Estimation of Fatty Acids in Intramuscular Fat of Beef by FT-MIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Meat Sampling
2.2. Extraction of the Intramuscular Fat of the Meat Samples
2.3. Mid-Infrared Spectra Measurements and Spectral Acquisition
2.4. Data Analysis
3. Results and Discussion
3.1. FTIR Spectra Measurement and Assignment of Representative Bands
3.2. Prediction Models
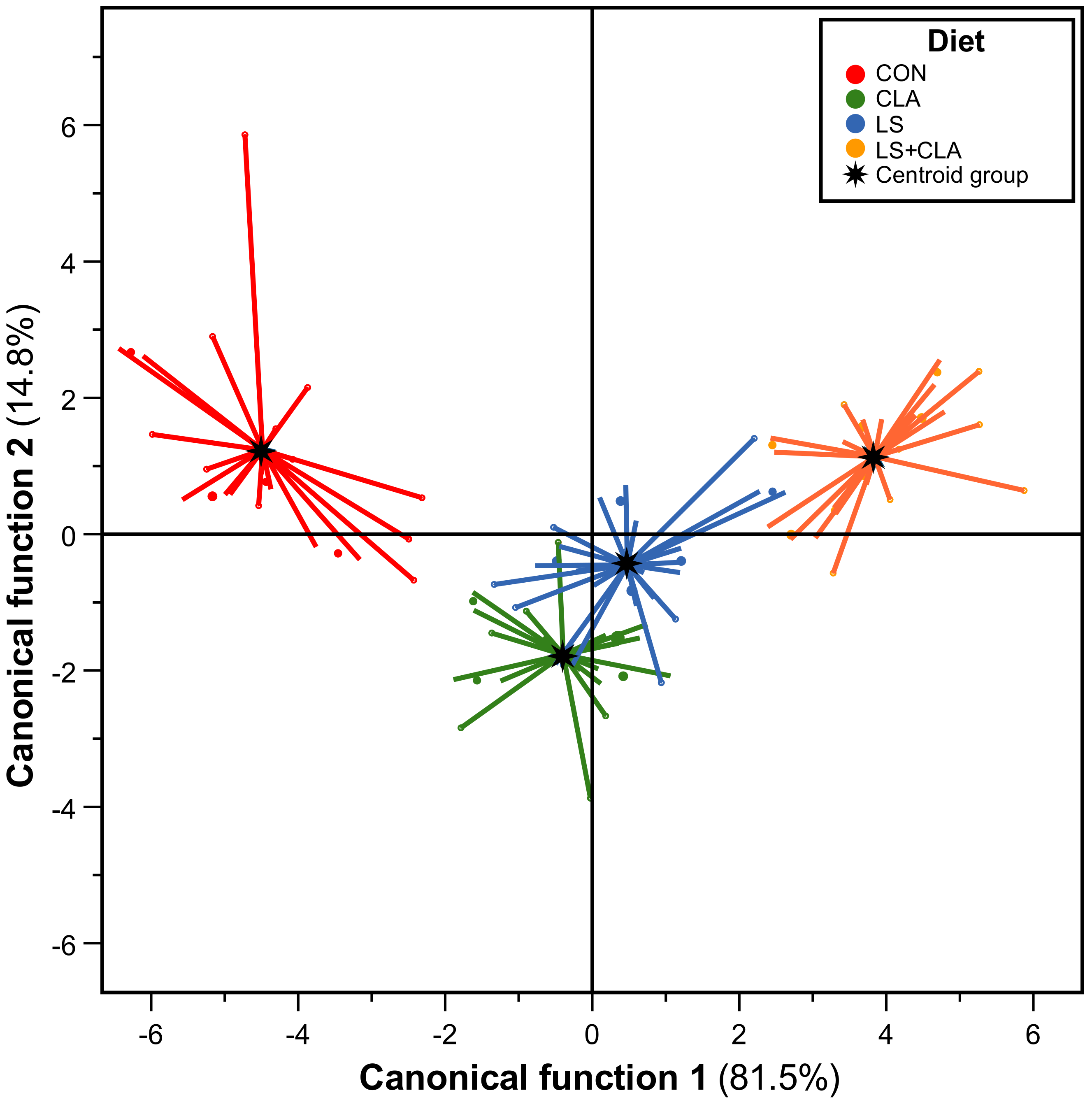
3.3. Discriminant Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| CON | LS | CLA | LS+CLA | SEM | p-Value | |
|---|---|---|---|---|---|---|
| C16:0 | 285.88 | 300.34 | 287.63 | 271.46 | 30.094 | 0.926 |
| C18:0 | 168.09 | 184.62 | 180.25 | 184.92 | 16.071 | 0.868 |
| C18:1t10+C18:1t11 | 14.19 b | 21.03 ab | 14.97 b | 26.31 a | 2.724 | 0.009 |
| C18:1c9 | 725.80 | 1203.22 | 856.82 | 984.51 | 143.514 | 0.127 |
| C18:2n6c9c12 (LA) | 106.71 | 95.07 | 106.36 | 111.46 | 7.116 | 0.422 |
| CLA9c11t (RA) | 2.29 b | 3.90 b | 3.83 b | 5.93 a | 0.513 | 0.000 |
| CLA10t12c | 0.95 | 0.90 | 0.93 | 0.97 | 0.108 | 0.970 |
| CLA9c10c | 1.07 | 0.76 | 1.32 | 0.75 | 0.219 | 0.210 |
| CLA9t11t | 0.93 c | 1.75 ab | 1.34 bc | 2.21 a | 0.191 | 0.000 |
| C18:3n-3 (ALA) | 1.84 b | 12.28 a | 2.08 b | 13.50 a | 0.688 | 0.000 |
| C20:5n-3 (EPA) | 2.39 b | 4.91 a | 2.54 b | 5.91 a | 0.456 | 0.000 |
| C22:5n-3 (DPA) | 6.62 b | 11.78 a | 6.91 b | 13.26 a | 0.911 | 0.000 |
| C22:6n-3 (DHA) | 0.97 | 0.84 | 0.98 | 0.92 | 0.121 | 0.834 |
| Σn-6 | 169.03 | 154.06 | 168.17 | 174.65 | 10.952 | 0.593 |
| Σn-3 | 11.83 b | 29.81 a | 12.51 b | 33.59 a | 1.837 | 0.000 |
| ΣCLA | 5.23 b | 7.30 ab | 7.43 ab | 9.86 a | 0.865 | 0.006 |
| ΣSFA | 532.71 | 581.62 | 551.10 | 546.36 | 55.314 | 0.936 |
| ΣMUFA | 873.13 | 1374.77 | 1008.04 | 1148.48 | 153.656 | 0.136 |
| ΣPUFA | 185.14 | 190.28 | 187.18 | 217.13 | 13.068 | 0.284 |
| ΣAG | 1590.98 | 2146.66 | 1746.32 | 1911.97 | 202.047 | 0.260 |

References
- Karoui, R.; Downey, G.; Blecker, C. Mid-Infrared Spectroscopy Coupled with Chemometrics: A Tool for the Analysis of Intact Food Systems and the Exploration of Their Molecular Structure-Quality Relationships-a Review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef]
