Enzymatic Reaction-Related Protein Degradation and Proteinaceous Amino Acid Metabolism during the Black Tea (Camellia sinensis) Manufacturing Process
Abstract
1. Introduction
2. Materials and Methods
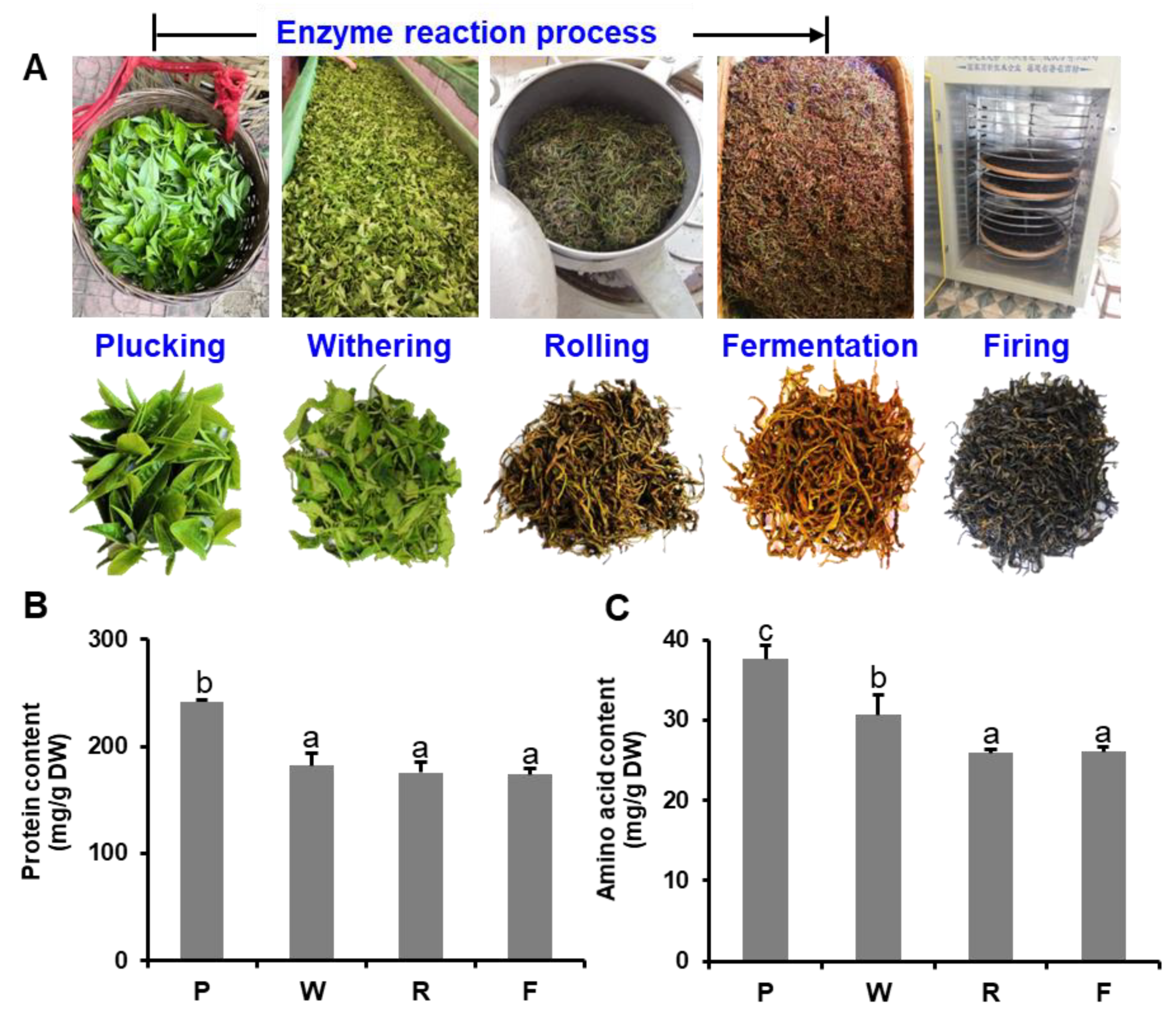
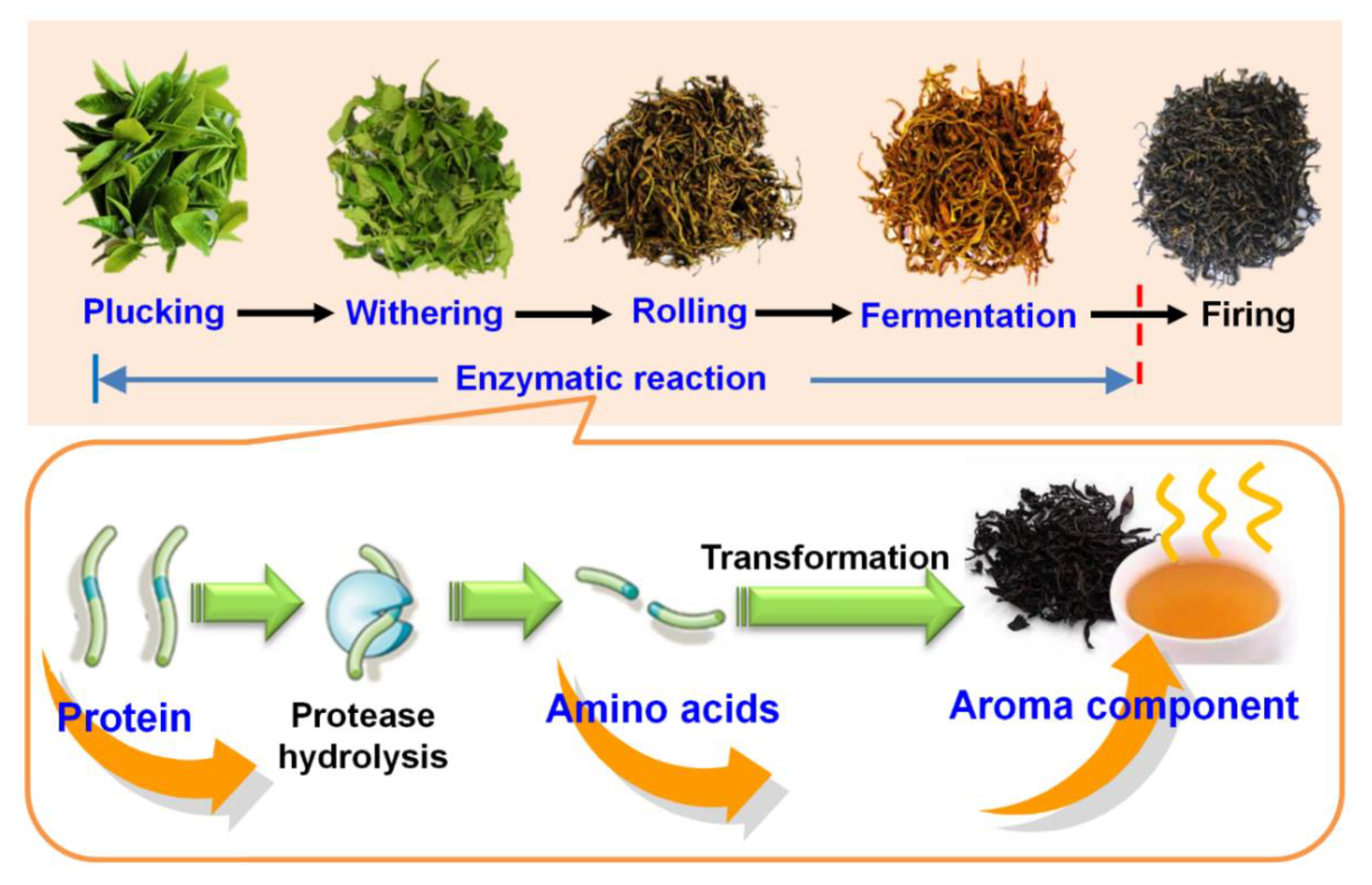
2.1. Plant Materials and Black Tea Manufacturing
2.2. Determination of Total Amino Acids and Total Soluble Protein Content at Each Step of Black Tea Manufacture
2.3. Free Amino Acid Content Analysis for Each Step of Black Tea Manufacture
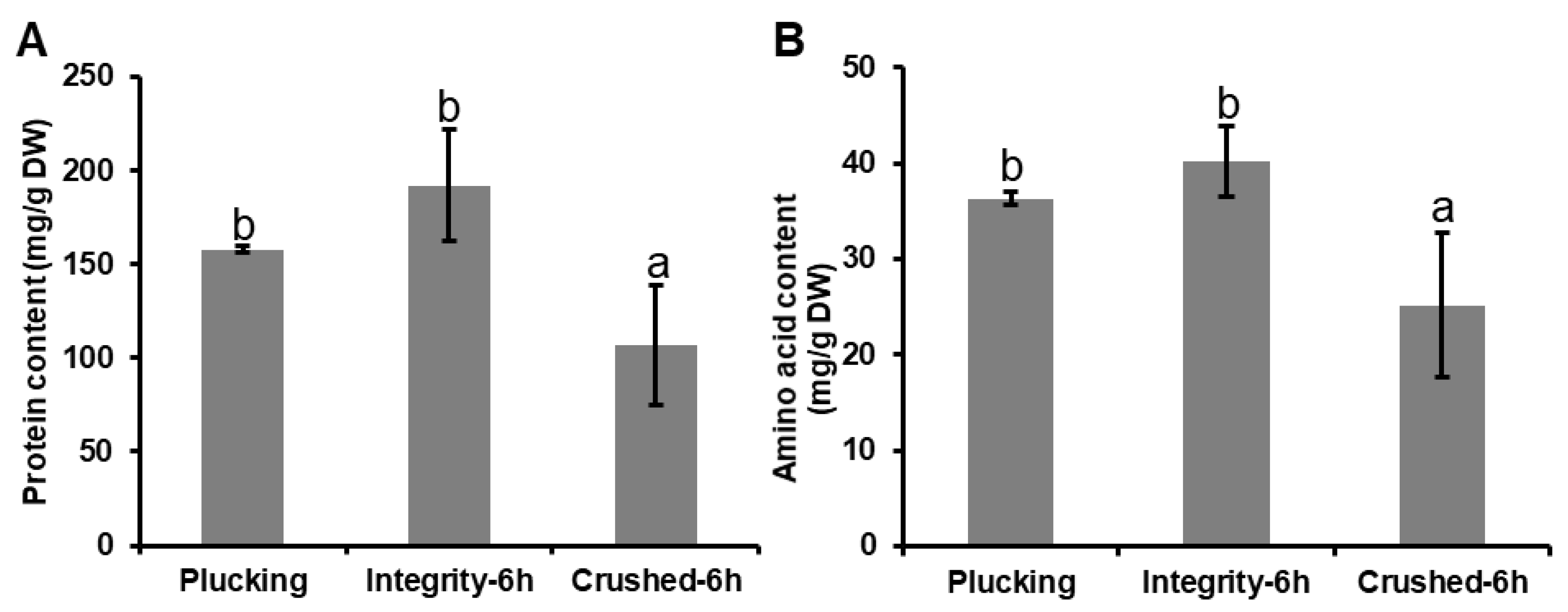
2.4. Simulation of Enzyme Reaction Step during Black Tea Manufacturing Processes and Analysis of Free Amino Acid Content
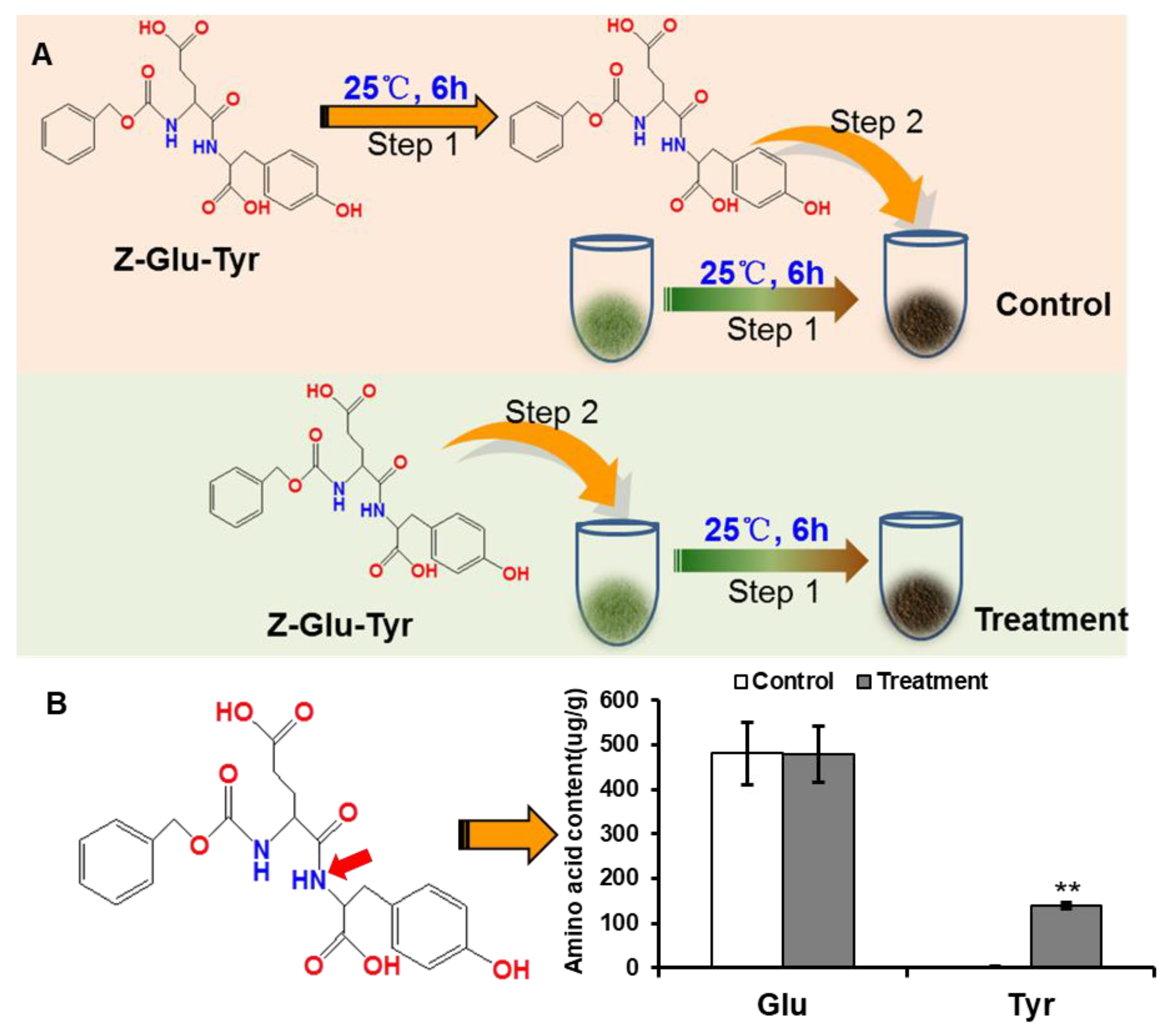
2.5. Investigation of Protein Degradation during Black Tea Fermentation with Artificially Synthesized Dipeptide Benzyloxycarbonyl Glutamyl-Tyrosine (Z-Glu-Tyr)
2.6. Analysis of Black Tea Aroma Compounds during the Manufacturing Processes
2.7. Investigation of the Amino Acid Catabolism during Black Tea Fermentation with Supplementation of [2H8] L-phenylalanine
2.8. Statistical Analysis
3. Results and Discussion
3.1. Changes in Proteins and Free Amino Acids during the Black Tea Manufacturing Process
