Abstract
In the present study, a novel Pediococcus pentosaceus SP2 strain, recently isolated from kefir grains, was evaluated as a starter culture in sourdough bread making. The novel starter was applied in fresh, freeze-dried, and freeze-dried immobilized (on wheat bran) form. The type of culture (fresh, freeze-dried, immobilized cells) influenced the bread characteristics. Specifically, the application of freeze-dried immobilized cells led to higher total titratable acidity (TTA) values (9.81 mL NaOH N/10), and the produced bread presented higher resistance to mold and rope spoilage. Moreover, the produced sourdough breads were significantly better in terms of pH, TTA, organic acids content, and resistance to mold and rope spoilage, compared to breads made with a commercial, wild microbiota, sourdough. The organic acids content was also significantly higher than the commercial sourdough sample (2.93 g/kg lactic acid; 1.01 g/kg acetic acid). Determination of volatile compounds through solid-phase microextraction (SPME) gas chromatography/mass spectrometry (GC/MS) analysis and sensorial assessments indicated no significant differences between the tested sourdough breads.
1. Introduction
The use of sourdough is well established as a natural, free of synthetic additives, method of bread making. Specifically, the advantages of sourdough are the production of breads with (i) higher nutritional value (minerals, free amino acids, and protein bioavailability), (ii) enhanced palatability, (iii) superior organoleptic characteristics (increased production of desirable volatile compounds), and (iv) increased shelf life (lower staling rate, high resistance to rope and mold spoilage) [1]. Sourdough is a food matrix with a complex microbial load that can be composed by lactic acid bacteria (LAB), acetic acid bacteria, and yeasts [2]. Consequently, its microbiological stability is critical and should be controlled in industrial production. A way to simplify this matter is the application of pure starters that will prevail over other species present in the dough. In addition, the control of fermentation conditions (e.g., pH, fermentation time and temperature) is also very significant because it can favor the growth of the starter culture [3,4].
Selected LAB are often used in sourdough starter culture preparations, because they are naturally present at high populations in the natural sourdough microflora and due to many advantages they offer in breads, such as enhanced flavor profile, higher preservation times, and increased nutritional value. Moreover, the potential health benefits (e.g., probiotic properties and antagonism against food-borne pathogens and spoilage organisms), has increased the consumer preference for foods containing LAB. Many such examples of LAB applications in sourdough preparations have been reported in the scientific literature [5,6,7,8,9,10].
Kefir grains have also been applied as mixed starters for sourdough bread making [11,12,13]. The kefir grains microbiota consist mainly of Lactobacillus, Streptococcus, Lactococcus species, and yeasts, among a variety of other species, which coexist in perfect symbiosis [14]. Likewise, kefir grains are considered a valuable source of beneficial microbes (mainly LAB) that can exhibit either probiotic properties or other functional properties of technological interest [11,15,16].
On the other hand, the food industry requires ready-to-use microbial starters that can be preserved for long periods, maintaining their functionality at high levels. Preservation methods, which are well established as being practical for industrial applications, are sub-cultivation, freezing, and drying [17]. However, preservation by drying seems to be the most appropriate, since it is a low-cost method, and because it produces compact, easily stored, and relatively lightweight final products [18]. Freeze-drying is also a widespread drying technique for microorganisms, which offers several advantages such as high stability and high viability of the cultures during storage [18,19].
Therefore, the efficiency of P. pentosaceus SP2, in free-form, freeze-dried form, and immobilized on wheat bran and freeze-dried form, as a starter culture in sourdough bread making is sought in the present study. Typical bread parameters were assessed, such as physicochemical characteristics, resistance to spoilage, flavor-related compounds, and consumer acceptance. The ultimate target will be to evaluate the technological performance of P. pentosaceus SP2 for application in probiotic food production in general, which is a significant prerequisite for candidate probiotic strains [20,21]. In this study, sourdough was selected as the food matrix because: (i) P. pentosaceus has been identified in the natural sourdough microbiota [22], and (ii) some P. pentosaceus strains seem to have antimicrobial properties against microbial bread spoilage [23]. For these reasons, P. pentosaceus SP2 was evaluated in this study as a potential starter culture in sourdough bread making. Moreover, the culture was also immobilized on wheat bran as a means to enhance its viability since immobilization has been previously shown to be a useful technique for the survival of cells during processing and storage [24].