- De Smet, S.; Vossen, E. Meat: The Balance between Nutrition and Health. A Review. Meat Sci. 2016, 120, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Beriain, M.J.; Gómez, I.; Ibanez, F.C.; Sarries, V.; Ordonez, A.I. Improvement of the functional and healthy properties of meat products. In Food Quality: Balancing Health and Disease; Grumezescu, A., Holban, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: London, UK, 2018; Volume 13, pp. 1–74. ISBN 978-0-12-811442-1. [Google Scholar]
- Olmedilla-Alonso, B.; Jiménez-Colmenero, F.; Sánchez-Muniz, F.J. Development and Assessment of Healthy Properties of Meat and Meat Products Designed as Functional Foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Choi, N.; Kurt, E.; Fisher, V.A.; Enser, M.; Wood, J.D. Manipulating the Fatty Acid Composition of Muscle and Adipose Tissue in Beef Cattle. Br. J. Nutr. 2001, 85, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Mapiye, C.; Aalhus, J.L.; Turner, T.D.; Rolland, D.C.; Basarab, J.A.; Baron, V.S.; McAllister, T.A.; Block, H.C.; Uttaro, B.; Lopez-Campos, O.; et al. Effects of Feeding Flaxseed or Sunflower-Seed in High-Forage Diets on Beef Production, Quality and Fatty Acid Composition. Meat Sci. 2013, 95, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Mapiye, C.; Turner, T.D.; Rolland, D.C.; Basarab, J.A.; Baron, V.S.; McAllister, T.A.; Block, H.C.; Uttaro, B.; Aalhus, J.L.; Dugan, M.E.R. Adipose Tissue and Muscle Fatty Acid Profiles of Steers Fed Red Clover Silage with and without Flaxseed. Livest. Sci. 2013, 151, 11–20. [Google Scholar] [CrossRef]
- Gillis, M.H.; Duckett, S.K.; Sackmann, J.R.; Realini, C.E.; Keisler, D.H.; Pringle, T.D. Effects of Supplemental Rumen-Protected Conjugated Linoleic Acid or Linoleic Acid on Feedlot Performance, Carcass Quality, and Leptin Concentrations in Beef Cattle. J. Anim. Sci. 2004, 82, 851–859. [Google Scholar] [CrossRef]
- Gillis, M.H.; Duckett, S.K.; Sackmann, J.R. Effects of Supplemental Rumen-Protected Conjugated Linoleic Acid or Corn Oil on Lipid Content and Palatability in Beef Cattle. J. Anim. Sci. 2007, 85, 1504–1510. [Google Scholar] [CrossRef][Green Version]
- Schlegel, G.; Ringseis, R.; Shibani, M.; Most, E.; Schuster, M.; Schwarz, F.J.; Eder, K. Influence of a Rumen-Protected Conjugated Linoleic Acid Mixture on Carcass Traits and Meat Quality in Young Simmental Heifers. J. Anim. Sci. 2012, 90, 1532–1540. [Google Scholar] [CrossRef]