3.2. Protein Degradation during Black Tea Fermentation with Artificially Synthesized Dipeptide Benzyloxycarbonyl Glutamyl-Tyrosin (Z-Glu-Tyr)
3.3. Proteinaceous Amino Acid Metabolism during Black Tea Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino acids |
| ANOVA | Analysis of variance |
| BSA | Bovine serum albumin |
| DW | Dry weight |
| GC-MS | Gas chromatography-mass spectrometry |
| 1PE | 1-Phenylethanol |
| 2PE | 2-Phenylethanol |
| Z-Glu-Tyr | Benzyloxycarbonyl glutamyl-tyrosin |
References
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Fagard, M.; Launay, A.; Clement, G.; Courtial, J.; Dellagi, A.; Farjad, M.; Krapp, A.; Soulié, M.C.; Masclaux-Daubresse, C. Nitrogen metabolism meets phytopathology. J. Exp. Bot. 2014, 65, 5643–5656. [Google Scholar] [CrossRef]
- Galili, G.; Avin-Wittenberg, T.; Angelovici, R.; Fernie, A.R. The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front. Plant Sci. 2014, 5, 447. [Google Scholar] [CrossRef] [PubMed]
- Häusler, R.E.; Ludewig, F.; Krueger, S. Amino acids—A life between metabolism and signaling. Plant Sci. 2014, 229, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive dissection of spatio-temporal metabolic shifts in primary, secondary and lipid metabolism during developmental senescence in Arabidopsis thaliana. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Douglas, A.E. The nutritional quality of phloem sap utilized by natural aphid populations. Ecol. Entomol. 1993, 18, 31–38. [Google Scholar] [CrossRef]
- Solomon, P.S.; Tan, K.C.; Oliver, R.P. The nutrient supply of pathogenic fungi; a fertile field for study. Mol. Plant Pathol. 2003, 4, 203–210. [Google Scholar] [CrossRef]
- Rico, A.; Preston, G.M. Pseudomonas syringae pv. tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol. Plant Microbe 2008, 21, 269–282. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie: 1.2 Aminosäuren, 5th ed.; Springer-Verlag: Berlin, Germany, 2001. [Google Scholar]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, G.M.; Wang, M.Q. The development and utilization of tea-substituting plant resources in China. Beverage Ind. 2008, 11, 4–6. (In Chinese) [Google Scholar]
- Liu, B.; Zhang, H.K.; Gong, H.Y. Determination of rutin and quercetin in tea of Lycium barbarum leaves by RP-HPLC. Chin. J. Exp. Tradit. Med. Formul. 2011, 14, 31. (In Chinese) [Google Scholar]
- Jia, Z.Y.; Luo, S.X.; Yan, H.; Wang, Y.J.; He, Y.Y.; Wang, J. Research on the bioavailability of Cd in Ilex kudingcha CJ Tseng Soil. Adv. Mater. Res. 2012, 356, 165–171. [Google Scholar]
- Thippeswamy, R.; Gouda, K.M.; Rao, D.H.; Martin, A.; Gowda, L.R. Determination of theanine in commercial tea by liquid chromatography with fluorescence and diode array ultraviolet detection. J. Agric. Food Chem. 2006, 54, 7014–7019. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Kobayashi, E.; Katsuno, T.; Asanuma, T.; Fujimori, T.; Ishikawa, T.; Tomomura, M.; Mochizuki, K.; Watase, T.; Nakamura, Y.; et al. Characterisation of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. Food Chem. 2012, 135, 2268–2276. [Google Scholar] [CrossRef]
- Wan, X.C. Tea Biochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Juneja, L.R.; Chu, D.C.; Okubo, T.; Nagato, Y.; Yokogoshi, H. L-theanine—A unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci. Technol. 1999, 10, 199–204. [Google Scholar] [CrossRef]
- Fowler, M.S.; Leheup, P.; Cordier, J.L. Cocoa, coffee and tea. In Microbiology of Fermented Foods; Springer: Boston, MA, USA, 1998; pp. 128–147. [Google Scholar]