2. Materials and Methods
2.1. Microorganisms
P. pentosaceus SP2, recently isolated from kefir grains [16], was grown in MRS (De Man, Rogosa and Sharpe) broth (Fluka, Buchs, Switzerland) at 37 °C for 24 h. Suitable amounts of harvested cell biomass (approximately 4% w/w wet weight basis) were then obtained and used for sourdough bread making. Baker’s yeast was a commercial Saccharomyces cerevisiae strain (S.I. Lesaffre, France), supplied in the form of compressed blocks.
2.2. Cell Immobilization
P. pentosaceus SP2 was immobilized on wheat bran, which was supplied by a local cereal processing company (Orestiada, Greece). The immobilization procedure included the mixing of 0.5 g of harvested cell mass with 5 g of wheat bran in 500 mL MRS broth and incubating at 37 °C for 48 h. Afterwards, the immobilized cells were washed twice with Ringers solution ¼ strength for the removal of free cells [24,25].
2.3. Freeze-Drying
Free and immobilized cells of P. pentosaceus SP2 were freeze-dried overnight on a freeze-drying system (FreeZone 4.5, Labconco, Kansas City, MO, USA). Subsequently, they were applied as starter cultures for sourdough bread production [25].
2.4. Determination of Cell Counts
The determination of viable cell counts of freshly harvested, freeze-dried, and freeze-dried/immobilized P. pentosaceus SP2 was done as follows: 1 g of each sample was homogenized in 9 mL of phosphate buffer (1.25 mL of 0.25 M solution of KH2PO4 per litre of distilled water). The suspension was serially diluted, plated on MRS agar (Fluka, Buchs, Switzerland) and incubated at 37 °C for 48–72 h. The cell counts were expressed as log cfu/g of freshly harvested P. pentosaceus SP2 cells or of wheat bran in the case of immobilized and freeze-dried/immobilized cells [25].
The determination of viable cell counts of LAB and yeasts in the sourdoughs was carried out in a similar manner. Specifically, 20 g of sourdough were homogenized in 200 mL of phosphate buffer. The suspension was serially diluted, and LAB was determined on MRS agar (Fluka, Buchs, Switzerland) after incubation at 37 °C for 48–72 h and yeasts were determined on malt agar (Fluka, Buchs, Switzerland) after incubation at 30 °C for 2 days [25].
2.5. Sourdough Bread Making
Commercial white flour was used for bread making (Hellenic Biscuit CO S.A., Athens, Greece), with the following composition (% w/w): Protein 11.0, carbohydrates 72.0, fat 1.5, fiber 2.2 and moisture 12.0. Mixing of the ingredients was performed mechanically, and the dough was molded manually in 1.5 L baking pans.
Three mother sponges were prepared by mixing 300 g wheat flour, 160 mL tap water, and 1% w/w (on flour basis) of (i) P. pentosaceus SP2 culture, or (ii) freeze-dried P. pentosaceus SP2 culture, or (iii) immobilized freeze-dried P. pentosaceus SP2 for 15 min. All the sponges were incubated at 30 °C for 24 h.
Sourdoughs were prepared by mixing, for 15 min, 250 g of the above-fermented mother sponges with 300 g wheat flour and 160 mL tap water, followed by incubation at 30 °C for 24 h. The sourdoughs were coded as Fresh SP2 (prepared with fresh P. pentosaceus SP2), Freeze-dried SP2 (prepared with freeze-dried P. pentosaceus SP2), and Immobilized SP2 (prepared with immobilized freeze-dried P. pentosaceus SP2). Subsequently, 3 respective sourdough breads were produced containing 30% w/w (on flour basis) of these sourdoughs (bread with Fresh SP2 sourdough, bread with Freeze-dried SP2 sourdough, and bread with Immobilized SP2 sourdough). The doughs of all the breads contained 150 g of sourdough, 500 g wheat flour, 270 mL tap water, and 4 g salt. In all cases, an amount of 1% w/w (on flour basis) of pressed baker’s yeast was also added as a leavening agent. All doughs were fermented at 30 °C for 2 h, proofed at 40 °C for 60 min and baked at 230 °C for approximately 40 min [4].
