Proximal Composition and Nutritive Value of Raw, Smoked and Pickled Freshwater Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Areas and Sampling
2.2. Fish Samples Preparation Procedure
2.3. Determination of Protein, Fat, Salt, Collagen and Moisture Content in Fish Samples and Energy Value by Near Infrared Spectroscopy Method (NIRS)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Proximal Composition of Fish Product Samples
3.2. Correlations Between Parameters Determined by the NIRS Method
3.3. Energy Value of Fish Product Samples
3.4. Calculated Reference Intake for Energy Value and Nutrients Based on the Consumption of Raw, Smoked and Pickled Fish (One Serving—150 g)
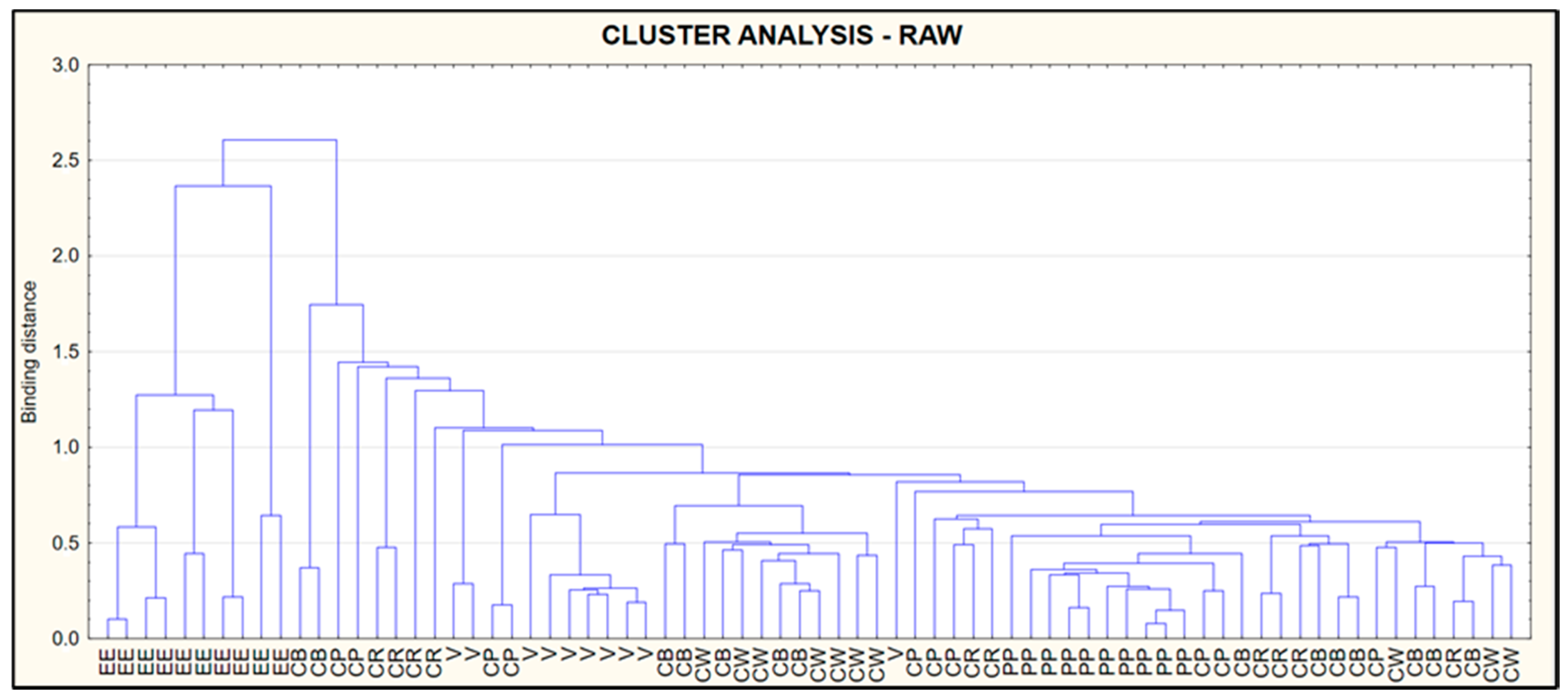
3.5. Cluster Analysis (CA)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases, Report of the Joint WHO/FAO Expert Consultation; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; p. 916. Available online: http://apps.who.int/iris/bitstream/10665/42665/1/WHO_TRS_916.pdf (accessed on 23 April 2003).
- Di Bella, G.; Potortì, A.G.; Turco, V.L.; Bua, D.; Licata, P.; Cicero, N.; Dugo, G. Trace elements in Thunnus thynnusfrom Mediterranean Sea and benefit–risk assessment for consumers. Food Addit. Contam. Part B 2015, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Turco, V.L.; Di Bella, G.; Furci, P.; Cicero, N.; Pollicino, G.; Dugo, G. Heavy metals content by ICP-OES in Sarda sarda, Sardinella aurita and Lepidopus caudatus from the Strait of Messina (Sicily, Italy). Nat. Prod. Res. 2013, 27, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.M.; García-Maldonado, E.; Gallego-Narbón, A.; Zapatera, B.; Vaquero, M.P. Fatty Acid Profile and Cardiometabolic Markers in Relation with Diet Type and Omega-3 Supplementation in Spanish Vegetarians. Nutrients 2019, 11, 1659. [Google Scholar] [CrossRef] [PubMed]
- Czarkowski, T.; Kupren, K.; Kwasiborska, D.; Jaczewski, J. Water and fish as significant elements of rural tourism in the Warmian-Masurian Voivodeship. Komun. Ryb. 2014, 4, 1–8, (In Polish with English abstract). [Google Scholar]
- Hliwa, P. Fish habitats and factors influencing contemporary fish fauna in Polish waters. In Proceedings of the Fish in Our Waters—Expectations, Reality, Challenges, Serock, Poland, 24 November 2018; pp. 2–14. Available online: https://ompzw.pl/files/filesfile/1/file-4652.pdf (accessed on 14 December 2020). (In Polish).
