Food Applications and Potential Health Benefits of Pomegranate and its Derivatives
Abstract
1. Introduction
2. General Description
2.1. History
2.2. Taxonomy
2.3. Cultivars
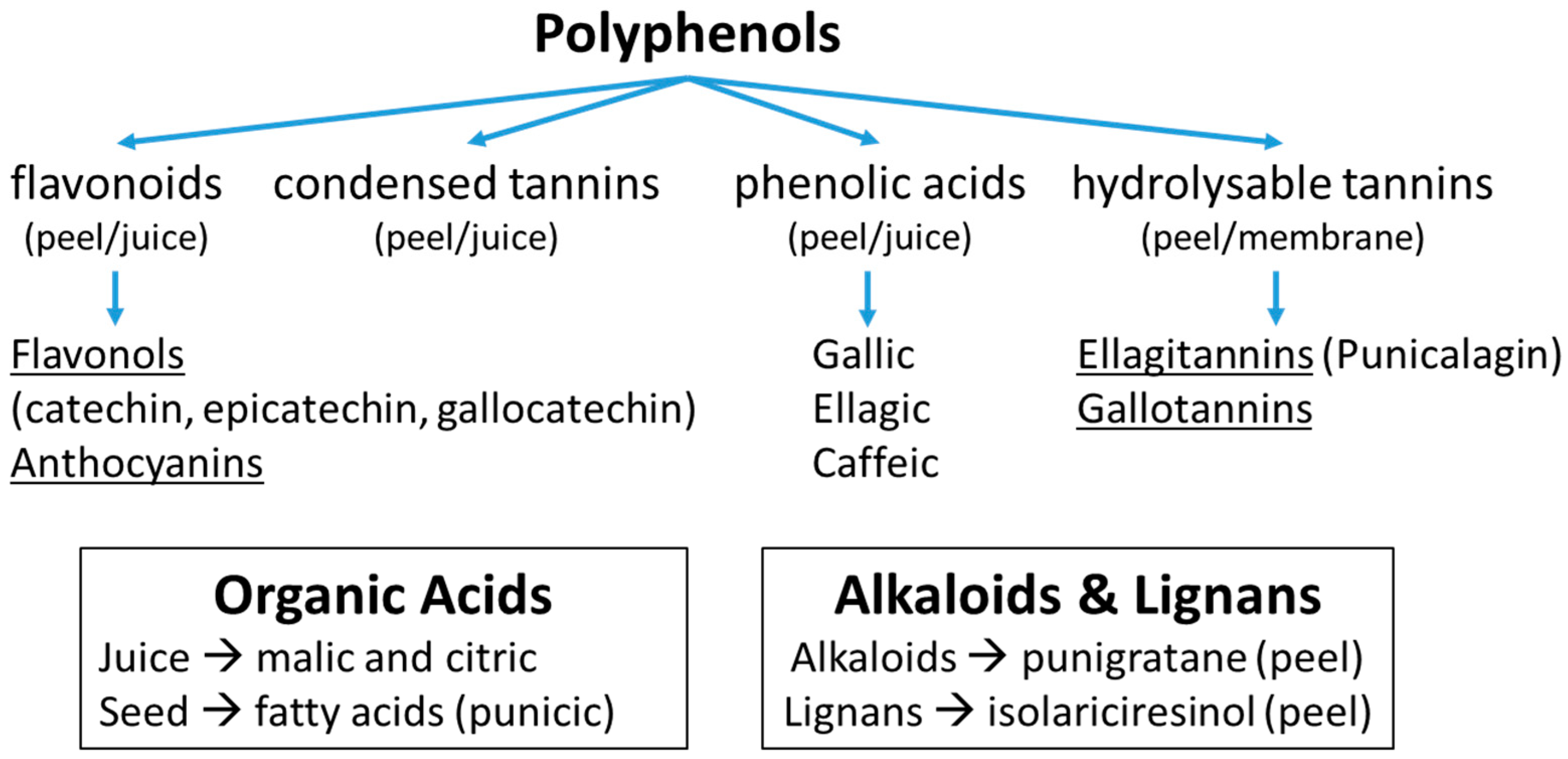
2.4. Composition
3. Health Benefits
3.1. In Vitro Studies
3.1.1. Prebiotic Effect and Antimicrobial Activity
3.1.2. Anticarcinogenic Effect
3.1.3. Skin Health
3.1.4. Obesity, Diabetes, Alzheimer’s Disease, Osteoporosis and Dental Health
3.2. Studies Using Mice Models
3.2.1. Obesity, Diabetes
3.2.2. Prevention and Treatment of Infections
3.2.3. Other Health Benefits
3.3. Human Studies
4. Applications in Food Products
4.1. Dairy Products
4.2. Films and Coatings
4.3. Antimicrobial and Antifungal Agent in Fruits and Juices
4.4. Meat and Fish Products
4.5. Cereal and Nuts Products
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.S.; Narzary, D.; Ranade, S.A. Systematics and taxonomic disposition of the genus Punica L. Pomegranate. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 19–25. [Google Scholar]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Aslanova, M.S.; Magerramov, M.A. Physicochemical parameters and amino acid composition of new pomological sorts of pomegranate fruits. Chem. Plant Raw Mater. 2012, 1, 165–169. [Google Scholar]
- da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Gunjan, J.; Rahul, N.; Divya, S.; Praveen, K.S.; Diwaker, K.A. Antioxidant activity of various parts of Punica granatum: A review. J. Drug Deliv. Ther. 2012, 2, 138–141. [Google Scholar] [CrossRef]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2379. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Chen, D.J.; Song, M.T.; Zhao, Y.L.; Li, Z.H. Assessment and utilization of pomegranate varieties resources. J. Fruit Sci. 1998, 15, 370–373. [Google Scholar]
- Saeed, W.T. Pomegranate cultivars as affected by Paclobutrazol, salt stress and change in fingerprints. Bull. Fac. Agric. Cairo Univ. 2005, 56, 581–615. [Google Scholar]
- Jalikop, S.H. Breeding of pomegranate and annonaceous fruits. Acta Hortic. 2011, 890, 191–197. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamočlija, J.; Ćirić, A.; Soković, M.; Heropoulos, G.; Proestos, C. Antiradical–antimicrobial activity and phenolic profile of pomegranate (Punica granatum L.) juices from different cultivars: A comparative study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Vasantha Kumar, G.K. Pomegranate cultivation in Karnataka state, India—A profitable venture. Acta Hortic. 2009, 818, 55–60. [Google Scholar] [CrossRef]
- Pande, G.; Akoh, C.C. Pomegranate cultivars (Punica granatum L.). In Nutritional Composition of Fruit Cultivars, 1st ed.; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2016; pp. 667–689. [Google Scholar]
- Varasteh, F.; Arzani, K.; Zamani, Z.; Mosheni. A. Evaluation of the most important fruit characteristics of some commercial pomegranate (Punica granatum L.) cultivars of Iran. Acta Hortic. 2009, 818, 103–108. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 2. [Google Scholar]
- Barone, E.; Caruso, T.; Marra, F.P.; Sottile, F. Preliminary observations on some Sicilian pomegranate (Punica granatum L.) varieties. In Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology, Proceedings of the Options Méditerranéennes: Série, A. Séminaires Méditerranéens, Orihuela, Spain, 15–17 October 1998; Melgarejo, P., Martínez-Nicolás, J.J., Martínez-Tomé, J., Eds.; CIHEAM: Zaragoza, Spain, 2000; Volume 42, pp. 137–141. [Google Scholar]
- Ferrara, G.; Giancaspro, A.; Mazzeo, A.; Giove, S.L.; Matarrese, A.M.S.; Pacucci, C.; Punzi, R.; Trani, A.; Gambacorta, G.; Blanco, A.; et al. Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci. Hortic. 2014, 178, 70–78. [Google Scholar] [CrossRef]
- LaRue, J.H. Growing Pomegranates in California. University of California, Leaflet 2459. 1980. Available online: http://ucanr.edu/sites/Pomegranates/files/122804.pdf (accessed on 30 December 2019).
- Hmid, I.; Elothmani, D.; Hanine, H.; Oukabli, A.; Mehinagic, E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 2017, 10, S2675–S2684. [Google Scholar] [CrossRef]
- Melgarejo, P.; Salazar, D.M.; Artes, F. Organic acids and sugars composition of harvested pomegranate fruits. Eur. Food Res. Tech. 2000, 211, 185–190. [Google Scholar] [CrossRef]