- Wu, D.; Feng, S.; He, C.; He, Y. NIRS and MIRS Technique for the Determination of Protein and Fat Content in Milk Powder. In Proceedings of the International Symposium on Photoelectronic Detection and Imaging 2007: Related Technologies and Applications, Beijing, China, 9–12 September 2007; Zhou, L., Ed.; International Society for Optics and Photonics: Beijing, China, 2008; Volume 6625, p. 66251S. [Google Scholar]
- Ripoche, A.; Guillard, A.S. Determination of Fatty Acid Composition of Pork Fat by Fourier Transform Infrared Spectroscopy. Meat Sci. 2001, 58, 299–304. [Google Scholar] [CrossRef]
- Flåtten, A.; Bryhni, E.A.; Kohler, A.; Egelandsdal, B.; Isaksson, T. Determination of C22:5 and C22:6 Marine Fatty Acids in Pork Fat with Fourier Transform Mid-Infrared Spectroscopy. Meat Sci. 2005, 69, 433–440. [Google Scholar] [CrossRef]
- Lozano, M.; Rodríguez-Ulibarri, P.; Echeverría, J.C.; Beruete, M.; Sorolla, M.; Beriain, M.J. Mid-Infrared Spectroscopy (MIR) for Simultaneous Determination of Fat and Protein Content in Meat of Several Animal Species. Food Anal. Methods 2017, 10, 3462–3470. [Google Scholar] [CrossRef]
- Ruiz, M.; Beriain, M.J.; Beruete, M.; Insausti, K.; Lorenzo, J.M.; Sarriés, M.V. Application of MIR Spectroscopy to the Evaluation of Chemical Composition and Quality Parameters of Foal Meat: A Preliminary Study. Foods 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Albertí, P.; Gómez, I.; Mendizabal, J.A.; Ripoll, G.; Barahona, M.; Sarriés, V.; Insausti, K.; Beriain, M.J.; Purroy, A.; Realini, C. Effect of Whole Linseed and Rumen-Protected Conjugated Linoleic Acid Enriched Diets on Feedlot Performance, Carcass Characteristics, and Adipose Tissue Development in Young Holstein Bulls. Meat Sci. 2013, 94, 208–214. [Google Scholar] [CrossRef]
- European Union. EU Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, L 276, 33–79. [Google Scholar]
- International Organization for Standardization (ISO). Meat and meat products—Determination of total fat content. In ISO 1443:1973; ISO (Meat, poultry, fish, eggs and their products); International Organization for Standardization: Geneva, Switzerland, 1973. [Google Scholar]
