Effects of Pre-Processing Hot-Water Treatment on Aroma Relevant VOCs of Fresh-Cut Apple Slices Stored in Sugar Syrup
Abstract
1. Introduction
2. Materials and Methods
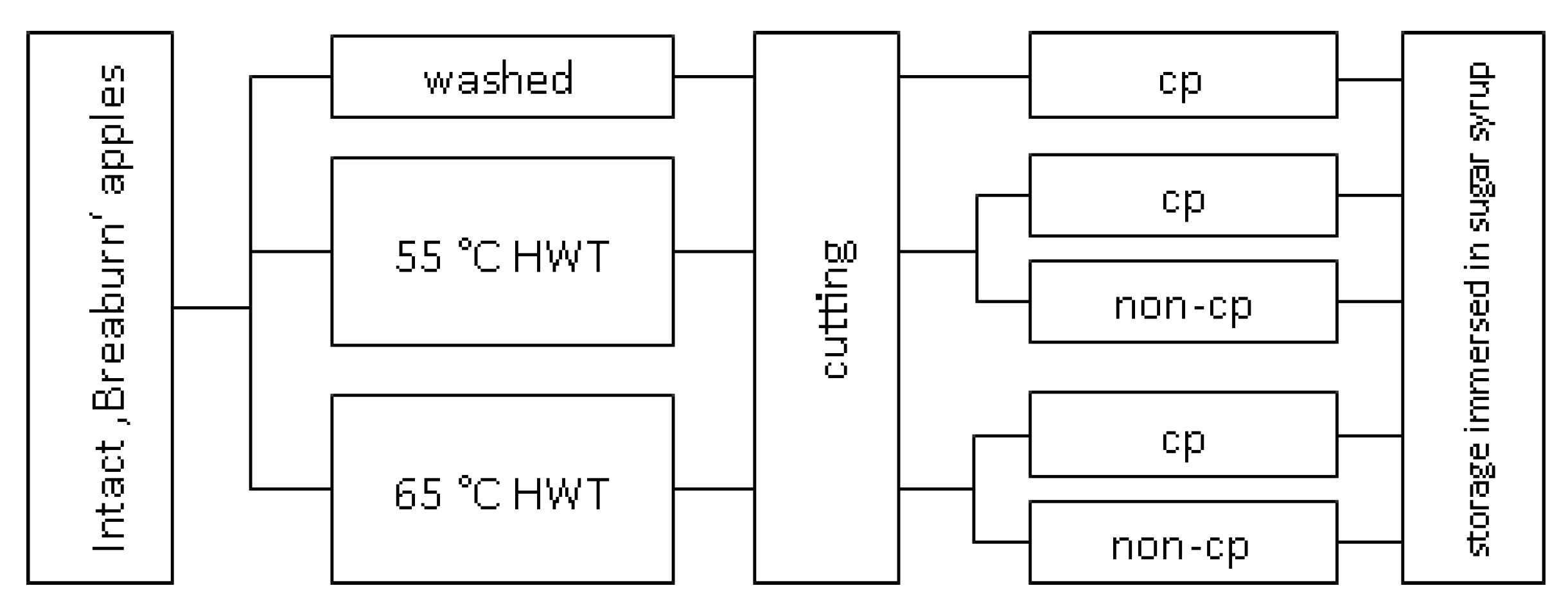
2.1. Material
2.2. Pre-Processing Short-Term Hot-Water Treatment
2.3. Fresh-Cut Preparation and Sampling
2.4. Sampling of Volatile Organic Compounds (VOCs) and Ethylene
2.5. Analysis of Volatile Organic Compounds
2.6. Quantification of Ethylene and CO2 Evolution
2.7. Statistical Analysis
3. Results
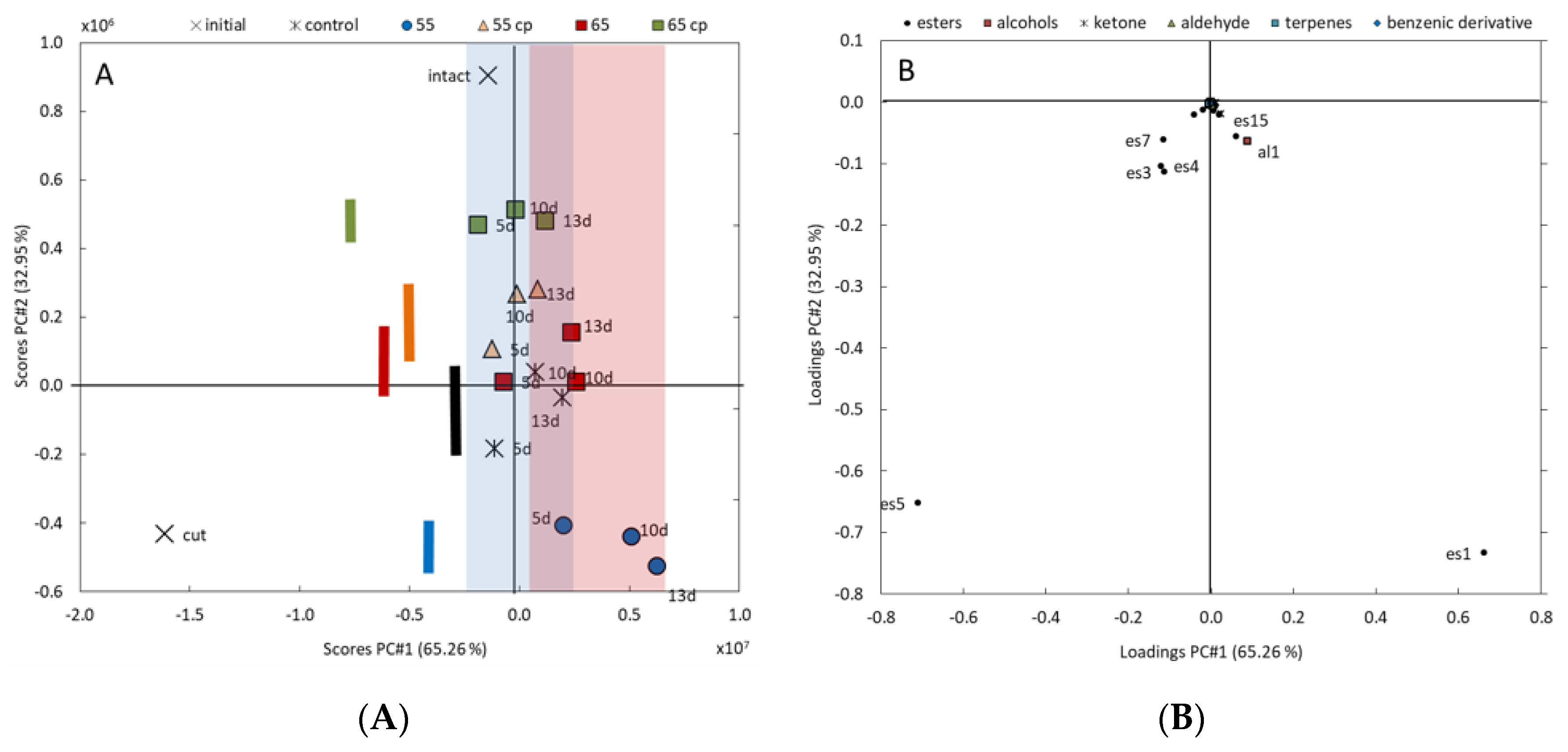
3.1. Volatile Organic Compounds
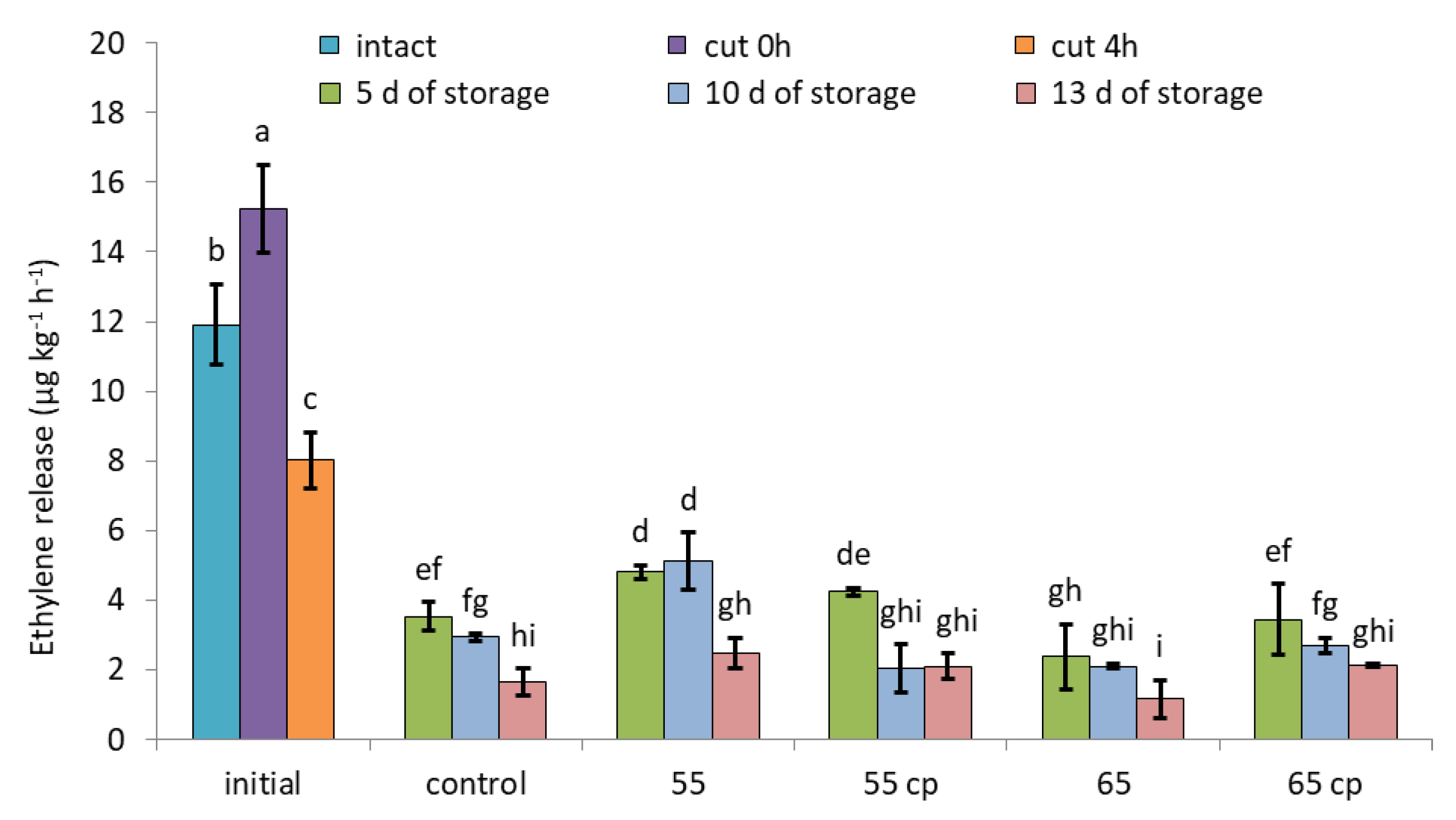
3.2. Ethylene Evolution
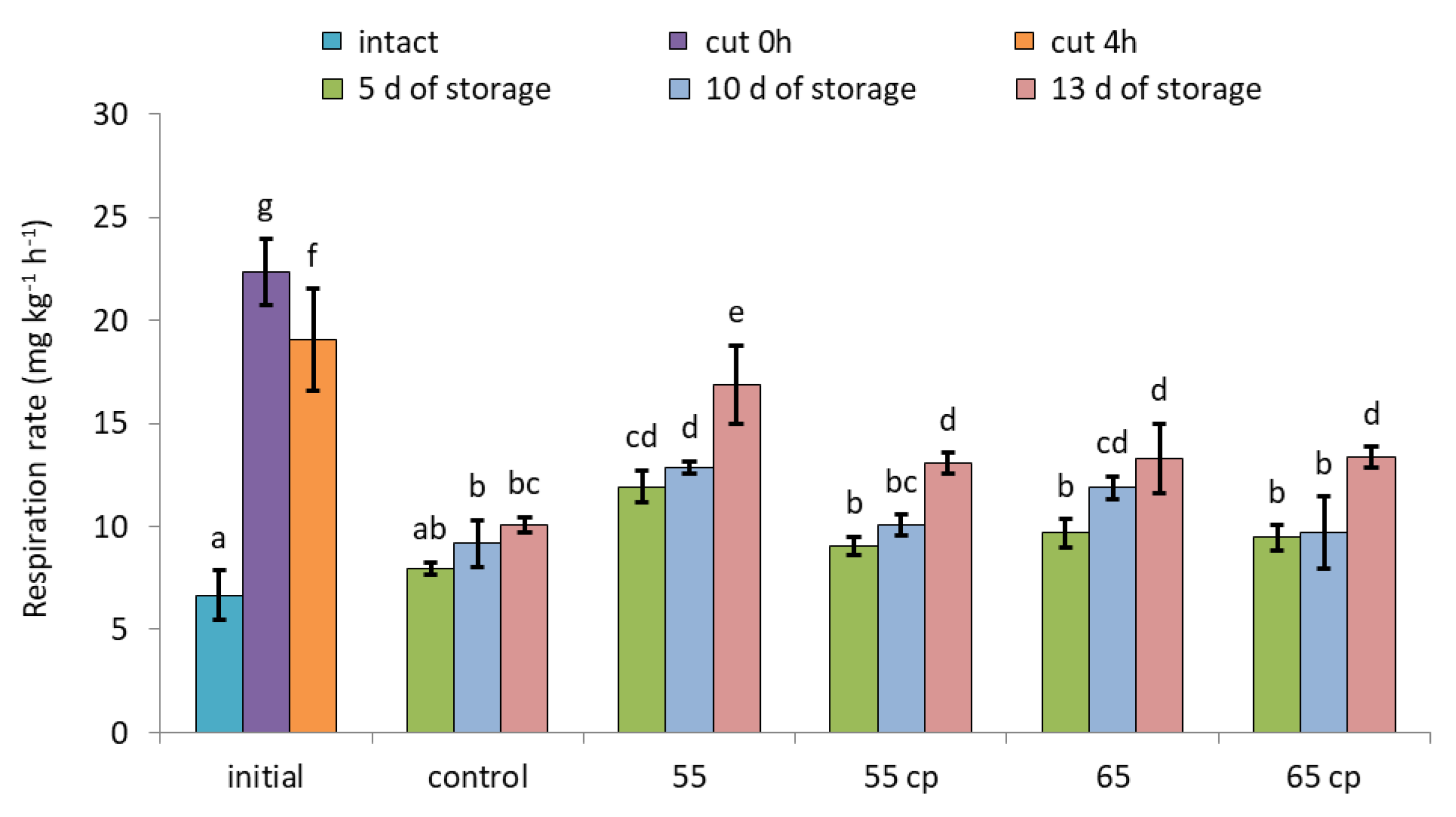
3.3. Respiration
4. Discussion
4.1. Effect of Cutting on the Release of VOCs, Ethylene and CO2
4.2. Impact of Hot-Water Treatment on the Release of VOCs, Ethylene and CO2
4.3. Effects of Storage in Sugar Syrup on the Release of VOCs, Ethylene and CO2
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watada, A.E.; Qi, L. Quality of fresh-cut produce. Postharvest Biol. Technol. 1999, 15, 201–205. [Google Scholar] [CrossRef]
- Beaulieu, J. Factors affecting sensory quality of fresh-cut produce. In Advances in Fresh-Cut Fruits and Vegetables; Martin-Belloso, O., Soliva-Fortuny, R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 115–143. [Google Scholar]
- Toivonen, P.M.; DeEll, J.R. Physiology of Fresh-Cut Fruits and Vegetables. In Fresh-Cut Fruits and Vegetables; Lamikanra, O., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 91–123. [Google Scholar]
- Alzamora, S.M.; Tapia, M.S.; Argaíz, A.; Welli, J. Application of combined methods technology in minimally processed fruits. Food Res. Int. 1993, 26, 125–130. [Google Scholar] [CrossRef]
- Nishikawa, F.; Iwama, T.; Kato, M.; Hyodo, H.; Ikoma, Y.; Yano, M. Effect of sugars on ethylene synthesis and responsiveness in harvested broccoli florets. Postharvest Biol. Technol. 2005, 36, 157–165. [Google Scholar] [CrossRef]
- Coupe, S.A.; Sinclair, B.K.; Watson, L.M.; Heyes, J.A.; Eason, J.R. Identification of dehydration-responsive cysteine proteases during post-harvest senescence of broccoli florets. J. Exp. Bot. 2003, 54, 1045–1056. [Google Scholar] [CrossRef]
- Rux, G.; Caleb, O.J.; Fröhling, A.; Herppich, W.B.; Mahajan, P.V. Respiration and storage quality of fresh-cut apple slices immersed in sugar syrup and orange juice. Food Bioprocess Technol. 2017, 10, 2081–2091. [Google Scholar] [CrossRef]
- Pietrysiak, E.; Ganjyal, G.M. Apple peel morphology and attachment of Listeria innocua through aqueous environment as shown by scanning electron microscopy. Food Control 2018, 92, 362–369. [Google Scholar] [CrossRef]
- Lurie, S. Postharvest heat treatments. Postharvest Biol. Technol. 1998, 14, 257–269. [Google Scholar] [CrossRef]
- Shao, X.F.; Tu, K.; Zhao, Y.Z.; Chen, L.; Chen, Y.Y.; Wang, H. Effects of prestorage heat treatment on fruit ripening and decay development in different apple cultivars. J. Hortic. Sci. Biotech. 2007, 82, 297–303. [Google Scholar] [CrossRef]
- Spadoni, A.; Guidarelli, M.; Phillips, J.; Mari, M.; Wisniewski, M. Transcriptional profiling of apple fruit in response to heat treatment: Involvement of a defense response during Penicillium expansum infection. Postharvest Biol. Technol. 2015, 101, 37–48. [Google Scholar] [CrossRef]