- Mars, M.; Marrakchi, M. Diversity of pomegranate (Punica granatum L.) germplasm in Tunisia. Genet. Resour Crop Evol. 1999, 46, 461–476. [Google Scholar] [CrossRef]
- Stover, E.W.; Mercure, E.W. The pomegranate: A new look at the fruit of paradise. HortScience 2007, 42, 1088–1092. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, Á.; Vázquez-Araújo, L.; Pérez-López, A.J.; Frutos-Fernández, M.J.; Carbonell-Barrachina, Á.A. Processing pomegranates for juice and impact on bioactive components. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: New York, NY, USA, 2015; pp. 629–636. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxid. Med. Cell. Longev. 2015, 2015, 1–19. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Tundis, R.; Sicari, V.; Loizzo, M.R. The juice of pomegranate (Punica granatum L.): Recent studies on its bioactivities. In Quality Control in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: New York, NY, USA, 2019; Volume 17, pp. 459–489. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef] [PubMed]
- Fadavi, A.; Barzegar, M.; Azizi, M.H.; Bayat, M. Note. Physicochemical composition of ten pomegranate cultivars (Punica granatum L.) grown in Iran. Food Sci. Technol. Int. 2005, 11, 113–119. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Caleb, O.J.; Fawole, O.A.; Opara, U.L. Effects of different maturity stages and growing locations on changes in chemical, biochemical and aroma volatile composition of ‘Wonderful’ pomegranate juice. J. Sci. Food Agric. 2016, 96, 1002–1009. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of maturity status on biochemical content, polyphenol composition and antioxidant capacity of pomegranate fruit arils (cv. ‘Bhagwa’). S. Afr. J. Bot. 2013, 85, 23–31. [Google Scholar] [CrossRef]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Bioavailable constituents/metabolites of pomegranate (Punica granatum L.) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE 2 production in human chondrocytes in vitro. J. Inflamm. 2008, 5, 9. [Google Scholar] [CrossRef]
- Cerrato, A.; Cannazza, G.; Capriotti, A.L.; Citti, C.; La Barbera, G.; Laganà, A.; Montone, C.M.; Piovesana, S.; Cavaliere, C. A new software-assisted analytical workflow based on high-resolution mass spectrometry for the systematic study of phenolic compounds in complex matrices. Talanta 2019, 120573. [Google Scholar] [CrossRef]
- Paul, A.; Banerjee, K.; Goon, A.; Saha, S. Chemo-profiling of anthocyanins and fatty acids present in pomegranate aril and seed grown in Indian condition and its bioaccessibility study. J. Food Sci. Technol. 2018, 55, 2488–2496. [Google Scholar] [CrossRef]
- Syed, D.N.; Afaq, F.; Mukhtar, H. Pomegranate derived products for cancer chemoprevention. Semin. Cancer Biol. 2007, 17, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.H.; Chen, H.D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Finegold, S.M.; Summanen, P.H.; Corbett, K.; Downes, J.; Henning, S.M.; Li, Z. Pomegranate extract exhibits in vitro activity against Clostridium difficile. Nutrition 2014, 30, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Carpéné, C.; Arbonés-Mainar, J.M.; Decaunes, P.; Valero, M.S.; López, V. Pomegranate juice and its main polyphenols exhibit direct effects on amine oxidases from human adipose tissue and inhibit lipid metabolism in adipocytes. J. Funct. Foods 2017, 33, 323–331. [Google Scholar] [CrossRef]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- An, J.; Guo, Y.; Wang, T.; Pantuck, A.J.; Rettig, M.B. Pomegranate extract inhibits EMT in clear cell renal cell carcinoma in a NF-κB and JNK dependent manner. Asian J. Urol. 2015, 2, 38–45. [Google Scholar] [CrossRef]
- Šavikin, K.; Živković, J.; Alimpić, A.; Zdunić, G.; Janković, T.; Duletić-Laušević, S.; Menković, N. Activity guided fractionation of pomegranate extract and its antioxidant, antidiabetic and antineurodegenerative properties. Ind. Crops Prod. 2018, 113, 142–149. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.; Yang, F.; Zeng, A.; Yang, S.; Luo, Y.; Zhang, Y.; Xie, Y.; Ye, T.; Xia, Y.; et al. The extract from Punica granatum (pomegranate) peel induces apoptosis and impairs metastasis in prostate cancer cells. Biomed. Pharm. 2017, 93, 976–984. [Google Scholar] [CrossRef]