- District of the Polish Angling Association in Olsztyn. List of Fish Most Often Caught in Masuria. Available online: https://infomazury.com.pl/informacje-dla-wedkarzy (accessed on 14 December 2020). (In Polish).
- Prieto, N.; Juarez, M.; Larsen, I.; López-Campos, Ó.; Zijlstra, R.; Aalhus, J. Rapid discrimination of enhanced quality pork by visible and near infrared spectroscopy. Meat Sci. 2015, 110, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; Informa UK Limited: London, UK, 2007. [Google Scholar]
- Krähmer, A.; Engel, A.; Kadow, D.; Ali, N.A.; Umaharan, P.; Kroh, L.W.; Schulz, H. Fast and neat—Determination of biochemical quality parameters in cocoa using near infrared spectroscopy. Food Chem. 2015, 181, 152–159. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Martínez-Marín, A.; Polvillo, O.; Fernández-Cabanás, V.M.; Carrizosa, J.; Urrutia, B.; Serradilla, J. Near Infrared Spectroscopy (NIRS) for the determination of the milk fat fatty acid profile of goats. Food Chem. 2016, 190, 244–252. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Singh, M.; Rennick, K.A.; Bakota, E.L.; Jham, G.N.; Liu, S.X.; Vaughn, S.F. Detection of Corn Adulteration in Brazilian Coffee (Coffea arabica) by Tocopherol Profiling and Near-Infrared (NIR) Spectroscopy. J. Agric. Food Chem. 2015, 63, 10662–10668. [Google Scholar] [CrossRef] [PubMed]
- Cascant, M.M.; Breil, C.; Fabiano-Tixier, A.S.; Chemat, F.; Garrigues, S.; De La Guardia, M. Determination of fatty acids and lipid classes in salmon oil by near infrared spectroscopy. Food Chem. 2018, 239, 865–871. [Google Scholar] [CrossRef]
- Grassi, S.; Casiraghi, E.; Alamprese, C. Handheld NIR device: A non-targeted approach to assess authenticity of fish fillets and patties. Food Chem. 2018, 243, 382–388. [Google Scholar] [CrossRef]
- Karlsdottir, M.G.; Arason, S.; Kristinsson, H.G.; Sveinsdottir, K. The application of near infrared spectroscopy to study lipid characteristics and deterioration of frozen lean fish muscles. Food Chem. 2014, 159, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ottavian, M.; Facco, P.; Fasolato, L.; Novelli, E.; Mirisola, M.; Perini, M.; Barolo, M. Use of Near-Infrared Spectroscopy for Fast Fraud Detection in Seafood: Application to the Authentication of Wild European Sea Bass (Dicentrarchus labrax). J. Agric. Food Chem. 2012, 60, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Pochanagone, S.; Rittiron, R. Preliminary Study on the Determination of ppm-Level Concentration of Histamine in Tuna Fish Using a Dry Extract System for Infrared Coupled with Near-Infrared Spectroscopy. ACS Omega 2019, 4, 19164–19171. [Google Scholar] [CrossRef] [PubMed]
- Ritthiruangdej, P.; Suwonsichon, T. Relationships between NIR Spectra and Sensory Attributes of Thai Commercial Fish Sauces. Anal. Sci. 2006, 23, 809–814. [Google Scholar] [CrossRef]
- Tito, N.; Rodemann, T.; Powell, S.M. Use of near infrared spectroscopy to predict microbial numbers on Atlantic salmon. Food Microbiol. 2012, 32, 431–436. [Google Scholar] [CrossRef]
- 39.1.01 AOAC Official Method 983.18, Meat and Meat Products Preparation of Sample Procedure. Meat and Meat Products David L. Soderberg, Chapter Editor, U.S. Department of Agriculture. Available online: https://www.ams.usda.gov/sites/default/files/media/QAD%20610E%20Exhibit_AOAC%20Official%20Method.pdf (accessed on 14 December 2020).
- Anderson, S. Determination of fat, moisture and protein in meat and meat products by using the FOSS FoodScan™ Near infrared Spectrophotometr with FOSS Artificial Neural Network Calibration Model and Associated Database: Collaborative study. J. AOAC Int. 2007, 90, 1073–1083. [Google Scholar] [CrossRef]
- REGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj (accessed on 14 December 2020).
- USDA, U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th Edition. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 6 June 2019).