- Kabelitz, T.; Hassenberg, K. Control of apple surface microflora for fresh-cut produce by post-harvest hot-water treatment. LWT Food Sci. Technol. 2018, 98, 492–499. [Google Scholar] [CrossRef]
- Fallik, E. Prestorage hot water treatments (immersion, rinsing and brushing). Postharvest Biol. Technol. 2004, 32, 125–134. [Google Scholar] [CrossRef]
- Maxin, P.; Williams, M.; Weber, R.W. Control of fungal storage rots of apples by hot-water treatments: A Northern European perspective. Erwerbs-Obstbau 2014, 56, 25–34. [Google Scholar] [CrossRef]
- Roy, S.; Conway, W.S.; Watada, A.E.; Sams, C.E.; Erbe, E.F.; Wergin, W.P. Heat treatment affects epicuticular wax structure and postharvest calcium uptake in Golden Delicious apples. HortScience 1994, 29, 1056–1058. [Google Scholar] [CrossRef]
- Lurie, S.; Fallik, E.; Klein, J.D. The effect of heat treatment on apple epicuticular wax and calcium uptake. Postharvest Biol. Technol. 1996, 8, 271–277. [Google Scholar] [CrossRef]
- Kabelitz, T.; Schmidt, B.; Herppich, W.B.; Hassenberg, K. Effects of hot water dipping on apple heat transfer and post-harvest fruit quality. LWT Food Sci. Technol. 2019, 108, 416–420. [Google Scholar] [CrossRef]
- Rux, G.; Efe, E.; Ulrichs, C.; Huyskens-Keil, S.; Hassenberg, K.; Herppich, W.B. Effects of pre-processing short-term hot-water treatments on quality and shelf life of fresh-cut apple slices. Foods 2019, 8, 653. [Google Scholar] [CrossRef]
- Biegańska-Marecik, R.; Czapski, J. The effect of selected compounds as inhibitors of enzymatic browning and softening of minimally processed apples. Acta Sci. Pol. Technol. Aliment. 2007, 6, 37–49. [Google Scholar]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R.L. Color, flavor, texture and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Shewmaker, C.K.; Schuch, W. Flavor trivia and tomato aroma: Biochemistry and possible mechanisms for control of important aroma components. HortScience 2000, 35, 1013–1022. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotech. 2016, 54, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, R.J.; Friel, E.N.; Souleyre, E.J.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J.H.; Ma, J.; Nain, B.; Cohan, D.; et al. A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Lamikanra, O.; Chen, J.C.; Banks, D.; Hunter, P.A. Biochemical and microbial changes during the storage of minimally processed cantaloupe. J. Agric. Food Chem. 2000, 48, 5955–5961. [Google Scholar] [CrossRef] [PubMed]
- Rux, G.; Luca, A.; Mahajan, P.V. Changes in volatile organic compounds in the headspace of modified atmosphere packed and unpacked white sausages. Food Packag. Shelf Life 2019, 19, 167–173. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- NIST. Chemistry WebBook: NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: https://webbook.nist.gov/chemistry/ (accessed on 25 August 2019). [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Paillard, N.M.M. The flavour of apples, pears and quinces. In Food Flavours. Part C: The Flavour of Fruits; Morton, I.D., MacLeod, A.J., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1990; pp. 1–41. ISBN 0-444-87362-7. [Google Scholar]
