAC International, 19th ed.; Official Method 991.43; AOAC International: Gaithersburg, MD, USA, 2012.
- Manini, F.; Brasca, M.; Plumed-Ferrer, C.; Morandi, S.; Erba, D.; Casiraghi, M.C. Study of the Chemical Changes and Evolution of Microbiota During Sourdoughlike Fermentation of Wheat Bran. Cereal Chem. J. 2014, 91, 342–349. [Google Scholar] [CrossRef]
- Oberleas, D.; Harland, B. Validation of a column liquid chromatographic method for phytate. J. AOAC Int. 2007, 90, 1635–1638. [Google Scholar]
- Lebesi, D.M.; Tzia, C. Staling of cereal bran enriched cakes and the effect of an endoxylanase enzyme on the physicochemical and sensorial characteristics. J. Food Sci. 2011, 76, S380–S387. [Google Scholar] [CrossRef]
- Zanoletti, M.; Marti, A.; Marengo, M.; Iametti, S.; Pagani, M.A.; Renzetti, S. Understanding the influence of buckwheat bran on wheat dough baking performance: Mechanistic insights from molecular and material science approaches. Food Res. Int. 2017, 102, 728–737. [Google Scholar] [CrossRef]
- International Association for Cereal Science and Technology. Standard No. 115/1, Method for Using the Brabender Farinograph; International Association for Cereal Science and Technology: Vienna, Austria, 1992. [Google Scholar]
- Marti, A.; Torri, L.; Casiraghi, M.C.; Franzetti, L.; Limbo, S.; Morandin, F.; Quaglia, L.; Pagani, M.A. Wheat germ stabilization by heat-treatment or sourdough fermentation: Effects on dough rheology and bread properties. LWT Food Sci. Technol. 2014, 59, 1100–1106. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Gu, G.; Shi, Z.; Mao, Z. Studies on water-extractable arabinoxylans during malting and brewing. Food Chem. 2005, 93, 33–38. [Google Scholar] [CrossRef]
- Hübner, F.; Arendt, E.K. Germination of Cereal Grains as a Way to Improve the Nutritional Value: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Augst, E.; Cox, S.; Koehler, P. Correlations between gluten aggregation properties defined by the GlutoPeak test and content of quality-related protein fractions of winter wheat flour. J. Cereal Sci. 2015, 66, 89–95. [Google Scholar] [CrossRef]
- Malegori, C.; Grassi, S.; Ohm, J.-B.; Anderson, J.; Marti, A. GlutoPeak profile analysis for wheat classification: Skipping the refinement process. J. Cereal Sci. 2018, 79, 73–79. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Biliaderis, C.G. Effect of barley β-glucan molecular size and level on wheat dough rheological properties. J. Food Eng. 2009, 91, 594–601. [Google Scholar] [CrossRef]
- Michniewicz, J.; Biliaderis, G.G.; Bushuk, W. Effect of added pentosans on some properties of wheat bread. Food Chem. 1992, 43, 251–257. [Google Scholar] [CrossRef]
- Enneking, D.; Wink, M. Towards the elimination of anti-nutritional factors in grain legumes. In Linking Research and Marketing Opportunities for Pulses in the 21st Century; Springer: New York, NY, USA, 2000; pp. 671–683. [Google Scholar]