- Kiczorowska, B.; Samolińska, W.; Grela, E.R.; Bik-Małodzińska, M. Nutrient and Mineral Profile of Chosen Fresh and Smoked Fish. Nutrients 2019, 11, 1448. [Google Scholar] [CrossRef]
- Jankowska, B.; Zakęś, Z.; Żmijewski, T.; Szczepkowski, M.; Kowalska, A. Slaughter yield, proximate composition and flesh colour of cultivated and wild perch (Perca fluviatilis L.). Czech J. Anim. Sci. 2008, 52, 260–267. [Google Scholar] [CrossRef]
- Łuczyńska, Ł.; Tońska, E.; Krejszeff, S.; Żarski, D. Comparison of Fatty Acids in the Muscles and Liver of Pond-Cultured and Wild Perch, Perca fluviatilis (L.), in Poland. Turk. J. Fish. Aquat. Sci. 2016, 16, 19–27. [Google Scholar] [CrossRef]
- Polak-Juszczak, L.; Adamczyk, M. Quality and amino acid composition of protein of fish from the Vistula Lagoon. Żywność. Nauka Technol. Jakość 2009, 3, 75–83. (In Polish) [Google Scholar]
- Tilami, S.K.; Sampels, S.; Zajíc, T.; Krejsa, J.; Másílko, J.; Mráz, J. Nutritional value of several commercially important river fish species from the Czech Republic. PeerJ 2018, 6, e5729. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kang, S.I.; Kim, Y.J.; Kim, M.J.; Heu, M.S.; Choi, B.D.; Kim, J.-S. Comparison of collagen characteristics of sea- and freshwater-rainbow trout skin. Food Sci. Biotechnol. 2016, 25, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Nematollahi, A.; Saei-Dehkordi, S.S. Proximate composition and fatty acid profile of edible tissues of Capoeta damascina (Valenciennes, 1842) reared in freshwater and brackish water. J. Food Compos. Anal. 2013, 32, 150–154. [Google Scholar] [CrossRef]
- Badiani, A.; Anfossi, P.; Fiorentini, L.; Gatta, P.P.; Manfredini, M.; Nanni, N.; Stipa, S.; Tolomelli, B. Nutritional composition of cultured Sturgeon (Acipenser spp.). J. Food Compos. Anal. 1996, 9, 171–190. [Google Scholar] [CrossRef]
- Bayse, S.M.; Regish, A.M.; McCormick, S.D. Proximate composition, lipid utilization and validation of a non-lethal method to determine lipid content in migrating American shad Alosa sapidissima. J. Fish Biol. 2018, 92, 1832–1848. [Google Scholar] [CrossRef]
- Chandrashekar, K.; Deosthale, Y. Proximate Composition, Amino Acid, Mineral and Trace Element Content of the Edible Muscle of 20 Indian Fish Species. J. Food Compos. Anal. 1993, 6, 195–200. [Google Scholar] [CrossRef]
- Bogard, J.R.; Thilsted, S.H.; Marks, G.C.; Wahab, A.; Hossain, M.A.; Jakobsen, J.; Stangoulis, J.C.R. Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J. Food Compos. Anal. 2015, 42, 120–133. [Google Scholar] [CrossRef]
- Ciborowska, H.; Rudnicka, A. Dietetics. Nutrition of a Healthy and Sick Person; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 2007. [Google Scholar]
- Gawęcki, J.; Hryniewiecki, L. Human Nutrition. Basics of Nutrition Science; WSiP: Warsaw, Poland, 2007. (In Polish) [Google Scholar]
- Polak-Juszczak, P.; Komar-Szymczak, K. Fatty acid profiles and fat contents of commercially important fish from Vistula Lagoon. Pol. J. Food Nutr. Sci. 2009, 59, 225–229. [Google Scholar]
- Aggelousis, G.; Lazos, E.S. Fatty acid composition of the lipids from eight freshwater fish species from Greece. J. Food Compos. Anal. 1991, 4, 68–76. [Google Scholar] [CrossRef]
- Suloma, A.; Ogata, H.Y.; Garibay, E.S.; Chavez, D.R.; El-Haroun, E.R. Fatty acid composition of Nile tilapia (Oreochromis niloticus) muscles: A comparative study with commercially important tropical freshwater fish in Philippines. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 921–932. [Google Scholar]
- Branciari, R.; Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Miraglia, D.; Bailetti, L.; Franceschini, R. Nutritional quality, safety and sensory properties of smoked tench (Tinca tinca) pâté from Trasimeno Lake, Italy. Ital. J. Food Saf. 2019, 8, 8130. [Google Scholar] [CrossRef]
- VanderJagt, D.J.; Glew, R.H.; Owolawashe, H.M.; Huang, Y.-S.; Chuang, L.-T. Fatty Acid Content of the Smoked, Fresh-Water Fish Clairas Gariepinus (Wanka Harwada, Hausa) in Northern Nigeria. Highl. Med. Res. J. 2005, 2, 8–13. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Mielcarek, K.; Nowakowski, P.; Gromkowska-Kępka, K.; Naliwajko, S.K.; Parobczak, A.; Markiewicz-Żukowska, R.; Socha, K.; Borawska, M.H. Assessment of salt content in smoked freshwater fish. Bromatol. Chem. Toksykol. 2018, 51, 24–30, (In Polish with English Abstract). [Google Scholar]
- Tokarczyk, G.; Szymczak, B.; Szymczak, M.; Domiszewski, Z. Changes in selected chemical and microbiological indicators during warm smoking process of thawed Whitefish (Coregonis clupeaformis). Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Breck, J.E. Body composition in fishes: Body size matters. Aquaculture 2014, 433, 40–49. [Google Scholar] [CrossRef]
- Filho, M.M.R.; Ramos, M.I.L.; Hiane, P.A.; De Souza, E.M.T. Nutritional Value of Seven Freshwater Fish Species From the Brazilian Pantanal. J. Am. Oil Chem. Soc. 2010, 87, 1461–1467. [Google Scholar] [CrossRef]
- GUS, Główny Urząd Statystyczny (Central Statistical Office). Statistical Yearbook Agriculture; GUS: Warsaw, Poland, 2019. Available online: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-agriculture-2018,6,13.html (accessed on 14 December 2020).
- Cieślik, E.; Siembida, A.; Cieślik, I. Nutritional awareness of fish and fish preserves consumption among the Malopolska Voivodeship’s residents. Bromatol. Chem. Toksykol. 2014, 47, 49–56, (In Polish with English Abstract). [Google Scholar]
- Hryszko, K. Fish market and consumption in 2016. In Proceedings of the Institute of Agricultural Economics and Food Economy, National Research Institute, XLII Training—Salmon Fish Breeders Conference, Gdynia, Poland, 5–7 December 2017; Available online: http://sprl.pl/userfiles/files/Krzysztof%20Hryszko.pdf (accessed on 14 December 2020). (In Polish).