- Fellman, J.K.; Mattinson, D.S.; Bostick, B.C.; Mattheis, J.P.; Patterson, M.E. Ester biosynthesis in “Rome” apples subjected to low-oxygen atmospheres. Postharvest Biol. Technol. 1993, 3, 201–214. [Google Scholar] [CrossRef]
- Poll, L. Evaluation of 18 apple varieties for their suitability for juice production. J. Sci. Food Agric. 1981, 32, 1081–1090. [Google Scholar] [CrossRef]
- Aaby, K.; Haffner, K.; Skrede, G. Aroma quality of Gravenstein apples influenced by regular and controlled atmosphere storage. LWT Food Sci. Technol. 2002, 35, 254–259. [Google Scholar] [CrossRef]
- Baiamonte, I.; Raffo, A.; Nardo, N.; Moneta, E.; Peparaio, M.; D’Aloise, A.; Kelderer, M.; Casera, C.; Paoletti, F. Effect of the use of anti-hail nets on codling moth (Cydia pomonella) and organoleptic quality of apple (cv. Braeburn) grown in Alto Adige Region (northern Italy). J. Sci. Food Agric. 2016, 96, 2025–2032. [Google Scholar] [CrossRef]
- Young, H.; Gilbert, J.M.; Murray, S.H.; Ball, R.D. Causal effects of aroma compounds on royal gala apple flavours. J. Sci. Food Agric. 1996, 71, 329–336. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.C.; Ricart-Coll, M.; Martín-Belloso, O. Sensory quality and internal atmosphere of fresh-cut golden delicious apples. Int. J. Food Sci. Tech. 2005, 40, 369–375. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.C.; Oms-Oliu, G.; Martín-Belloso, O. Effects of ripeness stages on the storage atmosphere, color, and textural properties of minimally processed apple slices. J. Food Sci. 2002, 67, 1958–1963. [Google Scholar] [CrossRef]
- Bett, K.L.; Ingram, D.A.; Grimm, C.C.; Lloyd, S.W.; Spanier, A.M.; Miller, J.M.; Gross, E.A.; Baldwin, B.T.; Vinyard, B.T. Flavor of fresh-cut gala apples in barrier film packaging as affected by storage time. J. Food Qual. 2001, 24, 141–156. [Google Scholar] [CrossRef]
- Finnegan, E.; Mahajan, P.V.; O’Connell, M.; Francis, G.A.; O’Beirne, D. Modelling respiration in fresh-cut pineapple and prediction of gas permeability needs for optimal modified atmosphere packaging. Postharvest Biol. Technol. 2013, 79, 47–53. [Google Scholar] [CrossRef]
- Schreier, P. Chromatographic Studies of Biogenesis of Plant Volatiles; Alfred Hüthig Verlag GmbH: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- De Pooter, H.L.; Montens, J.P.; Willaert, G.A.; Dirinck, P.J.; Schamp, N.M. Treatment of Golden Delicious apples with aldehydes and carboxylic acids: Effect on the headspace composition. J. Agric. Food Chem. 1983, 31, 813–818. [Google Scholar] [CrossRef]
- Rowan, D.D.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of straight-chain ester volatiles in Red Delicious and Granny Smith apples using deuterium-labeled precursors. J. Agric. Food Chem. 1999, 47, 2553–2562. [Google Scholar] [CrossRef]
- Beaulieu, J.C. Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. HortScience 2006, 41, 65–73. [Google Scholar] [CrossRef]
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Câmara, J.S. Characterization of volatile substances in apples from rosaceae family by headspace solid-phase microextraction followed by GC-qMS. J. Sep. Sci. 2009, 32, 1875–1888. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Jordan, M.A. The composition of strawberry aroma is influenced by cultivar, maturity, and storage. HortScience 2000, 35, 1022–1026. [Google Scholar] [CrossRef]