In addition, trials were carried out for comparison using traditional (wild microbiota) sourdough provided by a local bakery (sourdough coded as C). This sourdough is used 2–3 times per week and is, respectively, refreshed in order to obtain the appropriate acidity and viability of LAB. The produced bread (bread sample C) contained 30% (on flour basis) of a traditional wild microbiota sourdough. The recipe and the procedure followed was the same as described above for the P. pentosaceus SP2 sourdoughs. All trials were carried out in triplicate.
2.6. Organic Acids Analysis
Lactic, acetic, formic, propionic, n-valeric, and caproic acid were determined by ion-exchange liquid chromatography, as described previously by [25]. Determinations of organic acid concentrations were carried out by means of standard curves.
2.7. Determination of pH and Total Titratable Acidity
The pH and total titratable acidity (TTA) values of sourdough bread samples were determined as described previously [4]. The TTA was expressed as the volume (mL) of NaOH N/10 consumed.
2.8. Determination of Specific Loaf Volume
Loaf volume was measured by the rapeseed displacement method. The specific loaf volume was calculated as mL/g [25].
2.9. Analysis of Flavor Volatiles
Determination of volatile compounds was done by gas chromatography/mass spectrometry (GC/MS) analysis. Initially, volatile compounds were isolated by the headspace solid-phase microextraction (SPME) sampling technique, as described before [13]. The identification of volatile compounds was carried out through comparison with standard compounds (Sigma-Aldrich, Saint Louis, MO, USA) and MS data with those in NIST107, NIST21, and SZTERP libraries. 4-methyl-2-pentanol (Sigma-Aldrich) diluted in pure ethanol was used as the internal standard (IS) at various concentrations (4, 40, and 400μg/g of sample) for the semi-quantitative analysis of volatiles. The quantification of the volatile compounds was made by dividing the peak areas of the compounds of interest by the peak area of the IS and multiplying this ratio by the initial concentration of the IS. All assays were carried out in triplicate.
2.10. Rope and Mould Spoilage Observation
Sourdough breads made with Fresh SP2 sourdough, with Freeze-dried SP2 sourdough, and with Immobilized SP2 sourdough, as well as with the commercial sourdough (C), were examined for rope and mold spoilage. Specifically, sourdough bread samples of similar shape and size were cut from the same loaf of bread and stored at room temperature. The appearance of rope spoilage and mold spoilage was evaluated macroscopically as described before [4]. All assays were carried out in triplicate.
2.11. Sensory Evaluation
Sourdough breads made with Fresh SP2 sourdough, with Freeze-dried SP2 sourdough and with Immobilized SP2 sourdough, as well as with the commercial sourdough (C), were evaluated at a local bakery through a blind sensory evaluation test immediately after their production. Specifically, 15 randomly untrained testers (consumers) evaluated the breads providing scores between 0 (unacceptable) and 10 (exceptional) for attributes of flavor, taste, and overall quality such as volume, texture, and color. At least 3 samples were delivered to each tester. A scoring scale with 3 categories was applied: Class 1 related to high-quality bread without any off-odor or off-flavor, class 2 related to bread with slight off-odors or off-flavors and class 3 related to the bread of unacceptable quality. The results were expressed as average scores plus standard deviations [25].
2.12. Statistical Analysis
The effects of the different sourdough starters on the physicochemical characteristics of the breads, the volatile flavour compounds, and the scores of the sensory tests were analyzed by ANOVA followed by Duncan’s post hoc multiple range test to extract the specific differences between the various treatments. The statistical analysis was performed by using IMB SPSS v20 (IBM Corp., Armonk, NY, USA) at an alpha level of 5%.
3. Results and Discussion
3.1. Sourdough Bread Quality Characteristics
In general, no statistically significant differences were observed among the produced bread samples (p > 0.05) regarding specific loaf volumes and n-valeric and caproic acid levels. All other characteristics of the sourdough breads produced with any form of P. pentosaceus SP2 sourdough, were better compared to the bread produced with the commercial sourdough (C). Specifically, P. pentosaceus SP2 breads had statistically significant differences regarding pH values compared to the commercial sourdough breads (C) (Table 1).

Table 1.
Physicochemical characteristics of breads made with sourdough containing P. pentosaceus SP2 cells (Fresh SP2), freeze-dried P. pentosaceus SP2 cells (Freeze-dried SP2), immobilized freeze-dried P. pentosaceus SP2 cells (Immobilized SP2), as well as with the commercial sourdough (C).