- Gulube, Z.; Patel, M. Effect of Punica granatum on the virulence factors of cariogenic bacteria Streptococcus mutans. Microb. Pathog. 2016, 98, 45–49. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Scioscia, E.; Sateriale, D.; Pastore, G.; Colicchio, R.; Pagliuca, C.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; et al. In vitro antibacterial activity of pomegranate juice and peel extracts on cariogenic bacteria. Biomed. Res. Int. 2017, 2017, 2152749. [Google Scholar] [CrossRef]
- Spilmont, M.; Léotoing, L.; Davicco, M.J.; Lebecque, P.; Miot-Noirault, E.; Pilet, P.; Rios, L.; Wittrant, Y.; Coxam, V. Pomegranate peel extract prevents bone loss in a preclinical model of osteoporosis and stimulates osteoblastic differentiation in vitro. Nutrients 2015, 7, 9265–9284. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, E.M. Stimulation of osteoblastic differentiation and inhibition of interleukin-6 and nitric oxide in MC3T3-E1 cells by pomegranate ethanol extract. Phytother. Res. 2009, 23, 737–739. [Google Scholar] [CrossRef]
- Gavlighi, H.A.; Tabarsa, M.; You, S.; Surayot, U.; Ghaderi-Ghahfarokhi, M. Extraction, characterization and immunomodulatory property of pectic polysaccharide from pomegranate peels: Enzymatic vs. conventional approach. Int. J. Biol. Macromol. 2018, 116, 698–706. [Google Scholar] [CrossRef]
- Adaramoye, O.; Erguen, B.; Nitzsche, B.; Höpfner, M.; Jung, K.; Rabien, A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem. Biol. Interact. 2017, 274, 100–106. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Zheng, W.; Hu, M.; Wang, J.; Ma, S.; Deng, Y.; Luo, Y.; Ye, T.; Yin, W. Punica granatum (pomegranate) leaves extract induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in non-small cell lung cancer in vitro. Biomed. Pharm. 2016, 80, 227–235. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012, 49, 345–353. [Google Scholar] [CrossRef]
- Yao, X.; Cheng, X.; Zhang, L.; Yu, H.; Bao, J.; Guan, H.; Lu, R. Punicalagin from pomegranate promotes human papillary thyroid carcinoma BCPAP cell death by triggering ATM-mediated DNA damage response. Nutr. Res. 2017, 47, 63–71. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.B.; Kim, H.S.; Park, E.K.; et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef]
- Iwatake, M.; Okamoto, K.; Tanaka, T.; Tsukuba, T. Punicalagin attenuates osteoclast differentiation by impairing NFATc1 expression and blocking Akt-and JNK-dependent pathways. Mol. Cell. Biochem. 2015, 407, 161–172. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef]
- DaSilva, N.A.; Nahar, P.P.; Ma, H.; Eid, A.; Wei, Z.; Meschwitz, S.; Zawia, N.H.; Slitt, A.L.; Seeram, N.P. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr. Neurosci. 2019, 22, 185–195. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef]
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Rettig, M.B.; Heber, D.; An, J.; Seeram, N.P.; Rao, J.Y.; Liu, H.; Klatte, T.; Belldegrun, A.; Moro, A.; Henning, S.M.; et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-κB-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2662–2671. [Google Scholar] [CrossRef]
- Ahmadiankia, N. Molecular targets of pomegranate (Punica granatum) in preventing cancer metastasis. Iran. J. Basic Med. Sci. 2019, 22, 977. [Google Scholar] [CrossRef]
- Velagapudi, R.; Baco, G.; Khela, S.; Okorji, U.; Olajide, O. Pomegranate inhibits neuroinflammation and amyloidogenesis in IL-1β-stimulated SK-N-SH cells. Eur. J. Nutr. 2016, 55, 1653–1660. [Google Scholar] [CrossRef]
- Subash, S.; Braidy, N.; Essa, M.M.; Zayana, A.B.; Ragini, V.; Al-Adawi, S.; Al-Asmi, A.; Guillemin, G.J. Long-term (15 mo) dietary supplementation with pomegranates from Oman attenuates cognitive and behavioral deficits in a transgenic mice model of Alzheimer’s disease. Nutrition 2015, 31, 223–229. [Google Scholar] [CrossRef]
- Makled, M.N.; El-Awady, M.S.; Abdelaziz, R.R.; Atwan, N.; Guns, E.T.; Gameil, N.M.; Shebab El-Din, A.B.; Ammar, E.M. Pomegranate protects liver against cecal ligation and puncture-induced oxidative stress and inflammation in rats through TLR4/NF-κB pathway inhibition. Environ. Toxicol. Pharmacol. 2016, 43, 182–192. [Google Scholar] [CrossRef]
- Choudhury, S.; Ghosh, S.; Mukherjee, S.; Gupta, P.; Bhattacharya, S.; Adhikary, A.; Chattopadhyay, S. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J. Nutr. Biochem. 2016, 38, 25–40. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.P.; Zhang, Y.; Li, Y.; Sun, J.R. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J. Biol. Macromol. 2019, 137, 504–511. [Google Scholar] [CrossRef]