- Song, J.; Fan, L.; Forney, C.F.; Jordan, M.A. Using volatile emissions and chlorophyll fluorescence as indicators of heat injury in apples. J. Am. Soc. Hortic. Sci. 2001, 126, 771–777. [Google Scholar] [CrossRef]
- Fan, L.; Song, J.; Forney, C.F.; Jordan, M.A. Ethanol production and chlorophyll fluorescence predict breakdown of heat-stressed apple fruit during cold storage. J. Am. Soc. Hortic. Sci. 2005, 130, 237–243. [Google Scholar] [CrossRef]
- Klein, J.D. Ethylene biosynthesis in heat-treated apples. In Biochemical and Physiological Aspects of Ethylene Production in Lower and Higher Plants; Clijsters, H., de Proft, M., Marcelle, R., van Poucke, M., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 181–189. [Google Scholar]
- Singh, M.; Johnson-Flanagan, A. Co-ordination of photosynthetic gene expression during low-temperature acclimation and development in Brassica napus cv. jet neuf leaves. Plant Sci. 1998, 135, 171–181. [Google Scholar] [CrossRef]
- Pateraki, I.; Kanellis, A.K. Stress and developmental responses of terpenoid biosynthetic genes in Cistus creticus subsp. creticus. Plant Cell Rep. 2010, 29, 629–641. [Google Scholar] [CrossRef]
- Charron, C.S.; Cantliffe, D.J.; Heath, R.R. Volatile emissions from plants. Hort. Rev. 1995, 17, 43–72. [Google Scholar]
- Woodstock, L.W.; Taylorson, R.B. Ethanol and acetaldehyde in imbibing soybean seeds in relation to deterioration. Plant Physiol. 1981, 67, 424–428. [Google Scholar] [CrossRef]
- Bauchot, A.D.; Mottram, D.S.; Dodson, A.T.; John, P. Effect of aminocyclopropane-1-carboxylic acid oxidase antisense gene on the formation of volatile esters in cantaloupe Charentais melon (cv. Vedrandais). J. Agric. Food Chem. 1998, 46, 4787–4792. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Nicolai, B.; Lammertyn, J.; Bueso, M.C.; Monforte, A.J.; Fernández-Trujillo, J.P. Aroma volatiles associated with the senescence of climacteric or non-climacteric melon fruit. Postharvest Biol. Technol. 2009, 52, 146–155. [Google Scholar] [CrossRef]
- Larsen, M.; Watkins, C.B. Firmness and concentrations of acetaldehyde, ethyl acetate and ethanol in strawberries stored in controlled and modified atmospheres. Postharvest Biol. Technol. 1995, 5, 39–50. [Google Scholar] [CrossRef]
- Rizzolo, A.; Polesello, A.; Teleky-Vàmossy, G. CGC/Sensory analysis of volatile compounds developed from ripening apple fruit. J. High Resolut. Chromatogr. 1989, 12, 824–827. [Google Scholar] [CrossRef]
- Von Willert, D.J.; Matyssek, R.; Herppich, W.B. Experimentelle Pflanzenökologie: Grundlagen und Anwendungen; Georg Thieme Verlag: Stuttgart, Germany, 1995; p. 344. ISBN 3-13-134401-6. [Google Scholar]
- Rocculi, P.; Del Nobile, M.A.; Romani, S.; Baiano, A.; Dalla Rosa, M. Use of a simple mathematical model to evaluate dipping and MAP effects on aerobic respiration of minimally processed apples. J. Food Eng. 2006, 76, 334–340. [Google Scholar] [CrossRef]
- Lara, I.; Graell, J.; López, M.L.; Echeverría, G. Multivariate analysis of modifications in biosynthesis of volatile compounds after CA storage of ‘Fuji’ apples. Postharvest Biol. Technol. 2006, 39, 19–28. [Google Scholar] [CrossRef]