| Fish Species | Raw | Smoked | Pickled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | |
| Brown trout (1) (Salmo trutta m. lacustris L.) | NA a | NA | NA | NA | 10 | 20.98 ± 1.3 | 19.57 | 23.14 | NA | NA | NA | NA |
| Common bream (2) (Abramis brama L.) | 14 | 19.72 ± 1.5 | 16.35 | 21.12 | 10 | 23.40 ± 1.5 | 21.62 | 25.39 | 10 | 17.84 ± 0.4 | 17.29 | 18.53 |
| Common perch (3) (Perca fluviatilis L.) | 10 | 19.33 ± 0.6 | 18.47 | 19.90 | 10 | 22.79 ± 1.1 | 21.66 | 24.18 | 10 | 16.52 ± 2.9 | 12.22 | 20.25 |
| Common roach (4) (Rutilus rutilus L.) | 10 | 19.92 ± 0.9 | 18.8 | 21.13 | NA | NA | NA | NA | 12 | 16.96 ± 2.6 | 13.68 | 19.72 |
| Common whitefish (5) (Coregonus lavaretus L.) | 10 | 20.75 ± 0.9 | 19.3 | 21.74 | 12 | 23.01 ± 1.5 | 23.01 | 20.39 | 10 | 19.56 ± 1.9 | 16.62 | 21.85 |
| European eel (6) (Anguilla anguilla L.) | 10 | 17.72 ± 1.0 | 15.88 | 18.98 | 13 | 18.32 ± 3.0 | 12.80 | 21.54 | 10 | 13.12 ± 1.2 | 11.48 | 13.97 |
| Pike-perch (7) (Sander lucioperca L.) | 10 | 19.74 ± 0.1 | 19.48 | 19.88 | NA | NA | NA | NA | 10 | 18.31 ± 2.4 | 13.94 | 21.92 |
| Vendace (8) (Coregonus albula L.) | 10 | 20.08 ± 0.7 | 18.9 | 20.64 | 11 | 23.97 ± 1.5 | 22.21 | 26.18 | 10 | 18.31 ± 1.3 | 16.42 | 20.37 |
| Total | 74 | 19.61 ± 1.2 | 15.88 | 21.74 | 66 | 21.96 ± 2.7 | 12.80 | 26.18 | 72 | 17.22 ± 2.7 | 11.48 | 21.92 |
| Fish Species | Raw | Smoked | Pickled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | |
| Brown trout (1) (Salmo trutta m. lacustris L.) | NA a | NA | NA | NA | 10 | 18.81 ± 3.8 | 13.82 | 23.98 | NA | NA | NA | NA |
| Common bream (2) (Abramis brama L.) | 14 | 2.10 ± 0.6 | 1.24 | 3.34 | 10 | 3.50 ± 1.8 | 1.21 | 5.47 | 10 | 4.72 ± 1.1 | 3.04 | 6.52 |
| Common perch (3) (Perca fluviatilis L.) | 10 | 2.43 ± 1.9 | 0.89 | 6.20 | 10 | 1.03 ± 0.2 | 0.72 | 1.21 | 10 | 1.98 ± 2.4 | 0.01 | 5.37 |
| Common roach (4) (Rutilus rutilus L.) | 10 | 2.82 ± 2.0 | 1.47 | 6.86 | NA | NA | NA | NA | 12 | 3.11 ± 1.4 | 1.48 | 4.74 |
| Common whitefish (5) (Coregonus lavaretus L.) | 10 | 3.28 ± 0.3 | 2.83 | 3.77 | 12 | 3.11 ± 1.5 | 1.53 | 5.83 | 10 | 3.77 ± 0.9 | 2.84 | 5.46 |