- Zhai, X.; Zhu, C.; Zhang, Y.; Sun, J.; Alim, A.; Yang, X. Chemical characteristics, antioxidant capacities and hepatoprotection of polysaccharides from pomegranate peel. Carbohydr. Polym. 2018, 202, 461–469. [Google Scholar] [CrossRef]
- Nagano, T.; Ito, H. Diet containing a polyphenol concentrate from pomegranate juice attenuates contact hypersensitivity in mice. J. Funct. Foods 2018, 45, 247–253. [Google Scholar] [CrossRef]
- Chen, B.; Longtine, M.S.; Riley, J.K.; Nelson, D.M. Antenatal pomegranate juice rescues hypoxia-induced fetal growth restriction in pregnant mice while reducing placental cell stress and apoptosis. Placenta 2018, 66, 1–7. [Google Scholar] [CrossRef]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuźniak, V.; Mikołajczak, P.L.; Teissedre, P.-L.; et al. Neuroprotective effects of pomegranate juice against Parkinson’s disease and presence of ellagitannins-derived metabolite—Urolithin a—In the brain. Int. J. Mol. Sci. 2020, 21, 202. [Google Scholar] [CrossRef]
- Alkathiri, B.; El-Khadragy, M.; Metwally, D.; Al-Olayan, E.; Bakhrebah, M.; Abdel Moneim, A. Pomegranate (Punica granatum) juice shows antioxidant activity against cutaneous leishmaniasis-induced oxidative stress in female BALB/c mice. Int. J. Environ. Res. Public Health 2017, 14, 1592. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.P.; Lei, F.; Jiang, J.F.; Li, J.; Xing, D.M.; Du, L.J. Pomegranate leaf attenuates lipid absorption in the small intestine in hyperlipidemic mice by inhibiting lipase activity. Chin. J. Nat. Med. 2017, 15, 732–739. [Google Scholar] [CrossRef]
- Al-Megrin, W.A. In vivo study of pomegranate (Punica granatum) peel extract efficacy against Giardia lamblia in infected experimental mice. Asian Pac. J. Trop. Biomed. 2017, 7, 59–63. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef]
- Rodriguez, J.; Gilson, H.; Jamart, C.; Naslain, D.; Pierre, N.; Deldicque, L.; Francaux, M. Pomegranate and green tea extracts protect against ER stress induced by a high-fat diet in skeletal muscle of mice. Eur. J. Clin. Nutr. 2015, 54, 377–389. [Google Scholar] [CrossRef]
- Parmar, H.S.; Kar, A. Antidiabetic potential of Citrus sinensis and Punica granatum peel extracts in alloxan treated male mice. Biofactors 2007, 31, 17–24. [Google Scholar] [CrossRef]
- Parmar, H.S.; Kar, A. Medicinal values of fruit peels from Citrus sinensis, Punica granatum, and Musa paradisiaca with respect to alterations in tissue lipid peroxidation and serum concentration of glucose, insulin, and thyroid hormones. J. Med. Food 2008, 11, 376–381. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, S.; Henning, S.M.; Lee, R.; Hsu, M.; Grojean, E.; Pisegna, R.; Ly, A.; Heber, D.; Li, Z. Cholesterol-lowering effects of dietary pomegranate extract and inulin in mice fed an obesogenic diet. J. Nutr. Biochem. 2018, 52, 62–69. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Pu, W.; Dong, Z.; Sun, L.; Zang, W.; Gao, F.; Zhang, Y.; Feng, Z.; Liu, J. Punicalagin, an active component in pomegranate, ameliorates cardiac mitochondrial impairment in obese rats via AMPK activation. Sci. Rep. 2015, 5, 14014. [Google Scholar] [CrossRef]
- George, N.S.; Cheung, L.; Luthria, D.L.; Santin, M.; Dawson, H.D.; Bhagwat, A.A.; Smith, A.D. Pomegranate peel extract alters the microbiome in mice and dysbiosis caused by Citrobacter rodentium infection. Food Sci. Nutr. 2019, 7, 2565–2576. [Google Scholar] [CrossRef]
- Amer, O.S.; Dkhil, M.A.; Hikal, W.M.; Al-Quraishy, S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed Res. Int. 2015, 2015, 219670. [Google Scholar] [CrossRef]
- Smith, A.D.; George, N.S.; Cheung, L.; Bhagavathy, G.V.; Luthria, D.L.; John, K.M.; Bhagwat, A.A. Pomegranate peel extract reduced colonic damage and bacterial translocation in a mouse model of infectious colitis induced by Citrobacter rodentium. Nutr. Res. 2020, 73, 27–37. [Google Scholar] [CrossRef]
- Barati Boldaji, R.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. J. Sci. Food Agric. 2020, 100, 846–854. [Google Scholar] [CrossRef]
- Banihani, S.A.; Shuaibu, S.M.; Al-Husein, B.A.; Makahleh, S.S. Fresh pomegranate juice decreases fasting serum erythropoietin in patients with type 2 diabetes. Int. J. Food Sci. 2019, 1269341. [Google Scholar] [CrossRef]