- Fellman, J.K.; Mattheis, J.P.; Matthinson, D.S.; Bostick, B.C. Assay of acetyl-CoA alcohol transferase in delicious apples. HortScience 1991, 27, 773–776. [Google Scholar]
- Cortellino, G.; Gobbi, S.; Bianchi, G.; Rizzolo, A. Modified atmosphere packaging for shelf life extension of fresh-cut apples. Trends Food Sci. Technol. 2015, 46, 320–330. [Google Scholar] [CrossRef]
| Chemical Group | VOCs | CAS-Number | RI | RIliterature | Quantifier (Qualifier) Ions (m z−1) |
|---|---|---|---|---|---|
| esters (21) | Ethyl acetate | 141-78-6 | 903 | 863–908 | 43 (45–61) |
| Ethyl propionate | 105-37-3 | 963 | 939–976 | 57 (74–75) | |
| Ethyl 2-methylpropanoate | 97-62-1 | 968 | 957–969 | 43 (41–71) | |
| Propyl acetate | 109-60-4 | 977 | 952–996 | 43 (61–73) | |
| Methyl butyrate | 623-42-7 | 988 | 969–993 | 74 (43–71) | |
| Methyl 2-methylbutyrate | 868-57-5 | 1011 | 1000–1010 | 57 (41–88) | |
| Isobutyl acetate | 110-19-0 | 1014 | 1000–1031 | 43 (56–73) | |
| Ethyl butyrate | 105-54-4 | 1035 | 1000–1073 | 71 (43–88) | |
| Ethyl 2-methylbutanoate | 7452-79-1 | 1050 | 1022–1073 | 57 (41–102) | |
| Butyl acetate | 123-86-4 | 1069 | 1049–1105 | 43 (56–73) | |
| 2-methylbutyl acetate | 624-41-9 | 1116 | 1111–1125 | 43 (55–70) | |
| Ethyl valerate | 539-82-2 | 1129 | 1131–1139 | 88 (57–85) | |
| Ethyl 2-butenoate | 10544-63-5 | 1154 | 1158–1158 | 69 (41–99) | |
| Pentyl acetate | 628-63-7 | 1165 | 1175–1181 | 43 (55–70) | |
| Methyl hexanoate | 106-70-7 | 1178 | 1176–1189 | 74 (43–99) | |
| Ethyl hexanoate | 123-66-0 | 1222 | 1196–1245 | 71 (43–89) | |
| Hexyl acetate | 142-92-7 | 1258 | 1251–1311 | 43 (56–61) | |
| 2-Hexen-1-yl acetate | 10094-40-3 | 1317 | - | ||
| Hexyl butyrate | 2639-63-6 | 1397 | 1393–1410 | 71 (43–89) | |
| Hexyl 2-methylbutanoate | 10032-15-2 | 1414 | 1415–1416 | ||
| Hexyl hexanoate | 6378-65-0 | 1610 | 1596–1599 | 43 (56–117) | |
| ketones (2) | 2-Butanone | 78-93-3 | 908 | 875–926 | 43 (57–72) |
| 1-Penten-3-one | 1629-58-9 | 1020 | 1019–1056 | ||
| alcohols (6) | Ethanol | 64-17-5 | 943 | 900–955 | 45 (43–46) |
| 2-Methyl-1-propanol | 78-83-1 | 1096 | 1092–1114 | 43 (41–42) | |
| 1-Butanol | 71-36-3 | 1143 | 1116–1166 | ||
| 2-Methyl-1-butanol | 1565-80-6 | 1200 | - | 57 (41–56) | |
| 1-Hexanol | 111-27-3 | 1341 | 1339–1396 | ||
| 2-(2-Ethoxyethoxy)-ethanol | 111-90-0 | 1628 | 1615–1619 | 45 (59–72) | |
| aldehydes | Hexanal | 66-25-1 | 1077 | 1048–1120 | 44 (41–56) |
| terpenes (2) | D-Limonene | 5989-27-5 | 1186 | 1176–1238 | 68 (67–93) |
| α-Farnesene | 502-61-4 | 1747 | 1720–1764 | 93 (41–69) | |
| benzenic derivatives | Estragole | 140-67-0 | 1724 | 1624–1661 | 148 (117–147) |
| VOC | Time | Initial | Time | C | 55 | 55_cp | 65 | 65_cp |
|---|---|---|---|---|---|---|---|---|
| Cumulative VOC concentration | intact | 87 a | 5 d | 445 b–d | 515 c–e | 388 b | 415 b–c | 425 b–c |
| cut | 618 e | 10 d | 364 b | 493 c–e | 320 b | 379 b | 354 b | |
| 13 d | 385 b | 502 d–e | 305 b | 317 b | 369 b | |||
| Ethyl acetate | intact | 0.86 a | 5 d | 109 c–d | 152 e | 80.9 b–c | 93.3 b–d | 83.5 b–c |
| es1 | cut | 1.98 a | 10 d | 104 b–d | 180 f | 75.8 b | 121 d | 83.2 b–c |
| 13 d | 121 d | 196 f | 82 b–c | 106 b–d | 105 b–d | |||