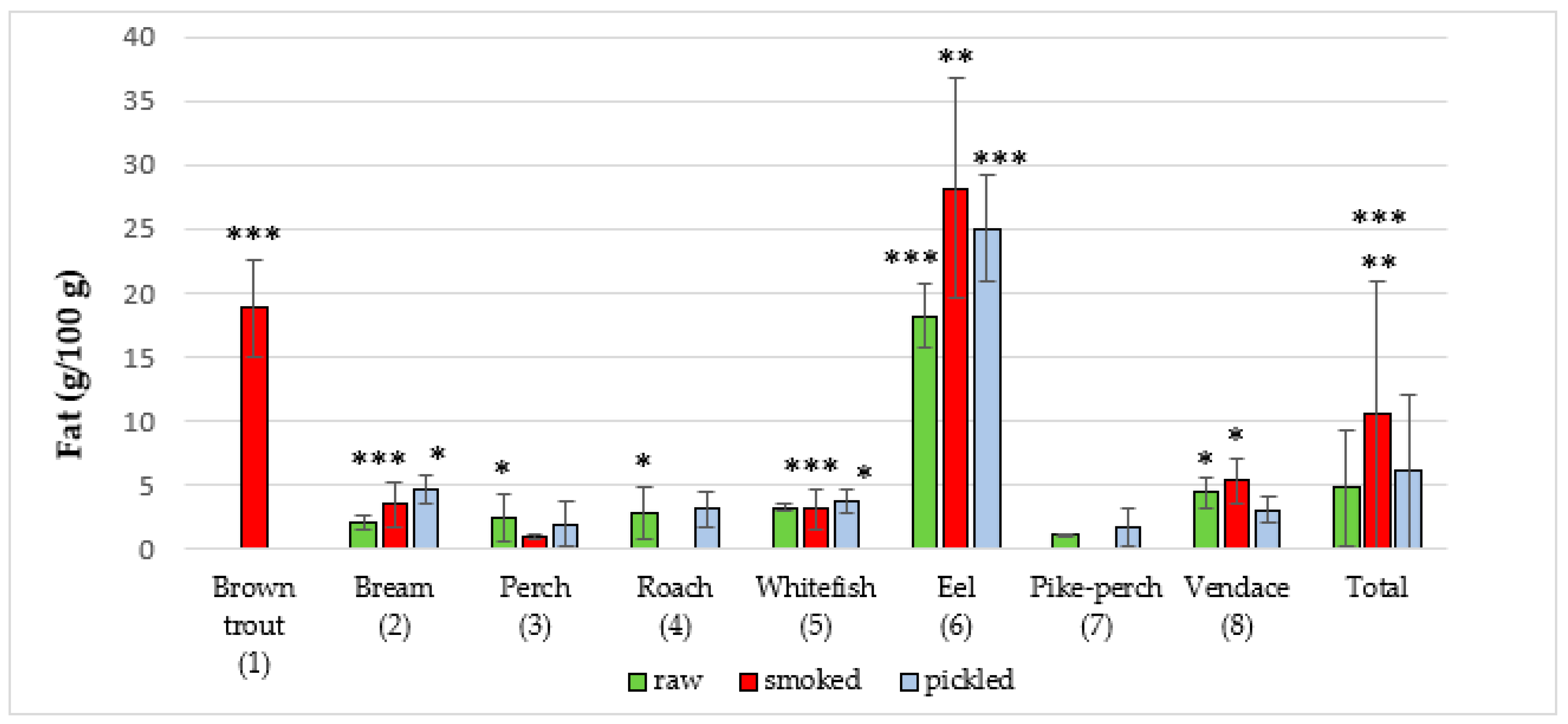
| European eel (6) (Anguilla anguilla L.) | 10 | 18.20 ± 2.5 | 14.89 | 22.33 | 13 | 28.16 ± 8.6 | 18.70 | 43.12 | 10 | 25.04 ± 4.1 | 18.26 | 29.72 |
| Pike-perch (7) (Sander lucioperca L.) | 10 | 1.11 ± 0.1 | 1.01 | 1.26 | NA | NA | NA | NA | 10 | 1.72 ± 2.0 | 0.01 | 6.02 |
| Vendace (8) (Coregonus albula L.) | 10 | 4.46 ± 1.2 | 2.18 | 5.33 | 11 | 5.34 ± 1.7 | 2.19 | 7.38 | 10 | 3.04 ± 1.0 | 1.66 | 4.45 |
| Total | 74 | 4.76 ± 5.6 | 0.89 | 22.33 | 66 | 10.54 ± 11.2 | 0.72 | 43.12 | 72 | 6.11 ± 8.0 | 0.01 | 29.72 |
| Fish Species | Raw | Smoked | Pickled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | |
| Brown trout (1) (Salmo trutta m. lacustris L.) | NA a | NA | NA | NA | 10 | 1.09 ± 0.2 | 0.86 | 1.36 | NA | NA | NA | NA |
| Common bream (2) (Abramis brama L.) | 14 | 0.91 ± 0.1 | 0.73 | 1.09 | 10 | 1.45 ± 0.2 | 1.04 | 1.72 | 10 | 2.03 ± 0.1 | 1.89 | 2.24 |
| Common perch (3) (Perca fluviatilis L.) | 10 | 1.01 ± 0.1 | 0.89 | 1.16 | 10 | 2.45 ± 0.5 | 2.07 | 3.39 | 10 | 2.04 ± 0.2 | 1.82 | 2.24 |
| Common roach (4) (Rutilus rutilus L.) | 10 | 1.11 ± 0.1 | 0.90 | 1.24 | NA | NA | NA | NA | 12 | 1.76 ± 0.1 | 1.69 | 1.88 |
| Common whitefish (5) (Coregonus lavaretus L.) | 10 | 0.85 ± 0.1 | 0.77 | 0.95 | 12 | 1.96 ± 0.3 | 1.55 | 2.63 | 10 | 1.70 ± 0.1 | 1.47 | 1.83 |
| European eel (6) (Anguilla anguilla L.) | 10 | 1.34 ± 0.1 | 1.12 | 1.48 | 13 | 1.94 ± 0.5 | 0.77 | 2.46 | 10 | 1.69 ± 0.1 | 1.65 | 1.76 |
| Pike-perch (7) (Sander lucioperca L.) | 10 | 1.01 ± 0.1 | 0.97 | 1.09 | NA | NA | NA | NA | 10 | 2.02 ± 0.2 | 1.69 | 2.29 |
| Vendace (8) (Coregonus albula L.) | 10 | 1.02 ± 0.1 | 0.96 | 1.13 | 11 | 1.61 ± 0.7 | 1.08 | 2.93 | 10 | 1.92 ± 0.1 | 1.74 | 2.06 |
| Total | 74 | 1.03 ± 0.2 | 0.73 | 1.48 | 66 | 1.76 ± 0.6 | 0.77 | 3.39 | 72 | 1.88 ± 0.2 | 1.47 | 2.29 |
| Fish Species | Raw | Smoked | Pickled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | |
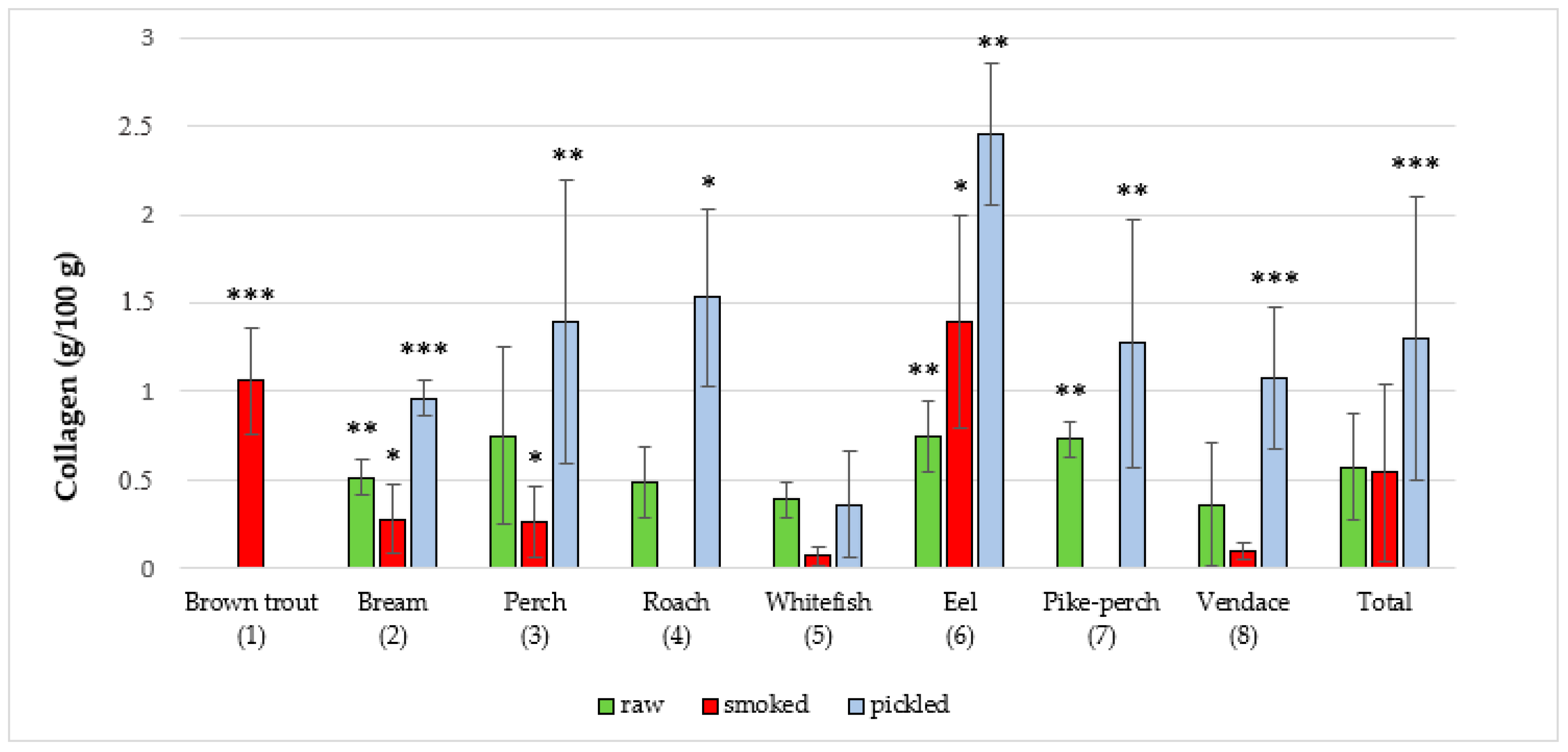