- Sohrab, G.; Roshan, H.; Ebrahimof, S.; Nikpayam, O.; Sotoudeh, G.; Siasi, F. Effects of pomegranate juice consumption on blood pressure and lipid profile in patients with type 2 diabetes: A single-blind randomized clinical trial. Clin. Nutr. ESPEN 2019, 29, 30–35. [Google Scholar] [CrossRef]
- Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.M.; Merril, D.A.; Henning, S.M.; Heber, D.; Small, G.W. Randomized placebo-controlled study of the memory effects of pomegranate juice in middle-aged and older adults. Am. J. Clin. Nutr. 2019, nqz241. [Google Scholar] [CrossRef]
- Fuster-Muñoz, E.; Roche, E.; Funes, L.; Martínez-Peinado, P.; Sempere, J.M.; Vicente-Salar, N. Effects of pomegranate juice in circulating parameters, cytokines, and oxidative stress markers in endurance-based athletes: A randomized controlled trial. Nutrition 2016, 32, 539–545. [Google Scholar] [CrossRef]
- Achraf, A.; Hamdi, C.; Turki, M.; Abdelkarim, O.; Ayadi, F.; Hoekelmann, A.; Yaich, S.; Souissi, N. Natural pomegranate juice reduces inflammation, muscle damage and increase platelets blood levels in active healthy Tunisian aged men. Alex. J. Med. 2018, 54, 45–48. [Google Scholar] [CrossRef]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Rudić-Grujić, V.; Paunović, M.; Arsić, A.; Petrović, S.; Vučić, V.; Mirjanić-Azarić, B.; Šavikin, K.; et al. Beneficial effects of pomegranate peel extract on plasma lipid profile, fatty acids levels and blood pressure in patients with diabetes mellitus type-2: A randomized, double-blind, placebo-controlled study. J. Funct. Foods 2019, 103692. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Hamishehkar, H.; Mobasseri, M.; Ebrahimzadeh, V.; Alipour, M.; Alipour, B. Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: A randomized controlled clinical trial. J. Cell Physiol. 2019, 234, 19621–19628. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Hamishehkar, H.; Alivand, M.; Alipour, M.; Solhi, M.; Alipour, B. Effect of pomegranate seed oil on the expression of PPAR-γ and pro-inflammatory biomarkers in obese type 2 diabetic patients. Food Sci. Nutr. 2019, 49, 854–865. [Google Scholar] [CrossRef]
- Haghighian, M.K.; Rafraf, M.; Moghaddam, A.; Hemmati, S.; Jafarabadi, M.A.; Gargari, B.P. Pomegranate (Punica granatum L.) peel hydro alcoholic extract ameliorates cardiovascular risk factors in obese women with dyslipidemia: A double blind, randomized, placebo controlled pilot study. Eur. J. Integr. Med. 2016, 8, 676–682. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated pomegranate reverts high-density lipoprotein (hdl)-induced endothelial dysfunction and reduces postprandial triglyceridemia in women with acute coronary syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef] [PubMed]
- Dimitreli, G.; Petridis, D.; Kapageridis, N.; Mixiou, M. Effect of pomegranate juice and fir honey addition on the rheological and sensory properties of kefir-type products differing in their fat content. LWT 2019, 111, 799–808. [Google Scholar] [CrossRef]
- Pan, L.H.; Liu, F.; Luo, S.Z.; Luo, J.P. Pomegranate juice powder as sugar replacer enhanced quality and function of set yogurts: Structure, rheological property, antioxidant activity and in vitro bioaccessibility. LWT 2019, 115, 108479. [Google Scholar] [CrossRef]
- Mahajan, D.; Bhat, Z.F.; Kumar, S. Pomegranate (Punica granatum) rind extract as a novel preservative in cheese. Food Biosci. 2015, 12, 47–53. [Google Scholar] [CrossRef]
- Chan, C.L.; Gan, R.Y.; Shah, N.P.; Corke, H. Enhancing antioxidant capacity of Lactobacillus acidophilus-fermented milk fortified with pomegranate peel extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Sandhya, S.; Khamrui, K.; Prasad, W.; Kumar, M.C.T. Preparation of pomegranate peel extract powder and evaluation of its effect on functional properties and shelf life of curd. LWT 2018, 92, 416–421. [Google Scholar] [CrossRef]
- Kennas, A.; Amellal-Chibane, H.; Kessal, F.; Halladj, F. Effect of pomegranate peel and honey fortification on physicochemical, physical, microbiological and antioxidant properties of yoghurt powder. J. Saudi Soc. Agric. Sci. 2018. [Google Scholar] [CrossRef]