- Gómez, I.; Mendizabal, J.A.; Sarriés, V.M.; Insausti, K.; Albertí, P.; Realini, C.; Pérez-Juan, M.; Oliver, M.A.; Purroy, A.; Beriain, M.J. Fatty Acid Composition of Young Holstein Bulls Fed Whole Linseed and Rumen-Protected Conjugated Linoleic Acid Enriched Diets. Livest. Sci. 2015, 180, 106–112. [Google Scholar] [CrossRef]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Evaluation of the Use of Attenuated Total Reflectance Mid Infrared Spectroscopy to Determine Fatty Acids in Intact Seeds of Barley (Hordeum Vulgare). LWT-Food Sci. Technol. 2014, 56, 478–483. [Google Scholar] [CrossRef]
- De Marchi, M.; Penasa, M.; Cecchinato, A.; Mele, M.; Secchiari, P.; Bittante, G. Effectiveness of Mid-Infrared Spectroscopy to Predict Fatty Acid Composition of Brown Swiss Bovine Milk. Animal 2011, 5, 1653–1658. [Google Scholar] [CrossRef]
- Arce, L.; Domínguez-Vidal, A.; Rodríguez-Estévez, V.; López-Vidal, S.; Ayora-Cañada, M.J.; Valcárcel, M. Feasibility Study on the Use of Infrared Spectroscopy for the Direct Authentication of Iberian Pig Fattening Diet. Anal. Chim. Acta 2009, 636, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mahesar, S.A.; Sherazi, S.T.H.; Kandhro, A.A.; Bhanger, M.I.; Khaskheli, A.R.; Talpur, M.Y. Evaluation of Important Fatty Acid Ratios in Poultry Feed Lipids by ATR FTIR Spectroscopy. Vib. Spectrosc. 2011, 57, 177–181. [Google Scholar] [CrossRef]
- Rohman, A.; Sismindari; Erwanto, Y.; Che Man, Y.B. Analysis of Pork Adulteration in Beef Meatball Using Fourier Transform Infrared (FTIR) Spectroscopy. Meat Sci. 2011, 88, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, C.; Rodriguez-Saona, L. Application of NIR and MIR Spectroscopy in Quality Control of Potato Chips. J. Food Compos. Anal. 2009, 22, 596–605. [Google Scholar] [CrossRef]
- Hernández-Martínez, M.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Almaraz-Abarca, N.; Ponce-Mendoza, A.; Vásquez-Murrieta, M.S. Prediction of Total Fat, Fatty Acid Composition and Nutritional Parameters in Fish Fillets Using MID-FTIR Spectroscopy and Chemometrics. LWT-Food Sci. Technol. 2013, 52, 12–20. [Google Scholar] [CrossRef]
- Papadopoulou, O.; Panagou, E.Z.; Tassou, C.C.; Nychas, G.J.E. Contribution of Fourier Transform Infrared (FTIR) Spectroscopy Data on the Quantitative Determination of Minced Pork Meat Spoilage. Food Res. Int. 2011, 44, 3264–3271. [Google Scholar] [CrossRef]