| Propyl acetate | intact | nd | 5 d | 3.79 g | 2.53 d–e | 2.45 c–e | 1.78 b–c | 2.59 d–e |
| es2 | cut | 2.91 e–f | 10 d | 3.35 f–g | 2.27 b–e | 2.31 b–e | 1.71 b | 2.39 b–e |
| 13 d | 3.74 g | 2.21 b–e | 2.11 b–d | 0.39 a | nd | |||
| Butyl acetate * | intact | 2.53 a | 5 d | 27.6 b | 25.3 b | 24.4 b | 26.3 b | 24.7 b |
| es3 | cut | 56.0 c | 10 d | 20.0 b | 18.7 b | 18.4 b | 17.7 b | 17.8 b |
| 13 d | 19.0 b | 17.3 b | 16.9 b | 14.9 b | 17.2 b | |||
| Isobutyl acetate | intact | 1.52 a | 5 d | 21.9 b | 18.1 b | 17.9 b | 22.2 b | 17.9 b |
| es4 | cut | 64.1 c | 10 d | 16.7 a–b | 15.1 a–b | 13.7 a–b | 16.1 a–b | 13.7 a–b |
| 13 d | 15.9 a–b | 15.8 a–b | 12.3 a–b | 13.9 a–b | 14.2 a–b | |||
| 2-methylbutyl acetate * | intact | 25.2 a | 5 d | 139 d | 127 c–d | 110 b–d | 114 b–d | 128 c–d |
| es5 | cut | 323 e | 10 d | 94.1 b–d | 94.7 b–d | 79.5 a–c | 74.8 a–c | 91.1 b–d |
| 13 d | 87.3 b–d | 89.9 b–d | 66.5 a–b | 62 a–b | 87.8 b–d | |||
| Pentyl acetate * | intact | 0.78 a | 5 d | 4.36 b | 3.34 a–b | 3.36 a–b | 4.04 b | 3.16 a–b |
| es6 | cut | 13.1 c | 10 d | 2.64 a–b | 2.44 a–b | 2.02 a–b | 2.37 a–b | 1.91 a–b |
| 13 d | 2.38 a–b | 1.83 a–b | 1.61 a–b | 1.80 a–b | 1.43 a–b | |||
| Hexyl acetate * | intact | 3.15 a | 5 d | 5.98 a | 4.64 a | 4.45 a | 5.19 a | 3.73 a |
| es7 | cut | 59.8 b | 10 d | 3.48 a | 3.56 a | 2.31 a | 2.85 a | 2.14 a |
| 13 d | 3.15 a | 2.56 a | 1.73 a | 2.13 a | 1.88 a | |||
| 2-Hexen-1-yl acetate | intact | nd | 5 d | nd | nd | nd | nd | nd |
| es8 | cut | 22.9 b | 10 d | nd | nd | nd | nd | nd |
| 13 d | nd | nd | nd | nd | nd | |||
| Ethyl propionate | intact | 1.46 b–c | 5 d | 3.17 e | 3.16 e | 2.03 c–d | 1.22 b | 1.65 b–d |
| es9 | cut | nd | 10 d | 3.27 e | 4.23 f | 2.34 d | 1.63 b–d | 1.79 b–d |
| 13 d | 5.01 g | 4.49 f–g | 2.33 d | 1.34 b–c | 1.82 b–d | |||
| Ethyl 2-methylpropanoate | intact | nd | 5 d | 1.06 b–c | 2.88 e | 1.32 b–c | 1.64 c–d | 1.24 b–c |
| es10 | cut | nd | 10 d | 0.97 b–c | 3.94 f | 1.18 b–c | 2.20 d–e | 1.31 b–c |
| 13 d | 1.35 b–c | 4.76 g | 0.63 a–b | 0.82 a–c | 0.89 a–c | |||
| Methyl butyrate | intact | 0.63 a | 5 d | 1.37 a–b | 1.06 a–b | 1.39 a–b | 0.77 a–b | 1.79 b |
| es11 | cut | 4.20 c | 10 d | 1.13 a–b | 0.93 a–b | 1.36 a–b | 0.63 a | 1.48 a–b |
| 13 d | 1.06 a–b | 0.93 a–b | 1.14 a–b | 0.57 a | 1.5 a–b | |||
| Methyl 2-methylbutyrate * | intact | nd | 5 d | 2.27 c–e | 1.60 b–c | 2.78 d–g | 1.09 b | 2.90 e–g |
| es12 | cut | nd | 10 d | 1.97 b–d | 1.62 b–c | 3.48 g | 1.09 b | 3.27 f–g |
| 13 d | 2.44 c–f | 1.80 b–c | 2.92 e–g | 1.06 b | 2.94 e–g | |||
| Ethyl butyrate * | intact | nd | 5 d | 12.1 b–c | 16.8 c–d | 12.4 b–c | 13.4 b–c | 12.5 b–c |
| es13 | cut | 1.64 a | 10 d | 10.9 b | 18.4 d | 11.9 b | 13.3 b–c | 11.6 b |
| 13 d | 12.9 b–c | 18.2 d | 11.0 b | 10.2 b | 12.3 b–c | |||
| Ethyl 2-butenoate | intact | nd | 5 d | 0.54 b–d | 0.62 b–e | 0.51 b–c | 0.91 e–f | 0.93 e–f |
| es14 | cut | nd | 10 d | 0.70 b–f | 0.82 c–f | 0.55 b–d | 0.98 f | 0.78 b–f |
| 13 d | 0.84 d–f | 0.98 f | 0.49 b | 0.79 b–f | nd | |||
| Ethyl 2-methylbutanoate * | intact | nd | 5 d | 25.1 b–c | 37.9 d–e | 30.4 b–d | 23.3 b | 31.9 b–d |
| es15 | cut | 0.98 a | 10 d | 25.1 b–c | 43.3 e–f | 29.6 b–d | 33.6 c–d | 32.8 b–d |