- Roberts, G.R.; Sanderson, G.W. Changes undergone by free amino-acids during the manufacture of black tea. J. Sci. Food Agric. 1966, 17, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, R.; Swain, T. Studies of the quality and flavour of Ceylon tea. J. Sci. Food Agric. 1965, 16, 57–64. [Google Scholar] [CrossRef]
- Wickremasinghe, R.L.; Swain, T. The flavour of black tea. Chem. Ind. 1964, 37, 1574–1575. [Google Scholar]
- Bokuchava, M.A.; Popov, V.R.; Bakh, A.N. The significance of amino acids in the formation of tea aroma in its interaction with tannin substances under conditions of elevated temperature. Dokl. Akad. Nauk. SSSR 1954, 99, 145–148. [Google Scholar]
- Chen, X.H.; Chen, D.J.; Jiang, H.; Sun, H.Y.; Zhang, C.; Zhao, H.; Li, X.S.; Yan, F.; Chen, C.; Xu, Z.M. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography–mass spectrometry and olfactometry and sensory analysis. Food Chem. 2019, 274, 130–136. [Google Scholar] [CrossRef]
- Doi, E.; Shibata, D.; Matoba, T. Modified colorimetric ninhydrin methods for peptidase assay. Anal. Biochem. 1981, 118, 173–184. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- GB 5009.3-2010. National Food Safety Standard Determination of Moisture in Foods; Ministry of Health: Beijing, China, 2010.