- Alamprese, C.; Casale, M.; Sinelli, N.; Lanteri, S.; Casiraghi, E. Detection of Minced Beef Adulteration with Turkey Meat by UB–VIS, Nir and Mir Spectroscopy. LWT-Food Sci. Technol. 2013, 53, 225–232. [Google Scholar] [CrossRef]
- Meza-Márquez, O.G.; Gallardo-Velázquez, T.; Osorio-Revilla, G. Application of Mid-Infrared Spectroscopy with Multivariate Analysis and Soft Independent Modeling of Class Analogies (SIMCA) for the Detection of Adulterants in Minced Beef. Meat Sci. 2010, 86, 511–519. [Google Scholar] [CrossRef]
- Doreau, M.; Chilliard, Y. Digestion and Metabolism of Dietary Fat in Farm Animals. Br. J. Nutr. 1997, 78 (Suppl. S1), S15–S35. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the Substantiation of Health Claims Related to Docosahexaenoic Acid (DHA), Eicosapentaenoic Acid (EPA) and Brain, Eye and Nerve Development (ID 501, 513, 540), Maintenance of Normal Brain Function (ID 497, 501, 510, 513, 519, 521, 534, 540, 688, 1323, 1360, 4294), Maintenance of Normal Vision (ID 508, 510, 513, 519, 529, 540, 688, 2905, 4294), Maintenance of Normal Cardiac Function (ID 510, 688, 1360), “Maternal Health; Pregnancy and Nursing” (ID 514), “to Fulfil Increased Omega-3 Fatty Acids Need during Pregnancy” (ID 539), “Skin and Digestive Tract Epithelial Cells Maintenance” (ID 525), Enhancement of Mood (ID 536), “Membranes Cell Structure” (ID 4295), “Anti-Inflammatory Action” (ID 4688) and Maintenance of Normal Blood LDL-Cholesterol Concentrations (ID 4719) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2078. [CrossRef]
- Deckelbaum, R.J.; Torrejon, C. The Omega-3 Fatty Acid Nutritional Landscape: Health Benefits and Sources. J. Nutr. 2012, 142, 587S–591S. [Google Scholar] [CrossRef] [PubMed]
- Tao, L. Oxidation of Polyunsaturated Fatty Acids and Its Impact on Food Quality and Human Health. Adv. Food Technol. Nutr. Sci. 2015, 1, 135–142. [Google Scholar] [CrossRef]
- Brugiapaglia, A.; Destefanis, G.; Lussiana, C. Characterization of Beef Fatty Acid Profile by Multivariate Analysis. In Proceedings of the 59th International Congress of Meat Science and Technology (ICoMST Proceedings), Izmir, Turkey, 18–23 August 2013; Serdaroğlu, M., Ed.; Elsevier: Izmir, Turkey, 2013; pp. S9–S17. [Google Scholar]