- Guillon, F.; Champ, M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Nelson, K.; Stojanovska, L.; Vasiljevic, T.; Mathai, M. Germinated grains: A superior whole grain functional food? Can. J. Physiol. Pharmacol. 2013, 91, 429–441. [Google Scholar] [CrossRef]
- Koehler, P.; Hartmann, G.; Wieser, H.; Rychlik, M. Changes of folates, dietary fiber, and proteins in wheat as affected by germination. J. Agric. Food Chem. 2007, 55, 4678–4683. [Google Scholar] [CrossRef]
- Boskov Hansen, H.; Andreasen, M.; Nielsen, M.; Larsen, L.; Knudsen, B.K.; Meyer, A.; Christensen, L.; Hansen, Å. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Shekiro, J.; Kuhn, E.M.; Selig, M.J.; Nagle, N.J.; Decker, S.R.; Elander, R.T. Enzymatic conversion of xylan residues from dilute acid-pretreated corn stover. Appl. Biochem. Biotechnol. 2012, 168, 421–433. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Study of hydration properties of wheat bran as a function of particle size. Food Chem. 2015, 179, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaur, V.; Kaler, R.S.S. A review on dietary fiber in cereals and its characterization. J. Appl. Nat. Sci. 2018, 10, 1216–1225. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, C.S. The effects of dietary fibre addition on the quality of common cereal products. J. Cereal Sci. 2013, 58, 216–227. [Google Scholar] [CrossRef]
- Lebesi, D.M.; Tzia, C. Use of endoxylanase treated cereal brans for development of dietary fiber enriched cakes. Innov. Food Sci. Emerg. Technol. 2012, 13, 207–214. [Google Scholar] [CrossRef]
- Gruppen, H.; Kormelink, F.J.M.; Voragen, A.G.J. Enzymic Degradation of Water-unextractable Cell Wall Material and Arabinoxylans from Wheat Flour. J. Cereal Sci. 1993, 18, 129–143. [Google Scholar] [CrossRef]
- Katina, K. High-fibre baking. In Bread Making: Improving Quality; Cauvain, S.P., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2003; pp. 487–499. ISBN 978-1-85573-553-8. [Google Scholar]
- Bucsella, B.; Molnár, D.; Harasztos, A.H.; Tömösközi, S. Comparison of the rheological and end-product properties of an industrial aleurone-rich wheat flour, whole grain wheat and rye flour. J. Cereal Sci. 2016, 69, 40–48. [Google Scholar] [CrossRef]
- Noort, M.W.J.; Van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre-protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Hídvégi, M.; Lásztity, R. Phytic acid content of cereals and legumes and interaction with proteins. Period. Polytech. Ser. Chem. 2003, 46, 59–64. [Google Scholar]
- Van Hung, P.; Hatcher, D.W.; Barker, W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef]
- Selinheimo, E.; Kruus, K.; Buchert, J.; Hopia, A.; Autio, K. Effects of laccase, xylanase and their combination on the rheological properties of wheat doughs. J. Cereal Sci. 2006, 43, 152–159. [Google Scholar] [CrossRef]
- Hartikainen, K.; Poutanen, K.; Katina, K. Influence of Bioprocessed Wheat Bran on the Physical and Chemical Properties of Dough and on Wheat Bread Texture. Cereal Chem. J. 2014, 91, 115–123. [Google Scholar] [CrossRef]