A higher TTA value (9.81 mL NaOH N/10), higher lactic acid (2.93 g/kg) and propionic acid content (0.08 g/kg) (p < 0.05), were determined in the sourdough bread made with Immobilized SP2 sourdough compared to all the other bread samples (Table 1). This may be explained by the fact that this sourdough had the lowest pH value (3.7) and highest TTA (19 mL NaOH N/10) than all the other sourdoughs revealing its high potential for bread acidification. Another explanation for this significant difference is that sourdough prepared with immobilized freeze-dried P. pentosaceus SP2, contained statistically higher viable cell counts of LAB (9.5 log cfu/g) compared to all the other sourdoughs (Figure 1). This result is quite interesting, since the initial viable cell counts of P. pentosaceus SP2 in the three different sourdoughs after their preparation were approximately the same (8.08 ± 0.12, 8.04 ± 0.09, and 8.04 ± 0.11 log cfu/g for the fresh P. pentosaceus SP2 sourdough, the freeze-dried SP2 sourdough, and the freeze-dried immobilized SP2 sourdough, respectively), while those of LAB in the commercial sourdough were 8.07 ± 0.11 log cfu/g (on wet weight basis). The initial cell counts of the biocatalysts used to prepare the sourdoughs were 8.23 ± 0.06, 8.18 ± 0.05, and 8.11 ± 0.8 log cfu/g for the fresh P. pentosaceus SP2 culture, the freeze-dried culture, and the freeze-dried immobilized biocatalyst, respectively. It should be underlined that the sourdough used in bakeries is appropriately activated and refreshed many times before its application for sourdough bread making. This is why, although no starter culture was added in the commercial sourdough, the initial cell counts for LAB were at about the same levels as those of the other tested sourdoughs. Likewise, the addition of starter culture in the sourdough and the application of immobilization clearly enhanced the viability of P. pentosaceus SP2 in the sourdoughs after incubation at 30 °C for 24 h, as shown in Figure 1. Specifically, wheat bran (a cereal processing by-product) that was used as the immobilization carrier is considered as a prebiotic substrate which enhances the viability of probiotic bacteria and can be incorporated in novel functional foods [24].
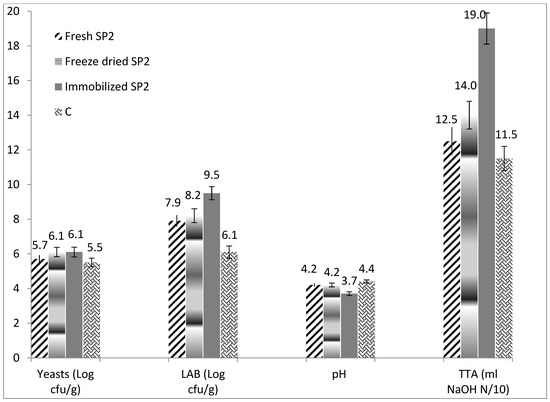
Figure 1.
Viable cell counts (log cfu/g) of yeasts and lactic acid bacteria (LAB), total titratable acidity (TTA), and pH values of the sourdoughs prepared with frsh P. pentosaceus SP2 cells (Fresh SP2), with freeze-dried P. pentosaceus SP2 cells (Freeze-dried SP2), with immobilized freeze-dried P. pentosaceus SP2 cells (Immobilized SP2), and commercial sourdough with wild microbiota (C), after incubation at 30 °C for 24 h.
3.2. Volatile Compounds
The composition of headspace volatile compounds of the produced breads was analyzed by SPME GC/MS, and the results are presented in Table 2. In total, 33 compounds were detected in all sourdough breads containing P. pentosaceus SP2, and 24 in the bread made by the commercial sourdough (C). Most of these compounds (especially those with low odor threshold values) are well known to affect bread flavor (sourdough and non-sourdough), such as benzaldehyde, heptanol, 2-phenylethyl acetate, hexanal, 2-methylbutanal, 2-phenylethanol, 1-octen-3-ol, 2-nonenal, furfural, etc., and their contribution to bread flavor has been widely reviewed [26]. The most important observation in this case, was the identification of more esters (10) in the case of the sourdough breads that contained P. pentosaceus SP2, compared to the commercial sourdough bread (5 compounds), which is a common observation for most fermented foods and is expected to affect flavor since esters are usually associated with pleasant fruity and flowery notes.