| Brown trout (1) (Salmo trutta m. lacustris L.) | NA a | NA | NA | NA | 10 | 1.06 ± 0.3 | 0.64 | 1.39 | NA | NA | NA | NA |
| Common bream (2) (Abramis brama L.) | 14 | 0.51 ± 0.1 | 0.34 | 0.75 | 10 | 0.28 ± 0.2 | 0.05 | 0.54 | 10 | 0.96 ± 0.1 | 0.67 | 1.11 |
| Common perch (3) (Perca fluviatilis L.) | 10 | 0.75 ± 0.5 | 0.34 | 1.81 | 10 | 0.26 ± 0.2 | 0.01 | 0.57 | 10 | 1.39 ± 0.8 | 0.70 | 2.45 |
| Common roach (4) (Rutilus rutilus L.) | 10 | 0.49 ± 0.2 | 0.20 | 0.94 | NA | NA | NA | NA | 12 | 1.53 ± 0.5 | 0.99 | 2.26 |
| Common whitefish (5) (Coregonus lavaretus L.) | 10 | 0.39 ± 0.1 | 0.30 | 0.47 | 12 | 0.07 ± 0.1 | 0.00 | 0.22 | 10 | 0.36 ± 0.4 | 0.00 | 0.88 |
| European eel (6) (Anguilla anguilla L.) | 10 | 0.75 ± 0.2 | 0.41 | 1.02 | 13 | 1.39 ± 0.6 | 0.45 | 2.25 | 10 | 2.45 ± 0.4 | 1.58 | 2.94 |
| Pike-perch (7) (Sander lucioperca L.) | 10 | 0.73 ± 0.1 | 0.65 | 0.86 | NA | NA | NA | NA | 10 | 1.27 ± 0.7 | 0.40 | 2.52 |
| Vendace (8) (Coregonus albula L.) | 10 | 0.36 ± 0.4 | 0.09 | 1.06 | 11 | 0.1 ± 0.1 | 0.00 | 0.28 | 10 | 1.07 ± 0.4 | 0.57 | 1.84 |
| Total | 74 | 0.57 ± 0.3 | 0.09 | 1.81 | 66 | 0.54 ± 0.6 | 0.00 | 2.25 | 72 | 1.30 ± 0.8 | 0.01 | 2.94 |
| Fish Species | Raw | Smoked | Pickled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | n | Mean ± SD | Min. | Max. | |
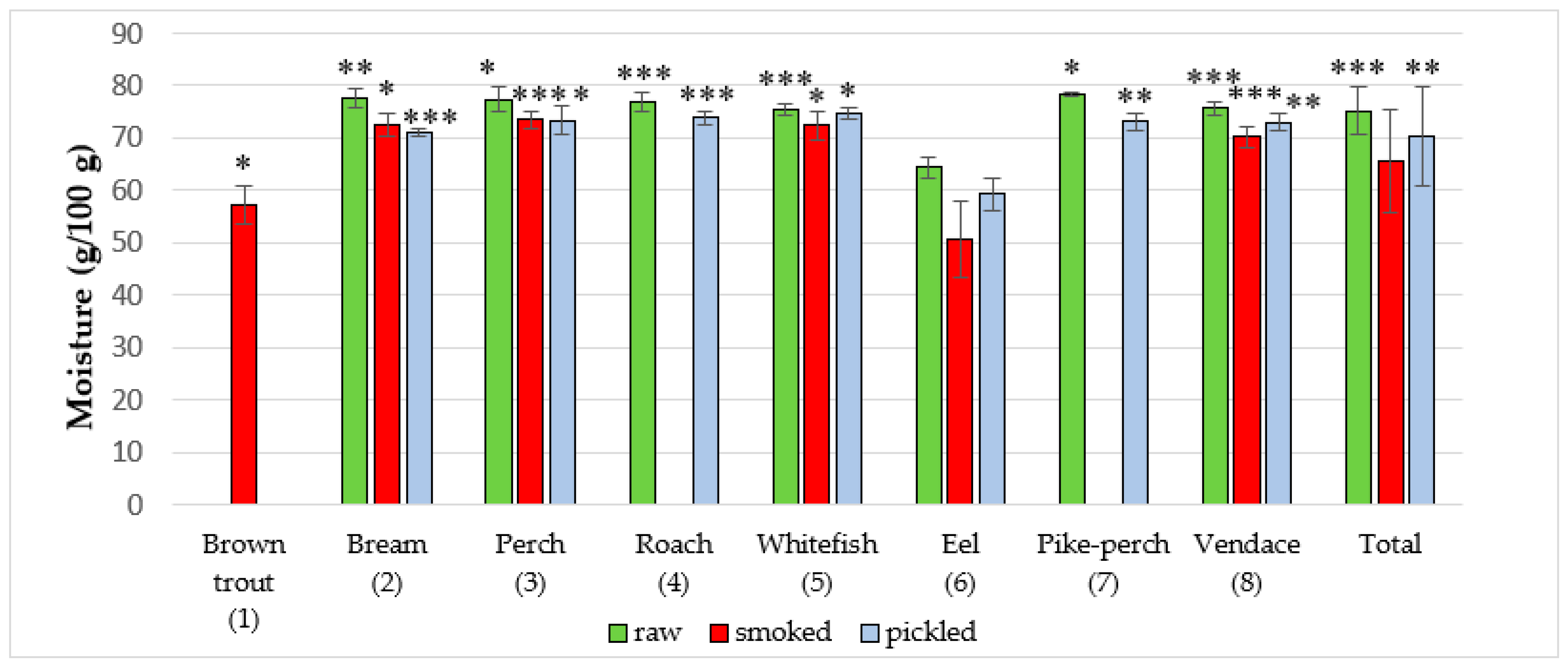
| Brown trout (1) (Salmo trutta m. lacustris L.) | NA | NA a | NA | NA | 10 | 57.13 ± 3.6 | 52.27 | 60.67 | NA | NA | NA | NA |
| Common bream (2) (Abramis brama L.) | 14 | 77.61 ± 1.7 | 75.03 | 80.66 | 10 | 72.46 ± 2.1 | 69.31 | 74.56 | 10 | 71.06 ± 0.6 | 70.12 | 72.40 |
| Common perch (3) (Perca fluviatilis L.) | 10 | 77.41 ± 2.5 | 72.82 | 80.00 | 10 | 73.55 ± 1.6 | 70.91 | 75.31 | 10 | 73.42 ± 2.7 | 68.55 | 76.02 |
| Common roach (4) (Rutilus rutilus L.) | 10 | 76.93 ± 1.9 | 73.51 | 79.51 | NA | NA | NA | NA | 12 | 73.83 ± 1.3 | 71.27 | 75.39 |
| Common whitefish (5) (Coregonus lavaretus L.) | 10 | 75.50 ± 1.1 | 74.2 | 77.11 | 12 | 72.37 ± 2.6 | 68.69 | 76.35 | 10 | 74.83 ± 1.1 | 72.51 | 76.27 |