- Moreno, T.; Varela, A.; Oliete, B.; Carballo, J.A.; Sánchez, L.; Montserrat, L. Nutritional Characteristics of Veal from Weaned and Unweaned Calves: Discriminatory Ability of the Fat Profile. Meat Sci. 2006, 73, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Oliete, B.; Moreno, T.; Carballo, J.; Montserrat, L.; Sánchez, L. Estudio de La Calidad de La Carne de Ternera de Raza Rubia Gallega a Lo Largo de La Maduración al Vacío. Arch. Zootec. 2006, 55, 3–14. [Google Scholar]
- Alfaia, C.P.M.; Alves, S.P.; Martins, V.S.I.; Costa, A.S.H.; Fontes, C.M.G.A.; Lemos, J.P.C.; Bessa, R.J.B.; Prates, J.A.M. Effect of the Feeding System on Intramuscular Fatty Acids and Conjugated Linoleic Acid Isomers of Beef Cattle, with Emphasis on Their Nutritional Value and Discriminatory Ability. Food Chem. 2009, 114, 939–946. [Google Scholar] [CrossRef]
- Martínez Marín, A.L.; Peña Blanco, F.; Avilés Ramírez, C.; Pérez Alba, L.M.; Polvillo Polo, O. Selecting the Best Set of Gas Chromatography-Derived Fatty Acids to Discriminate between Two Finishing Diets Using Linear Discriminant Analysis. Meat Sci. 2013, 95, 173–176. [Google Scholar] [CrossRef]


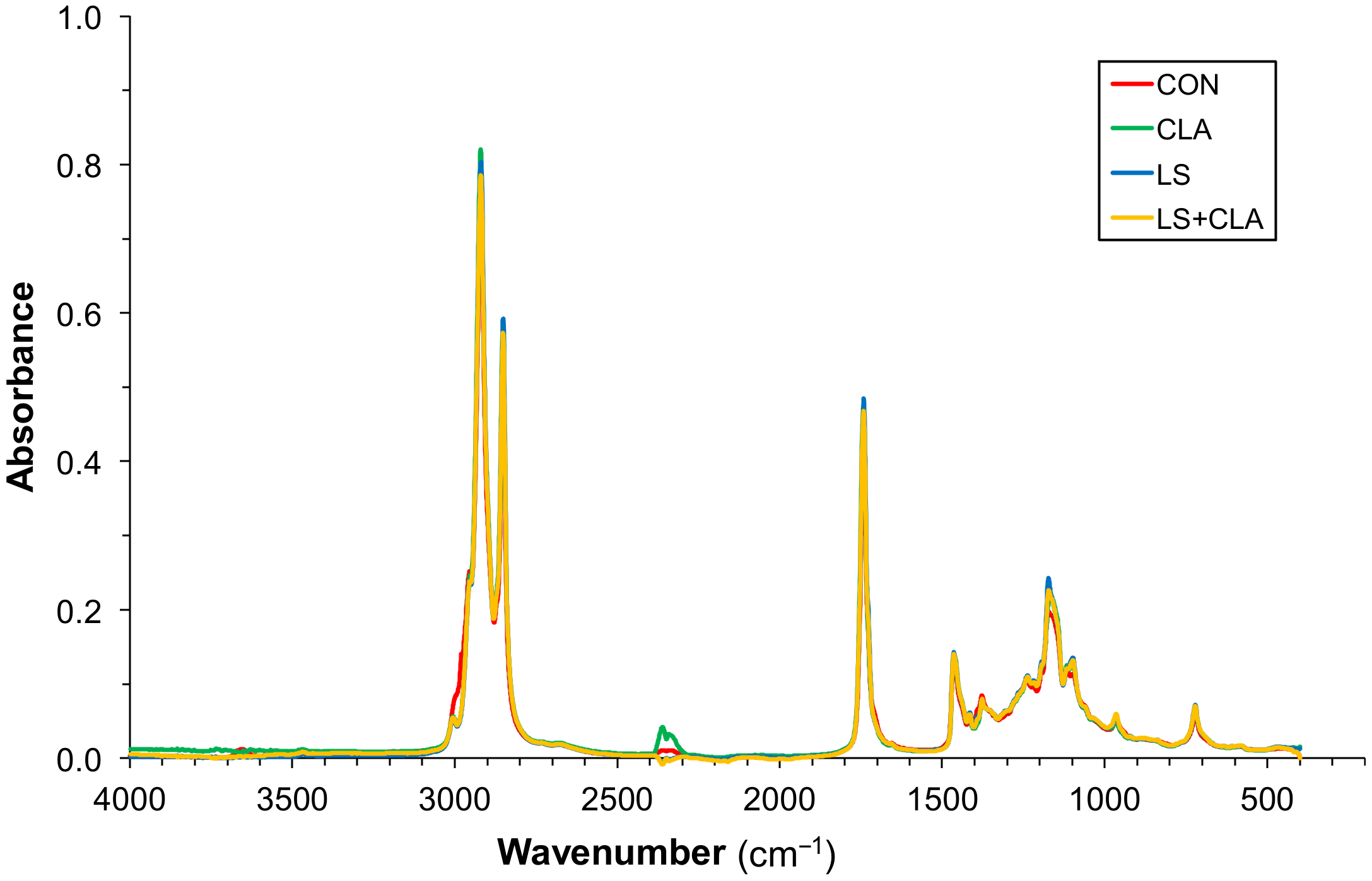



| Wavenumber (cm−1) | Functional Group | References |
|---|---|---|
| 3005 | CH stretching vibration in cis double bond =CH; unsaturated fatty acids | [24,25,26,27] |
| 2920 | C-H asymmetric stretching of CH2 and CH3; aliphatic groups | [14,24,25,26,27] |
| 2854 | C-H symmetric stretching of CH2 and CH3; aliphatic groups | [14,24,25,26,27,28] |
| 1743 | C=O stretching of esters: free fatty acids | [14,24,25,26,27,29] |
| 1465 | C-H scissoring vibration | [24,25,26,28,30,31] |
| 1377 | Symmetric bending vibrations of CH3 groups | [24,25,26,27] |
| 1160 | C-O stretching vibration y C-H bending | [24,28,31] |
| 721 | Overlapping of the methylene (-CH2) rocking vibration and to the out of plane vibration of cis-disubstituted olefins | [14,24,25,26,27] |
| Fatty Acid | Intramuscular Fat Extracted from Beef | Beef | ||||||
|---|---|---|---|---|---|---|---|---|
| Validation | Calibration | Validation | Calibration | |||||
| R2 | RMSECV | R2 | RMSEC | R2 | RMSECV | R2 | RMSEC | |
| C16:0 | 78.73 | 0.94 | 88.55 | 0.72 | 1.30 | 40.20 | 7.02 | 39.30 |
| C18:0 | 62.47 | 0.89 | 83.26 | 0.63 | 33.38 | 23.60 | 41.27 | 22.70 |
| C18:1c9 | 53.85 | 2.16 | 82.52 | 1.41 | 32.99 | 28.40 | 53.58 | 24.60 |