| 13 d | 33.7 c–d | 51.0 f | 27.4 b–c | 24.8 b–c | 33.4 c–d | |||
| Hexyl butyrate * | intact | 5.24 a | 5 d | 1.55 b–d | 1.92 b–d | 2.45 b–c | 2.66 b | 2.51 b–c |
| es16 | cut | 5.39 a | 10 d | 1.00 d–e | 1.16 c–e | 1.44 b–e | 1.38 b–e | 1.65 b–d |
| 13 d | 0.63 d–e | nd | nd | nd | 1.28 c–e | |||
| Hexyl 2-methylbutanoate * | intact | 5.56 a | 5 d | 11 b–e | 12.9 e | 12.6 d–e | 11.3 c–e | 11.2 c–e |
| es17 | cut | 8.20 b | 10 d | 11.2 c–e | 11.1 b–e | 10.2 b–e | 9.76 b–d | 12.3 d–e |
| 13 d | 11.4 c–e | 9.96 b–d | 9.80 b–d | 8.67 b–c | 12.1 d–e | |||
| Ethyl valerate | intact | nd | 5 d | 0.37 d–e | 0.47 f–g | nd | 0.39 e–f | nd |
| es18 | cut | nd | 10 d | 0.34 c–e | 0.54 g | 0.26 b–c | 0.28 b–d | nd |
| 13 d | 0.38 e | 0.48 g | 0.21 b | nd | 0.3 b–e | |||
| Methyl hexanoate | intact | nd | 5 d | 0.58 b–d | 1.80 e–f | 0.54 a–d | 1.44 e | 1.01 d |
| es19 | cut | 2.09 f | 10 d | 0.41 a–b | 1.89 e–f | 0.39 a–b | 0.98 c–d | 0.44 a–b |
| 13 d | 0.49 a–c | 2.17 f | nd | nd | nd | |||
| Ethyl hexanoate | intact | nd | 5 d | 13.3 i–j | 15.7 j | 9.95 g–h | 8.04 f–g | 7.67 e–g |
| es20 | cut | 1.56 a–b | 10 d | 7.15 d–g | 11.6 h–i | 4.88 c–e | 4.74 b–e | 4.28 b–d |
| 13 d | 6.34 d–f | 7.72 e–g | 2.86 a–c | 2.74 a–c | 3.22 a–c | |||
| Hexyl hexanoate * | intact | 2.11 a–b | 5 d | 0.98 d–g | 1.28 c–g | 1.57 b–e | 2.48 a | 1.75 a–d |
| es21 | cut | 1.92 a–c | 10 d | 0.71 f–g | 1.07 d–g | 0.82 e–g | 1.27 c–g | 1.43 b–f |
| 13 d | 0.56 g | 0.71 f–g | 0.73 f–g | 0.92 e–g | 1.14 c–g | |||
| Ethanol | intact | 9.72 b | 5 d | 13.1 c | 27.7 f | 14.9 c | 22.0 d–e | 20.5 d |
| al1 | cut | 2.21 a | 10 d | 13.1 c | 27.8 f | 14.0 c | 20.9 d–e | 15.6 c |
| 13 d | 15.0 c | 32.4 g | 19.7 d | 23.8 e | 20.1 d | |||
| 2-(2-Ethoxyethoxy)ethanol | intact | 0.65 a | 5 d | 0.35 b–d | 0.45 b | 0.43 b | 0.37 b–d | 0.38 b–c |
| al2 | cut | 0.62 a | 10 d | 0.30 c–e | 0.25 d–e | 0.22 e | 0.29 c–e | 0.22 e |
| 13 d | 0.26 c–e | 0.25 d–e | 0.28 c–e | 0.27 c–e | 0.29 c–e | |||
| 2-Methyl-1-propanol | intact | 0.86 a | 5 d | 1.02 a–b | 0.96 a | 0.94 a | 1.21 a–c | 1.13 a–b |
| al3 | cut | 2.99 e | 10 d | 0.92 a | 1.01 a–b | 0.88 a | 1.07 a–b | 0.93 a |
| 13 d | 1.07 a–b | 1.68 d | 1.50 c–d | 1.36 b–d | 1.09 a–b | |||
| 1-Butanol * | intact | 1.51 a | 5 d | 1.56 a | 2.01 b–c | 1.72 a–b | 2.13 c | 1.99 b–c |
| al4 | cut | 2.82 d | 10 d | 1.59 a | 1.82 a–c | 1.72 a–b | 1.79 a–c | 1.66 a–b |
| 13 d | 1.46 a | 1.67 a–b | 1.67 a–b | 1.67 a–b | 1.64 a–b | |||
| 2-Methyl-1-butanol | intact | 3.24 a | 5 d | 4.25 a–b | 5.32 b–d | 4.44 a–c | 5.14 b–d | 5.89 c–d |
| al5 | cut | 9.02 e | 10 d | 4.63 a–c | 5.58 b–d | 4.67 b–c | 4.60 a–c | 5.21 b–d |
| 13 d | 4.51 a–c | 6.3 d | 4.77 b–c | 4.67 b–c | 5.55 b–d | |||
| 1-Hexanol * | intact | 1.44 a | 5 d | 2.90 b | 3.99 b–c | 3.07 b | 4.54 c | 3.60 b–c |
| al6 | cut | 3.94 b–c | 10 d | 3.06 b | 4.21 b–c | 3.08 b | 3.39 b–c | 2.84 b |
| 13 d | 2.88 b | 3.93 b–c | 3.27 b–c | 3.52 b–c | 3.11 b | |||
| 2-Butanone | intact | 2.67 a–e | 5 d | 0.86 a–b | 4.71 e–f | 3.62 c–e | 8.89 g | 6.75 f–g |
| k1 | cut | 0.33 a | 10 d | 0.73 a–b | 7.32 g | 1.96 a–d | 4.68 e–f | 4.22 d–e |
| 13 d | 1.32 a–c | 7.12 g | 2.44 a–e | 3.30 b–e | 3.76 c–e | |||
| 1-Penten-3-one | intact | nd | 5 d | 1.35 b | 2.29 b–d | 1.77 b–c | 3.35 e–f | 1.98 b–c |