| European eel (6) (Anguilla anguilla L.) | 10 | 64.36 ± 2.1 | 61.01 | 66.67 | 13 | 50.54 ± 7.3 | 38.11 | 58.84 | 10 | 59.37 ± 3.1 | 53.30 | 64.55 |
| Pike-perch (7) (Sander lucioperca L.) | 10 | 78.43 ± 0.3 | 78 | 78.81 | NA | NA | NA | NA | 10 | 73.15 ± 1.7 | 70.48 | 76.30 |
| Vendace (8) (Coregonus albula L.) | 10 | 75.66 ± 1.4 | 74.72 | 78.34 | 11 | 70.25 ± 2.0 | 66.94 | 72.68 | 10 | 73.06 ± 1.7 | 70.20 | 75.68 |
| Total | 74 | 75.26 ± 4.7 | 61.01 | 80.66 | 66 | 65.60 ± 10.0 | 38.11 | 76.35 | 72 | 70.31 ± 9.6 | 53.30 | 76.30 |
| Fish Species | kJ | kcal | ||||
|---|---|---|---|---|---|---|
| Raw | Smoked | Pickled | Raw | Smoked | Pickled | |
| Brown trout (Salmo trutta m. lacustris L.) | NA | 1060.1 | NA | NA | 253.2 | NA |
| Common bream (Abramis brama L.) | 409.4 | 523.8 | 476.6 | 97.8 | 125.1 | 113.8 |
| Common perch (Perca fluviatilis L.) | 415.3 | 420.5 | 351.3 | 99.2 | 100.4 | 83.9 |
| Common roach (Rutilus rutilus L.) | 439.9 | NA | 401.2 | 105.1 | NA | 95.8 |
| Common whitefish (Coregonus lavaretus L.) | 471.1 | 502.5 | 469.6 | 112.5 | 120.1 | 112.2 |
| European eel (Anguilla anguilla L.) | 982.6 | 1367.9 | 1163.3 | 234.7 | 326.7 | 277.8 |
| Pike-perch (Sander lucioperca L.) | 372.4 | NA | 371.5 | 89.0 | NA | 88.7 |
| Vendace (Coregonus albula L.) | 504.3 | 602.7 | 421.2 | 120.5 | 143.9 | 100.6 |
| Total | 507.8 | 764.9 | 518.6 | 121.3 | 182.7 | 123.9 |
| (%) RI of kJ | (%) RI of kcal | (%) RI of Protein | (%) RI of Fat | (%) RI of Salt | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | S | P | R | S | P | R | S | P | R | S | P | R | S | P | |
| Brown trout (Salmo trutta m. lacustris L.) | NA | 18.9 | NA | NA | 19.0 | NA | NA | 62.9 | NA | NA | 40.3 | NA | NA | 27.3 | NA |
| Common bream (Abramis brama L.) | 7.31 | 9.35 | 8.51 | 7.33 | 9.38 | 8.54 | 59.2 | 70.2 | 53.5 | 4.50 | 7.50 | 10.1 | 22.8 | 36.3 | 50.8 |
| Common perch (Perca fluviatilis L.) | 7.41 | 7.51 | 6.27 | 7.44 | 7.53 | 6.29 | 58.0 | 68.4 | 49.6 | 5.21 | 2.21 | 4.24 | 25.3 | 61.3 | 51.0 |
| Common roach (Rutilus rutilus L.) | 7.85 | NA | 7.16 | 7.88 | NA | 7.19 | 59.8 | NA | 50.9 | 6.04 | NA | 6.66 | 27.8 | NA | 44.0 |