- Van Nieuwenhove, C.P.; Moyano, A.; Castro-Gómez, P.; Fontecha, J.; Sáez, G.; Zárate, G.; Pizarro, P.L. Comparative study of pomegranate and jacaranda seeds as functional components for the conjugated linolenic acid enrichment of yogurt. LWT 2019, 111, 401–407. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Bertolo, M.R.; Martins, V.C.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Berizi, E.; Hosseinzadeh, S.; Shekarforoush, S.S.; Barbieri, G. Microbial, chemical, textural and sensory properties of coated rainbow trout by chitosan combined with pomegranate peel extract during frozen storage. Int. J. Biol. Macromol. 2018, 106, 1004–1013. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce Penicillium digitatum postharvest decay of oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Munir, S.; Hu, Y.; Liu, Y.; Xiong, S. Enhanced properties of silver carp surimi-based edible films incorporated with pomegranate peel and grape seed extracts under acidic condition. Food Packag. Shelf Life 2019, 19, 114–120. [Google Scholar] [CrossRef]
- Arabestani, A.; Kadivar, M.; Shahedi, M.; Goli, S.A.H.; Porta, R. Characterization and antioxidant activity of bitter vetch protein-based films containing pomegranate juice. LWT 2016, 74, 77–83. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- El-Nashi, H.B.; Fattah, A.F.A.K.A.; Rahman, N.R.A.; El-Razik, M.A. Quality characteristics of beef sausage containing pomegranate peels during refrigerated storage. Ann. Agric. Sci. 2015, 60, 403–412. [Google Scholar] [CrossRef]
- Juneja, V.K.; Cadavez, V.; Gonzales-Barron, U.; Mukhopadhyay, S.; Friedman, M. Effect of pomegranate powder on the heat inactivation of Escherichia coli O104: H4 in ground chicken. Food Control 2016, 70, 26–34. [Google Scholar] [CrossRef]
- Basiri, S.; Shekarforoush, S.S.; Aminlari, M.; Akbari, S. The effect of pomegranate peel extract (PPE) on the polyphenol oxidase (PPO) and quality of Pacific white shrimp (Litopenaeus vannamei) during refrigerated storage. LWT 2015, 60, 1025–1033. [Google Scholar] [CrossRef]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and antimicrobial activity of rosemary, pomegranate and olive extracts in fish patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, D.; Roila, R.; Andoni, E.; Braconi, P.; Branciari, R. Punica granatum and Citrus spp. extract mix affects spoilage microorganisms growth rate in vacuum-packaged cooked sausages made from pork meat, emmer wheat (Triticum dicoccum Schübler), almond (Prunus dulcis Mill.) and hazelnut (Corylus avellana L). Foods 2019, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Pomegranate seed powder as a functional component of gluten-free bread (Physical, sensorial and antioxidant evaluation). Int. J. Food Sci. Technol. 2018, 53, 1906–1913. [Google Scholar] [CrossRef]
- Saeidi, Z.; Nasehi, B.; Jooyandeh, H. Optimization of gluten-free cake formulation enriched with pomegranate seed powder and transglutaminase enzyme. J. Food Sci. Technol. 2018, 55, 3110–3118. [Google Scholar] [CrossRef]
- Dib, A.; Kasprzak, K.; Wójtowicz, A.; Benatallah, L.; Waksmundzka-Hajnos, M.; Zidoune, M.N.; Oniszczuk, T.; Karakuła-Juchnowicz, H.; Oniszczuk, A. The effect of pomegranate seed powder addition on radical scavenging activity determined by TLC–DPPH test and selected properties of gluten-free pasta. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 364–372. [Google Scholar] [CrossRef]
- Nicosia, M.G.L.D.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Molva, Ç.; Baysal, A.H. Evaluation of bioactivity of pomegranate fruit extract against Alicyclobacillus acidoterrestris DSM 3922 vegetative cells and spores in apple juice. LWT 2015, 62, 989–995. [Google Scholar] [CrossRef]
- Fernández, J.; Pérez-Álvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Smaoui, S.; Hlima, H.B.; Mtibaa, A.C.; Fourati, M.; Sellem, I.; Elhadef, K.; Ennouria, K.; Mellouli, L. Pomegranate peel as phenolic compounds source: Advanced analytical strategies and practical use in meat products. Meat Sci. 2019, 158, 107914. [Google Scholar] [CrossRef] [PubMed]
- Keşkekoğlu, H.; Üren, A. Inhibitory effects of pomegranate seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs after cooking by four different methods. Meat Sci. 2014, 96, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
| Country | Variety | References |
|---|---|---|
| China | Dabaitian, Heyinruanzi, Tongpi, Bopi | [8] |
| Egypt | Arabi, Manfaloty, Nab ElGamal, Wardy | [9] |