Table 2.
SPME GC/MS analysis (semi-quantitative) of flavor-related compounds (μg/g) extracted from breads made with sourdoughs containing P. pentosaceus SP2 cells (Fresh SP2), freeze-dried P. pentosaceus SP2 cells (Freeze-dried SP2), immobilized freeze-dried P. pentosaceus SP2 cells (Immobilized SP2), as well as with the commercial sourdough (C).
3.3. Appearance of Spoilage
It is widely recognized that the microbial spoilage of bread determines its shelf life and causes considerable economic losses. Therefore, the role of LAB in sourdough bread spoilage is a widely studied topic. The published work has shown that organic acids produced by LAB species present strong antimicrobial activity in bread, depending on the sensitivity of the individual spoilage organisms, while they affect the activity of baker’s yeast, the rising of the dough, as well as the texture of the bread [4,5,6,7,8,23,25,27,28,29,30]. The appearance of mold and rope spoilage in all sourdough bread samples was monitored through daily macroscopic observations, which are depicted in Figure 2.
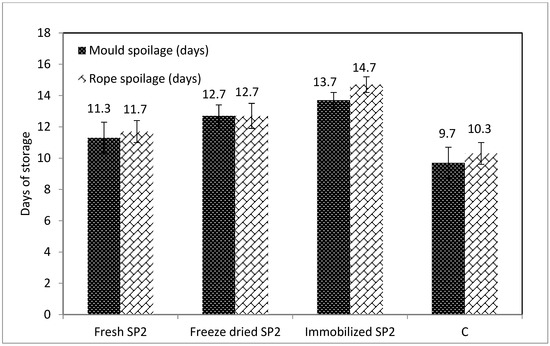
Figure 2.
Resistance against rope and mold spoilage of breads made with sourdoughs containing P. pentosaceus SP2 cells (Fresh SP2), freeze-dried P. pentosaceus SP2 cells (Freeze-dried SP2), immobilized freeze-dried P. pentosaceus SP2 cells (Immobilized SP2), as well as with the commercial sourdough (C).
Regarding mold spoilage, it seems that the sourdough bread made with immobilized SP2 sourdough was more resistant (p < 0.05) compared to the other samples, since spoilage was observable after the 13th day of storage. The sourdough bread made with Fresh SP2 sourdough developed observable spoilage after the 11th day, sourdough bread made with Freeze -dried SP2 sourdough after the 12th day, while the commercial sourdough bread (C) developed spoilage after the 9th day. The better resistance to spoilage of the sourdough bread made with immobilized SP2 sourdough may be attributed to its higher TTA and organic acid content, as also discussed in previous studies. Specifically, it has been reported that acetic acid presents higher antifungal activity than other organic acids produced by LAB, and it can also have a desirable effect on bread flavor at a certain concentration range [23].
In addition to their antifungal capacity, the organic acids and other compounds produced by LAB, also present effective antibacterial activities. This may explain the delayed (p < 0.05) development of rope spoilage in the sourdough bread made with Immobilized SP2 sourdough (after the 14th day), i.e., 2–4 days later than the other bread samples. This is in agreement with a recent study reporting that the use in sourdough bread making of a novel L. paracasei K5 strain delayed rope spoilage [4]. The antimicrobial effect of P. pentosaceus has also been previously shown in meat applications [31]. In addition, it has been reported that in sourdough fermented with Lactobacillus plantarum and P. pentosaceus, rope spoilage was delayed effectively, provided that the pH of the sourdoughs was below 4.0, and TTA was higher than 12 [27].
3.4. Consumer Acceptability
The results of the customer-oriented sensory evaluation of the produced sourdough breads are presented in Table 3. In general, the evaluation did not reveal significant differences among the different bread types. Likewise, the fact that the proposed starter culture did not receive lower scores compared to the commercial sourdough that was applied weekly in the bakery can be considered a positive outcome. In addition, even though wheat bran could affect the texture and volume of the bread negatively, as highlighted in previous studies [24], this was not observed by the evaluators.

Table 3.