| C18:1t10+C18:1t11 | 81.17 | 0.35 | 91.29 | 0.25 | 28.35 | 8.12 | 47.60 | 7.13 |
| C18:2n-6 (LA) | 59.63 | 1.80 | 82.58 | 1.23 | 34.45 | 15.60 | 46.73 | 14.60 |
| C18:3n-3 (ALA) | 96.21 | 0.15 | 98.68 | 0.09 | 48.72 | 4.67 | 68.94 | 3.78 |
| CLAc9,t11 (RA) | 82.03 | 0.04 | 87.80 | 0.03 | 48.09 | 1.58 | 66.81 | 1.30 |
| CLAt10,c12 | 15.48 | 0.01 | 78.07 | 0.006 | 28.33 | 0.17 | 55.09 | 0.14 |
| C20:4n-6 | 57.49 | 0.64 | 77.04 | 0.49 | 25.36 | 8.08 | 51.90 | 6.72 |
| C20:5n-3 (EPA) | 82.44 | 0.05 | 93.82 | 0.03 | 37.07 | 1.61 | 57.34 | 1.38 |
| C22:5n-3 (DPA) | 86.61 | 0.06 | 95.34 | 0.04 | 35.41 | 3.41 | 52.82 | 3.04 |
| C22:6n-3 (DHA) | 66.64 | 0.007 | 82.48 | 0.006 | 17.1 | 0.15 | 33.63 | 0.14 |
| Ʃn-6 | 57.72 | 2.53 | 82.98 | 1.70 | 29.02 | 23.80 | 47.98 | 21.00 |
| Ʃn-3 | 94.65 | 0.24 | 98.01 | 0.16 | 45.75 | 8.73 | 61.79 | 7.64 |
| ƩCLA | 80.31 | 0.06 | 90.10 | 0.04 | 36.67 | 2.97 | 53.93 | 2.60 |
| ƩSFA | 71.12 | 1.17 | 85.35 | 0.89 | 18.66 | 68.30 | 26.26 | 66.30 |
| ƩMUFA | 76.85 | 1.83 | 93.63 | 1.03 | 32.42 | 29.20 | 53.64 | 25.20 |
| ƩPUFA | 71.95 | 2.33 | 88.13 | 1.60 | 39.44 | 29.10 | 49.95 | 27.40 |
| Classified into Group | |||||
|---|---|---|---|---|---|
| CON | CLA | LS | LS+CLA | ||
| Actual group | CON | 95.2 | 4.8 | 0.0 | 0.0 |
| CLA | 0.0 | 95.8 | 4.2 | 0.0 | |
| LS | 0.0 | 16.7 | 79.2 | 8.3 | |
| LS+CLA | 0.0 | 0.0 | 0.0 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beriain, M.J.; Ibañez, F.C.; Beruete, E.; Gómez, I.; Beruete, M. Estimation of Fatty Acids in Intramuscular Fat of Beef by FT-MIR Spectroscopy. Foods 2021, 10, 155. https://doi.org/10.3390/foods10010155
Beriain MJ, Ibañez FC, Beruete E, Gómez I, Beruete M. Estimation of Fatty Acids in Intramuscular Fat of Beef by FT-MIR Spectroscopy. Foods. 2021; 10(1):155. https://doi.org/10.3390/foods10010155
Chicago/Turabian StyleBeriain, María José, Francisco C. Ibañez, Edurne Beruete, Inmaculada Gómez, and Miguel Beruete. 2021. "Estimation of Fatty Acids in Intramuscular Fat of Beef by FT-MIR Spectroscopy" Foods 10, no. 1: 155. https://doi.org/10.3390/foods10010155
APA StyleBeriain, M. J., Ibañez, F. C., Beruete, E., Gómez, I., & Beruete, M. (2021). Estimation of Fatty Acids in Intramuscular Fat of Beef by FT-MIR Spectroscopy. Foods, 10(1), 155. https://doi.org/10.3390/foods10010155