| Georgia | Pirosmani, Rubin, Shirvani, Slunar, Vedzisuri, Imeretis Sauketeso | [10] |
| Greece | Hermione, Persephone, Porphirogeneti | [11] |
| India | Ganesh, Mridula, Bhagwa, Ruby, Alandi | [12,13] |
| Iran | Malas-e-Saveh, Rabab-e-Neyriz, Malas-e-Yazdi, Sishe Kape-Ferdos, Naderi-e-Budrood | [14] |
| Israel | Rosh Hapered, Malisi, Wonderful, Asmar | [13,15] |
| Italy | Dente di Cavallo, Neirana, Profeta, A dente Molfetta, Ecotipo Turi, Maddaloni Dolce, Giardino Chiuso Dolce | [16,17] |
| Malta | Blance, Dulce Colourada, Cagin | [13,18] |
| Morocco | Gjebali, Djeibi, Grenade Jaune, Grenade rouge, Bzou, Sefri, Chelfi | [19] |
| Spain | Mollar de Elche, Agri de albatera, Valenciana | [13,20] |
| Tunisia | Gabsi, Tounsi, Zehri, Mezzi, Jebali, Garoussi, Kalaii, Zaghouani | [10,21] |
| Turkey | Cekirdksiz, Ernar, Fellahyemez, Hatay, Akanar, Hicaznar, Janarnar | [10,13] |
| USA | Wonderful, Early Foothill, Granada, Spanish sweet, Ruby red | [13,18,22] |
| Characteristic | Sweet 1 Varieties | Sour–Sweet 1 Varieties | Sour 1 Varieties | Wonderful 2 Variety | Bhagwa 3 Variety |
|---|---|---|---|---|---|
| TSS 4 (°Brix) | 10.0–16.5 | 12.0–15.0 | 13.0–16.0 | 15.7–17.5 | 16.2 ± 0.2 |
| pH | 4.0–4.2 | 3.6–3.7 | 2.9–3.6 | 2.8–3.6 | 3.6 ± 0.1 |
| TA 5 (g/L) | 4.0–6.8 | 8.2–11.4 | 14.8–24.5 | 11.0–13.0 | 3.8 ± 0.2 |
| Fructose (%w/v) | 4.1–6.0 | 3.9–4.0 | 3.5–4.0 | 7.8–9.1 | 8.2 |
| Glucose (%w/v) | 4.3–6.4 | 4.3–4.4 | 3.4–3.9 | 7.3–8.4 | 7.0 |
| Total sugars (%w/v) | 8.5–12.4 | 8.3–8.4 | 7.2–7.9 | 15.3–17.5 | 15.2 |
| Derivative | Effect | References |
|---|---|---|
| whole fruit extract | ↓H2O2-induced oxidative stress; ↓apoptosis; natural antioxidants for skin health | [37] |
| whole fruit extract | antimicrobial activity against 29 clinical Clostridium difficile isolates | [38] |
| juice extract | inhibition of a-glucosidase activity | [39] |
| extract and juice | prebiotic effect | [40] |
| juice | ↓lipogenesis and lipolysis | [41] |
| juice | inhibition of lipase, α-glucosidase and dipeptidyl peptidase-4 | [42] |
| peel extract | inhibition of renal cell carcinoma growth | [43] |
| peel extract | anti-neurodegenerative | [44] |
| peel extract | ↑apoptosis and ↓metastasis in prostate cancer cells | [45] |
| peel and juice extract | inhibition of cariogenic bacteria | [46,47] |
| peel and fruit extract | stimulates osteoblastic differentiation (osteoporosis) | [48,49] |
| peel polysaccharide | immunostimulatory effect | [50] |
| punicalagin | antiproliferative activity against human lung, breast, cervical and prostate cancer cells | [51,52,53] |
| punicalagin | ↑papillary thyroid human carcinoma cell death | [54] |
| punicalagin | inhibition of lipopolysaccharide-induced memory impairment (Alzheimer’s disease) | [55] |
| punicalagin | attenuates osteoclast differentiation (osteoporosis) | [56] |
| pomegranate-derived products (juice, extract, oil) | photo-chemopreventive effect in human reconstituted skin | [57] |
| urolithins | inhibition of neuroinflammation (Alzheimer’s disease) | [58] |
| Derivative | Effect | References |
|---|---|---|
| fruit | ↓of progression of cognitive and behavioral impairments in Alzheimer’s disease | [65] |
| whole fruit extract | anti-inflammatory and antioxidant effects | [66] |
| whole fruit extract | ↓apoptosis and inflammation in liver cells | [67] |
| peel polysaccharide | ↓weight loss and ↑immune organ index of immunosuppressed mice | [68] |
| peel polysaccharide | protection against CCl4-induced liver injury | [69] |
| pomegranate aril extract | inhibition of contact hypersensitivity of allergic dermatitis | [70] |
| juice | ↑hypoxia-induced fetal growth and ↓apoptosis in the placenta in pregnant mice | [71] |
| juice | neuroprotection and protection against oxidative damage in Parkinson’s disease rat model | [72] |
| juice | antileishmanial activity, probably by boosting the endogenous antioxidant activity in female BALB/c mice | [73] |
| leaf | ↓total serum cholesterol and triglycerides of hyperlipidemic mice | [74] |
| peel extract | contribution in prevention and treatment of Giardia lamblia infection | [75] |
| peel extract | preventing bone loss associated with ovariectomy in mice | [48] |
| Derivative | Subject | Effect | References |
|---|---|---|---|
| whole fruit extract | overweight and obese patients | anti-inflammatory effect; ↓body weight, serum glucose, total cholesterol, LDL; ↑HDL | [91] |