Scores of the customer-oriented sensory evaluation of the breads made with sourdoughs prepared with P. pentosaceus SP2 cells in free-form (Fresh SP2), freeze-dried form (Freeze-dried SP2), and immobilized freeze-dried form (Immobilized SP2), as well as with the commercial sourdough (C).
4. Conclusions
Control of bread spoilage by natural means is significant from economic, food safety, as well as consumer acceptance points of view. Specifically, the microbiological stability of sourdough is important and should be controlled in industrial bread production. This can be achieved by using selected, efficient LAB species. The aim of this study was to assess the technological performance in sourdough bread making of a novel P. pentosaceus SP2 strain, recently isolated from kefir grains. The results showed that P. pentosaceus SP2 can be efficiently used as a sourdough starter. The produced breads were better in terms of acidity, organic acid content, and resistance to spoilage, compared to commercial sourdough bread (wild microbiota) prepared under the same conditions. In addition, the novel starter was more effective in the immobilized, freeze-dried form (on wheat bran), while immobilization enhanced its viability in sourdoughs. These findings indicate the potential for commercialization of the P. pentosaceus SP2 strain in the form of dry, lightweight, and reservable immobilized preparations for industrial purposes. Future work is needed to evaluate if the novel strain is able to produce particular metabolites such as exopolysaccharides and bacteriocins, as well as its potential for probiotic food production [28].
Author Contributions
Conceptualization: S.P.; Data curation: I.M.; Methodology: I.M. and A.B.; Project administration: S.P.; Supervision: S.P.; Visualization: A.B.; Writing—review & editing: S.P. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that there is no conflict of interest in this research article.
References
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel Insights on the Functional/Nutritional Features of the Sourdough Fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of Sourdough Technology: From Production to Marketing. Food Bioprocess Technol. 2018, 11, 242–270. [Google Scholar] [CrossRef]
- Cocolin, L.; Alessandria, V.; Dolci, P.; Gorra, R.; Rantsiou, K. Culture Independent Methods to Assess the Diversity and Dynamics of Microbiota during Food Fermentation. Int. J. Food Microbiol. 2013, 167, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Bekatorou, A. Effect of a Novel Lactobacillus Paracasei Starter on Sourdough Bread Quality. Food Chem. 2019, 271, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, X.; Zhang, Y.; Yang, W.; Ma, G.; Ma, N.; Hu, Q.; Pei, F. A Novel Lactic Acid Bacterium for Improving the Quality and Shelf Life of Whole Wheat Bread. Food Control 2019, 109, 106914. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of Sourdough Lactobacilli–Do They Matter for Microbial Ecology and Bread Quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Ouiddir, M.; Bettache, G.; Salas, M.L.; Pawtowski, A.; Donot, C.; Brahimi, S.; Mabrouk, K.; Coton, E.; Mounier, J. Selection of Algerian Lactic Acid Bacteria for Use as Antifungal Bioprotective Cultures and Application in Dairy and Bakery Products. Food Microbiol. 2019, 82, 160–170. [Google Scholar] [CrossRef]
- Omedi, J.O.; Huang, W.; Zheng, J. Effect of Sourdough Lactic Acid Bacteria Fermentation on Phenolic Acid Release and Antifungal Activity in Pitaya Fruit Substrate. LWT 2019, 111, 309–317. [Google Scholar] [CrossRef]
- Eisenbach, L.; Geissler, A.J.; Ehrmann, M.A.; Vogel, R.F. Comparative Genomics of Lactobacillus Sakei Supports the Development of Starter Strain Combinations. Microbiol. Res. 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Gallo, G.; Settanni, L.; Berloco, M.G.; Siragusa, S.; Parente, E.; Corsetti, A.; Gobbetti, M. Genotypic and Phenotypic Diversity of Lactobacillus Rossiae Strains Isolated from Sourdough. J. Appl. Microbiol. 2007, 103, 821–835. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef]
- Plessas, S.; Pherson, L.; Bekatorou, A.; Nigam, P.; Koutinas, A.A. Bread Making Using Kefir Grains as Baker’s Yeast. Food Chem. 2005, 93, 585–589. [Google Scholar] [CrossRef]