- Van Vliet, T. Strain hardening as an indicator of bread-making performance: A review with discussion. J. Cereal Sci. 2008, 48, 1–9. [Google Scholar] [CrossRef]
- Courtin, C.M.; Gelders, G.G.; Delcour, J.A. Use of two endoxylanases with different substrate selectivity for understanding arabinoxylan functionality in wheat flour breadmaking. Cereal Chem. 2001, 78, 564–571. [Google Scholar] [CrossRef]
- Courtin, C.M.; Roelants, A.; Delcour, J.A. Fractionation−reconstitution experiments provide insight into the role of endoxylanases in bread-making. J. Agric. Food Chem. 1999, 47, 1870–1877. [Google Scholar] [CrossRef]
- Rouau, X.; El-Hayek, M.-L.; Moreau, D. Effect of an enzyme preparation containing pentosanases on the bread-making quality of flours in relation to changes in pentosan properties. J. Cereal Sci. 1994, 19, 259–272. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Dappolonia, B.L. Sprouting in hard red spring wheat. Bak. Dig. 1979, 53, 17–19. [Google Scholar]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Goesaert, H.; Slade, L.; Levine, H.; Delcour, J.A. Amylases and bread firming—An integrated view. J. Cereal Sci. 2009, 50, 345–352. [Google Scholar] [CrossRef]
- De Leyn, I. Functional additives. In Bakery Products Science and Technology; Hui, Y.H., Ed.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 233–244. [Google Scholar]
| Bran from Unsprouted Wheat (BUW) | Bran from Sprouted Wheat (BSW) | |
|---|---|---|
| Total starch | 18.3 ± 0.5 | 26.9 ± 2.0 ** |
| Arabinoxylans | ||
| Total | 14.4 ± 0.5 | 12.0 ± 0.7 |
| Soluble | 0.22 ± 0.01 | 0.29 ± 0.08 |
| Phytic acid | 13.98 ± 0.46 | 11.29 ± 0.02 * |
| Total Sugar | 3.0 ± 0.9 | 7.0 ± 2.2 * |
| Glucose | 0.14 ± 0.01 | 0.53 ± 0.02 * |
| Fructose | 0.05 ± 0.01 | 0.20 ± 0.00 * |
| Sucrose | 2.02 ± 0.20 | 4.99 ± 0.00 * |
| Raffinose | 0.79 ± 0.02 | n.d. |
| Maltose | n.d. | 1.29 ± 0.03 |
| Total Dietary Fiber (TDF) | 45.4 ± 0.6 | 40.2 ± 0.9 * |
| Soluble (SDF) | 2.2 ± 0.4 | 2.0 ± 0.3 |
| Insoluble (IDF) | 43.1 ± 0.2 | 38.2 ± 0.6 * |
| α-amylase activity | 0.094 ± 0.003 | 143 ± 16 * |
| Xylanase activity | 0.16 ± 0.04 | 0.35 ± 0.03 * |
| Water Holding Capacity | 4.9 ± 0.1 | 4.5 ± 0.1 * |
| Water Binding Capacity | 3.9 ± 0.2 | 3.7 ± 0.2 |
| Refined Wheat Flour (CTRL) | CTRL + BUW | CTRL + BSW | |||
|---|---|---|---|---|---|
| GLUTEN AGGREGATION PROPERTIES (GlutoPeak Test) | Peak maximum time | (s) | 192 ± 3 c | 65 ± 1 a | 93 ± 4 b |
| Maximum torque | (BE) | 40.3 ± 0.6 a | 45.7 ± 0.6 c | 43.7 ± 2 b | |
| Total Energy | (GPE) | 3567 ± 112 c | 1914 ± 25 a | 2059 ± 35 b | |
| MIXING PROPERTIES (Farinograph Test) | Water absorption | (%) | 58.1 ± 0.2 a | 65.6 ± 0.5 c | 61.8 ± 0.5 b |
| Development time | (min) | 10.8 ± 0.3 c | 5.6 ± 0.3 b | 4.5 ± 0.5 a | |
| Stability | (min) | 23 ± 2 c | 12 ± 2 b | 6.4 ± 0.07 a | |
| Degree of softening | (FU) | 15 ± 1 a | 44 ± 5 b | 103.5 ± 0.7 c |
| Resting Time | CTRL | CTRL + BUW | CTRL + BSW | |