| juice | hemodialysis patients | improved blood pressure, serum triglycerides, HDL, oxidative stress and inflammation | [85] |
| juice | humans with type 2 diabetes | ↓serum erythropoietin level | [86] |
| juice | humans with type 2 diabetes | ↓systolic and diastolic blood pressure | [87] |
| juice | healthy adults | maintains visual memory skills | [88] |
| juice | endurance-based athletes | modulation of fat and protein damage | [89] |
| juice | active healthy men | ↓systolic blood pressure, creatinine and muscle damage parameters | [90] |
| seed oil | humans with type 2 diabetes | ↓levels of fasting blood sugar | [93,94] |
| peel extract | humans with type 2 diabetes | hypolipemic, hypoglycemic, and antioxidative potential | [92] |
| peel extract | patients with dyslipidemia | ↓systolic blood pressure, LDL, total cholesterol; ↑HDL | [95] |
| microencapsulated pomegranate | women with acute coronary syndrome | reverts high-density lipoprotein-induced endothelial dysfunction and ↓postprandial triglyceridemia | [96] |
| Derivative | Product | Effect | References |
|---|---|---|---|
| juice | kefir-type | ↑viscosity; ↑acidity | [97] |
| juice powder | yogurt | ↑total phenolics; ↑antioxidant activity; ↑solid-like behavior | [98] |
| peel extract | cheese | ↑lipid oxidative stability; ↑storage quality | [99] |
| peel extract powder | fermented milk | ↑total phenolics; ↑antioxidant activity | [100] |
| peel extract powder | cheese | ↑antioxidant activity; ↑shelf life | [101] |
| peel extract powder | freeze-dried yogurt | ↑total phenolics; ↑antioxidant activity | [102] |
| seed powder | yogurt | ↑antioxidant activity; fatty acid profile improvement | [103] |
| Derivative | Product | Effect | References |
|---|---|---|---|
| peel extract | zein-based film (cheese) | ↑tensile strength; ↑antioxidant and antimicrobial activity | [104] |
| peel extract | chitosan coating (rainbow trout, pacific white shrimp, strawberry) | improvement of functional characteristics of coatings; improvement of sensory characteristics and ↑shelf life of food | [105,106,107,108] |
| peel powder | starch-based films | better mechanical properties; antimicrobial activity | [109] |
| peel extract | chitosan/locust gum coating (oranges) | inhibition of Penicillium digitatum; ↑shelf-life of oranges | [110] |
| peel extract | surimi-based films | superior mechanical properties; improved thermal stability | [111] |
| juice | bitter vetch seed protein films | antioxidant activity; improvement of physicochemical properties | [112] |
| Derivative | Product | Effect | References |
|---|---|---|---|
| peel powder | meatballs | ↑antioxidant activity; ↓lipid and protein oxidation; ↑microbial quality | [113] |
| peel powder | beef sausage | quality criteria improvement; improvement of cooking characteristics | [114] |
| juice powder | raw ground chicken | ↓heat resistance of E. coli | [115] |
| peel extract | pacific white shrimp | ↓lipid oxidation; ↓melanosis; ↓microbial growth | [116] |
| extract | fish patties | ↑shelf-life | [117] |
| extract | pork sausage | controlling microbial growth and oxidation; ↑shelf-life | [118] |
| Derivative | Product | Effect | References |
|---|---|---|---|
| peel extract | hazelnut paste | ↑shelf life; delay oxidation | [119] |
| peel extract | cookies | ↑shelf life; ↑antioxidant activity; ↑panelist acceptance (odor, color) | [120] |
| seed powder | gluten-free bread | ↑specific volume and springiness; ↑antioxidant activity | [121] |
| seed powder | gluten-free cake | ↑antioxidant activity, protein and fiber; ↓peroxide value | [122] |
| seed powder | gluten-free sheeted pasts | ↑antioxidant activity; ↓cooking and textural parameters | [123] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. https://doi.org/10.3390/foods9020122
Kandylis P, Kokkinomagoulos E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods. 2020; 9(2):122. https://doi.org/10.3390/foods9020122
Chicago/Turabian StyleKandylis, Panagiotis, and Evangelos Kokkinomagoulos. 2020. "Food Applications and Potential Health Benefits of Pomegranate and its Derivatives" Foods 9, no. 2: 122. https://doi.org/10.3390/foods9020122
APA StyleKandylis, P., & Kokkinomagoulos, E. (2020). Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods, 9(2), 122. https://doi.org/10.3390/foods9020122