| k2 | cut | nd | 10 d | 3.71 e–f | 3.98 f | 3.21 d–f | 5.16 g | 3.72 e–f |
| 13 d | 3.52 e–f | 2.78 c–e | 4.18 f | 4.06 f | 3.76 e–f | |||
| Hexanal * | intact | 0.50 a | 5 d | 3.23 b–d | 6.47 f–g | 4.66 d–f | 7.14 g | 5.25 d–g |
| ad1 | cut | 1.92 a–b | 10 d | 4.49 d–f | 5.08 d–f | 3.72 b–d | 6.26 e–g | 4.43 d–e |
| 13 d | 4.19 c–d | 2.30 a–c | 3.46 b–d | 3.77 b–d | 3.81 b–d | |||
| Estragole | intact | 0.30 b–e | 5 d | 0.61 g | 0.42 c–f | 0.45 d–g | 0.35 b–f | 0.47 e–g |
| bd1 | cut | 0.41 b–f | 10 d | 0.52 f–g | nd | nd | 0.29 b–d | 0.37 b–f |
| 13 d | 0.51 f–g | nd | 0.26 b–c | 0.24 b | 0.36 b–f | |||
| D-Limonene | intact | 3.27 a | 5 d | 6.26 a–d | 10.4 e | 9.77 e | 7.54 b–e | 8.45 d–e |
| tp1 | cut | 4.93 a–c | 10 d | 4.74 a–b | 4.54 a | 9.54 e | 7.65 c–e | 8.67 d–e |
| 13 d | 4.70 a–b | 4.40 a | 9.06 d–e | 5.23 a–c | 9.28 e | |||
| α-Farnesene | intact | 13.8 a–c | 5 d | 24.0 d–e | 18.8 a–d | 21.3 c–e | 16.8 a–d | 28.0 e |
| tp2 | cut | 19.1 a–d | 10 d | 17.4 a–d | 14.4 a–c | 14.9 a–c | 15.0 a–c | 20.5 b–e |
| 13 d | 16.5 a–d | 10.6 a | 12.1 a–b | 12.5 a–b | 17.7 a–d |
| Treatment | Reaction | VOCs | Aroma Threshold Values (nL L−1) |
|---|---|---|---|
| all sHWT | ↑ | Ethanol | 8–900 a/>1 × 105,b |
| ↓ | Propyl acetate | 2000–11,000 a,b | |
| Estragole | n/a | ||
| sHWT without cp | ↑ | Ethanol | 8–900 a/>1 × 105,b |
| sHWT combined with cp | ↑ | D-limonene | 4–229 a |
| ↓ | Ethyl 2-butenoate | n/a | |
| only 55 °C sHWT | ↑ | 2-methyl-1-propanol | 360–3300 a |
| only 55 °C sHWT without cp | ↑ | Ethyl acetate | 5–13,500 b |
| Ethyl 2-methylpropanoate | 0.01–1 a | ||
| Ethyl butyrate * | 0.1–18 a | ||
| Ethyl 2-methylbutanoate * | 0.006–0.1 a,b | ||
| Ethyl valerate | 1.5–5 a,b | ||
| Methyl hexanoate | 10–87 a | ||
| 2-butanone | n/a | ||
| ↓ | Estragole | n/a | |
| 1-penten-3-one | 400 a | ||
| only 55 °C sHWT combined with cp | ↓ | Ethyl acetate | 5–13,500 a,b |
| only 65 °C sHWT | ↓ | Propyl acetate | 2000–11,000 a,b |
| only sHWT at 65 °C or combined with cp | ↓ | Ethyl propionate | 9–45 a |
| Ethyl hexanoate | 0.3–5 a | ||
| Methyl hexanoate | 10–87 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rux, G.; Efe, E.; Ulrichs, C.; Huyskens-Keil, S.; Hassenberg, K.; Herppich, W.B. Effects of Pre-Processing Hot-Water Treatment on Aroma Relevant VOCs of Fresh-Cut Apple Slices Stored in Sugar Syrup. Foods 2020, 9, 78. https://doi.org/10.3390/foods9010078
Rux G, Efe E, Ulrichs C, Huyskens-Keil S, Hassenberg K, Herppich WB. Effects of Pre-Processing Hot-Water Treatment on Aroma Relevant VOCs of Fresh-Cut Apple Slices Stored in Sugar Syrup. Foods. 2020; 9(1):78. https://doi.org/10.3390/foods9010078
Chicago/Turabian StyleRux, Guido, Efecan Efe, Christian Ulrichs, Susanne Huyskens-Keil, Karin Hassenberg, and Werner B. Herppich. 2020. "Effects of Pre-Processing Hot-Water Treatment on Aroma Relevant VOCs of Fresh-Cut Apple Slices Stored in Sugar Syrup" Foods 9, no. 1: 78. https://doi.org/10.3390/foods9010078
APA StyleRux, G., Efe, E., Ulrichs, C., Huyskens-Keil, S., Hassenberg, K., & Herppich, W. B. (2020). Effects of Pre-Processing Hot-Water Treatment on Aroma Relevant VOCs of Fresh-Cut Apple Slices Stored in Sugar Syrup. Foods, 9(1), 78. https://doi.org/10.3390/foods9010078