|---|---|---|---|---|
| Extensibility (E) (mm) | 45 min | 209 ± 11 b | 119 ± 6 a | 132 ± 4 a |
| Resistance to extension (R) (BU) | 353 ± 46 a | 423 ± 29 a | 431 ± 1 a | |
| R/E ratio | 1.7 ± 0.3 a | 4.0 ± 0.4 b | 3.3 ± 0.1 b | |
| Energy (cm2) | 155 ± 9 b | 76 ± 2 a | 88 ± 2 a | |
| Extensibility (E) (mm) | 90 min | 182 ± 7 b | 104 ± 6 a | 109 ± 3 a |
| Resistance to extension (R) (BU) | 405 ± 63 a | 512 ± 26 a,b | 583 ± 41 b | |
| R/E ratio | 2.2 ± 0.3 a | 4.9 ± 0.5 b | 5.4 ± 0.2 b | |
| Energy (cm2) | 147 ± 31 b | 77 ± 4a | 94 ± 11 a,b | |
| Extensibility (E) (mm) | 135 min | 185 ± 2 b | 97 ± 2 a | 97 ± 1 a |
| Resistance to extension (R) (BU) | 430 ± 45a | 609 ± 32 b | 721 ± 7 c | |
| R/E ratio | 2.3 ± 0.3 a | 6.0 ± 0.3 b | 7.5 ± 0.1 c | |
| Energy (cm2) | 163 ± 4 b | 85 ± 5 a | 101 ± 3 a |
| CTRL | CTRL + BUW | CTRL + BSW | |
|---|---|---|---|
| Maximum dough height (mm) | 51 ± 3 b | 47 ± 1 a | 47 ± 1 a |
| Time of maximum dough development (min) | 180 ± 0 c | 161 ± 2 b | 131 ± 6 a |
| Dough height at 180 min (mm) | 51 ± 3 b | 44 ± 1 a | 39 ± 1 a |
| Weakening coefficient at 180 min (%) | n.d. | 6 ± 1 b | 17.5 ± 0.4 a |
| Total CO2 (mL) | 1129 ± 52 a | 1301 ± 42 a,b | 1476 ± 84 b |
| Retained CO2 (mL) | 1064 ± 45 a | 1197 ± 29 b | 1271 ± 46 b |
| Released CO2 (mL) | 65 ± 7 a | 104 ± 12 a | 205 ± 38 b |
| CO2 retention coefficient (%) | 94 ± 1 b | 92 ± 0.1 b | 86 ± 2 a |
| Porosity time (min) | n.d. | 103 ± 4 b | 76 ± 4 a |
| CTRL | CTRL + BUW | CTRL + BSW | ||
|---|---|---|---|---|
| Bread | Specific volume (mL/g) | 2.7 ± 0.6 b | 2.2 ± 0.1 a | 2.4 ± 0.1 a,b |
| Crust | Browning (100-L*) | 53 ± 4 a | 60 ± 4 b | 60 ± 1 b |
| Redness (a*) | 9 ± 3 a | 12 ± 1 b | 12 ± 1 b | |
| Yellowness (b*) | 25 ± 2 b | 20 ± 5 a | 19 ± 1 a | |
| Crumb | Browning (100-L*) | 48 ± 3 a | 52 ± 2 b | 57 ± 2 c |
| Redness (a*) | −1.3 ± 0.2 a | 3.80 ± 0.3 c | 3.2 ± 0.4 b | |
| Yellowness (b*) | 18 ± 1 b | 16 ± 1 a | 16 ± 1 a | |
| Crumb firming kinetics | A0 (N) | 6.48 | 6.62 | 3.12 |
| A∞ (N) | 66.01 | 34.55 | 31.35 | |
| A∞-A0 (N) | 59.53 | 27.93 | 28.23 | |
| k (h-n) | 0.186 | 0.480 | 0.252 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, G.; D’Incecco, P.; Casiraghi, M.C.; Marti, A. Exploiting Milling By-Products in Bread-Making: The Case of Sprouted Wheat. Foods 2020, 9, 260. https://doi.org/10.3390/foods9030260
Cardone G, D’Incecco P, Casiraghi MC, Marti A. Exploiting Milling By-Products in Bread-Making: The Case of Sprouted Wheat. Foods. 2020; 9(3):260. https://doi.org/10.3390/foods9030260
Chicago/Turabian StyleCardone, Gaetano, Paolo D’Incecco, Maria Cristina Casiraghi, and Alessandra Marti. 2020. "Exploiting Milling By-Products in Bread-Making: The Case of Sprouted Wheat" Foods 9, no. 3: 260. https://doi.org/10.3390/foods9030260
APA StyleCardone, G., D’Incecco, P., Casiraghi, M. C., & Marti, A. (2020). Exploiting Milling By-Products in Bread-Making: The Case of Sprouted Wheat. Foods, 9(3), 260. https://doi.org/10.3390/foods9030260