- Plessas, S.; Alexopoulos, A.; Bekatorou, A.; Mantzourani, I.; Koutinas, A. A.; Bezirtzoglou, E. Examination of Freshness Degradation of Sourdough Bread Made with Kefir through Monitoring the Flavor Volatile Composition during Storage. Food Chem. 2011, 124, 627–633. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk Kefir: Composition, Microbial Cultures, Biological Activities, and Related Products. Front. Μicrobiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Alexopoulos, A.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Microbial Ecology and Quality Assurance in Food Fermentation Systems. The Case of Kefir Grains Application. Anaerobe 2011, 17, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the Probiotic Potential of Lactic Acid Bacteria Isolated from Kefir Grains: Evaluation of Adhesion and Antiproliferative Properties in in Vitro Experimental Systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Alonso, S. Novel Preservation Techniques for Microbial Cultures. In Novel Food Fermentation Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–33. [Google Scholar]
- Maisnam, D.; Rasane, P.; Dey, A.; Kaur, S.; Sarma, C. Recent Advances in Conventional Drying of Foods. J. Food Technol. Preserv. 2017, 1, 25–34. [Google Scholar]
- Chávez, B.E.; Ledeboer, A.M. Drying of Probiotics: Optimization of Formulation and Process to Enhance Storage Survival. Dry. Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Karapetsas, A.; Kazakos, S.; Alexopoulos, A.; Mantzourani, I.; Chondrou, P.; Fournomiti, M.; Galanis, A.; Bezirtzoglou, E. Isolation, Characterization and Evaluation of the Probiotic Potential of a Novel Lactobacillus Strain Isolated from Feta-Type Cheese. Food Chem. 2017, 226, 102–108. [Google Scholar] [CrossRef]
- Celano, G.; De Angelis, M.; Minervini, F.; Gobbetti, M. Different Flour Microbial Communities Drive to Sourdoughs Characterized by Diverse Bacterial Strains and Free Amino Acid Profiles. Front. Microbiol. 2016, 7, 1770. [Google Scholar] [CrossRef]
- Ripari, V.; Gänzle, M.G.; Berardi, E. Evolution of Sourdough Microbiota in Spontaneous Sourdoughs Started with Different Plant Materials. Int. J. Food Microbiol. 2016, 232, 35–42. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial Activity of Lactic Acid Bacteria against Pathogenic and Spoilage Microorganism Isolated from Food and Their Control in Wheat Bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Bosnea, L.; Kanellaki, M.; Ganatsios, V.; Koutinas, A.A. Wheat Bran as Prebiotic Cell Immobilisation Carrier for Industrial Functional Feta-Type Cheese Making: Chemical, Microbial and Sensory Evaluation. Biocatal. Agric. Biotechnol. 2018, 13, 75–83. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Plessas, S. Assessment of Ready-to-Use Freeze-Dried Immobilized Biocatalysts as Innovative Starter Cultures in Sourdough Bread Making. Foods 2019, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Schieberle, P. Generation of Flavor Compounds during Sourdough Fermentation: Applied and Fundamental Aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Katina, K.; Sauri, M.; Alakomi, H.-L.; Mattila-Sandholm, T. Potential of Lactic Acid Bacteria to Inhibit Rope Spoilage in Wheat Sourdough Bread. Lwt Food Sci. Technol. 2002, 35, 38–45. [Google Scholar] [CrossRef]
- Gutiérrez-Cortés, C.; Suarez, H.; Buitrago, G.; Nero, L.A.; Todorov, S.D. Characterization of Bacteriocins Produced by Strains of Pediococcus Pentosaceus Isolated from Minas Cheese. Ann. Microbiol. 2018, 68, 383–398. [Google Scholar] [CrossRef]
- Debonne, E.; Vermeulen, A.; Bouboutiefski, N.; Ruyssen, T.; Van Bockstaele, F.; Eeckhout, M.; Devlieghere, F. Modelling and Validation of the Antifungal Activity of DL-3-phenyllactic Acid and Acetic Acid on Bread Spoilage Moulds. Food Microbiol. 2020, 88, 103407. [Google Scholar] [CrossRef]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting Synergies of Sourdough and Antifungal Organic Acids to Delay Fungal Spoilage of Bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef]
- Mattila-Sandholm, T.; Haikara, A.; Skyttä, E. The Effect of Pediococcus Damnosus and Pediococcus Pentosaceus on the Growth of Pathogens in Minced Meat. Int. J. Food Microbiol. 1991, 13, 87–94. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).