| Common whitefish (Coregonus lavaretus L.) | 8.41 | 8.97 | 8.38 | 8.44 | 9.01 | 8.41 | 62.3 | 69.1 | 58.7 | 7.03 | 6.66 | 8.08 | 21.3 | 49.0 | 42.5 |
| European eel (Anguilla anguilla L.) | 17.5 | 24.4 | 20.8 | 17.6 | 24.5 | 20.8 | 53.2 | 55.0 | 39.4 | 39.0 | 60.3 | 51.5 | 33.5 | 48.5 | 42.3 |
| Pike-perch (Sander lucioperca L.) | 6.65 | NA | 6.63 | 6.67 | NA | 6.54 | 59.2 | NA | 54.9 | 2.38 | NA | 3.69 | 25.3 | NA | 50.5 |
| Vendace (Coregonus albula L.) | 9.01 | 10.8 | 7.52 | 9.03 | 10.8 | 7.54 | 60.2 | 71.9 | 54.9 | 9.56 | 11.4 | 6.51 | 25.5 | 40.3 | 48.0 |
| Total | 9.07 | 13.6 | 9.26 | 9.10 | 13.7 | 9.29 | 58.8 | 65.9 | 51.7 | 10.2 | 22.6 | 13.1 | 25.8 | 44.0 | 47.0 |
| RI a | 8400 kJ | 2000 kcal | 50 g | 70 g | 6 g | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarek, K.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Moskwa, J.; Nowakowski, P.; Borawska, M.H.; Socha, K. Proximal Composition and Nutritive Value of Raw, Smoked and Pickled Freshwater Fish. Foods 2020, 9, 1879. https://doi.org/10.3390/foods9121879
Mielcarek K, Puścion-Jakubik A, Gromkowska-Kępka KJ, Soroczyńska J, Naliwajko SK, Markiewicz-Żukowska R, Moskwa J, Nowakowski P, Borawska MH, Socha K. Proximal Composition and Nutritive Value of Raw, Smoked and Pickled Freshwater Fish. Foods. 2020; 9(12):1879. https://doi.org/10.3390/foods9121879
Chicago/Turabian StyleMielcarek, Konrad, Anna Puścion-Jakubik, Krystyna J. Gromkowska-Kępka, Jolanta Soroczyńska, Sylwia K. Naliwajko, Renata Markiewicz-Żukowska, Justyna Moskwa, Patryk Nowakowski, Maria H. Borawska, and Katarzyna Socha. 2020. "Proximal Composition and Nutritive Value of Raw, Smoked and Pickled Freshwater Fish" Foods 9, no. 12: 1879. https://doi.org/10.3390/foods9121879
APA StyleMielcarek, K., Puścion-Jakubik, A., Gromkowska-Kępka, K. J., Soroczyńska, J., Naliwajko, S. K., Markiewicz-Żukowska, R., Moskwa, J., Nowakowski, P., Borawska, M. H., & Socha, K. (2020). Proximal Composition and Nutritive Value of Raw, Smoked and Pickled Freshwater Fish. Foods, 9(12), 1879. https://doi.org/10.3390/foods9121879