- Mei, X.; Chen, Y.Y.; Zhang, L.Y.; Fu, X.M.; Wei, Q.; Grierson, D.; Zhou, Y.; Huang, Y.H.; Dong, F.; Yang, Z.Y. Dual mechanisms regulating glutamate decarboxylases and accumulation of gamma-aminobutyric acid in tea (Camellia sinensis) leaves exposed to multiple stresses. Sci. Rep. 2016, 6, 23685. [Google Scholar] [CrossRef]
- Zeng, L.T.; Wang, X.Q.; Dong, F.; Watanabe, N.; Yang, Z.Y. Increasing postharvest high-temperatures lead to increased volatile phenylpropanoids/benzenoids accumulation in cut rose (Rosa hybrida) flowers. Postharvest Biol. Technol. 2019, 148, 68–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.X.; Zhang, N.; Ma, L.S. Antioxidant and functional properties of tea protein as affected by the different tea processing methods. J. Food Sci. Technol. 2015, 52, 742–775. [Google Scholar] [CrossRef]
- Harbowy, M.E.; Balentine, D.A.; Davies, A.P.; Cai, Y. Tea chemistry. Crit. Rev. Plant Sci. 1997, 16, 415–480. [Google Scholar] [CrossRef]
- Hufnagel, J.C.; Hofmann, T. Orosensory-directed identification of astringent mouthfeel and bitter-tasting compounds in red wine. J. Agric. Food Chem. 2008, 56, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Varner, J.E. Biochemistry of senescence. Annu. Rev. Plant Physiol. 1961, 12, 245–264. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of different drying methods on the sensory quality and chemical components of black tea. LWT 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Dev, C.; Bajaj, K.L. Role of chlorophylls, amino acids and sugars in tea. Two Bud 1980, 27, 16–20. [Google Scholar]
- Kumar, R.S.S.; Murugesan, S.; Kottur, G.; Gyamfl, D. Black tea: The plants, processing/manufacturing and production. In Tea in Health and Disease Prevention; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 41–57. [Google Scholar]
- Kumazawa, K.; Wada, Y.; Masuda, H. Characterization of epoxydecenal isomers as potent odorants in black tea (Dimbula) infusion. J. Agric. Food Chem. 2006, 54, 4795–4801. [Google Scholar] [CrossRef]
- Sanderson, G.W.; Roberts, G.R. Peptidase activity in shoot tips of the tea plant (Camellia sinensis L.). Biochem. J. 1964, 93, 419–423. [Google Scholar] [CrossRef]
- Stodt, U.W.; Blauth, N.; Niemann, S.; Stark, J.; Pawar, V.; Jayaraman, S.; Koek, J.; Engelhardt, U.H. Investigation of processes in black tea manufacture through model fermentation (oxidation) experiments. J. Agric. Food Chem. 2014, 62, 7854–7861. [Google Scholar] [CrossRef]
- Yamamura, H.; Rekharsky, M.V.; Ishihara, Y.; Kawai, M.; Inoue, Y. Factors controlling the complex architecture of native and modified cyclodextrins with dipeptide (Z-Glu-Tyr) studied by microcalorimetry and NMR spectroscopy: Critical effects of peripheral bis-trimethylamination and cavity size. J. Am. Chem. Soc. 2004, 126, 14224–14233. [Google Scholar] [CrossRef]
- Gui, J.D.; Fu, X.M.; Zhou, Y.; Katsuno, T.; Mei, X.; Deng, R.F.; Xu, X.L.; Zhang, L.Y.; Dong, F.; Watanabe, N.; et al. Does enzymatic hydrolysis of glycosidically bound volatile compounds really contribute to the formation of volatile compounds during the oolong tea manufacturing process? J. Agric. Food Chem. 2015, 63, 6905–6914. [Google Scholar] [CrossRef]
- Owuor, P.O.; Cheruiyot, D.K. Effects of nitrogen fertilizers on the aluminium contents of mature tea leaf and extractable aluminium in the soil. Plant Soil. 1989, 119, 342–345. [Google Scholar] [CrossRef]
- Co, H.; Sanderson, G.W. Biochemistry of tea fermentation: Conversion of amino acids to black tea aroma constituents. J. Food Sci. 1970, 35, 160–164. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Seth, S.; Tudu, B.; Tamuly, P.; Jana, A.; Ghosh, D.; Bandyopadhyay, R.; Bhuyan, M. Monitoring of black tea fermentation process using electronic nose. J. Food Eng. 2007, 80, 1146–1156. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zeng, L.T.; Liao, Y.Y.; Zhou, Y.; Xu, X.L.; Dong, F.; Yang, Z.Y. An alternative pathway for the formation of aromatic aroma compounds derived from L-phenylalanine via phenylpyruvic acid in tea (Camellia sinensis (L.) O. Kuntze) leaves. Food Chem. 2019, 270, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, Z.; Baldermann, S.; Kajitani, Y.; Ota, S.; Kasuga, H.; Imazeki, Y.; Ohnishi, T.; Watanabe, N. Characterization of L-phenylalanine metabolism to acetophenone and 1-phenylethanol in the flowers of Camellia sinensis using stable isotope labeling. J. Plant Physiol. 2012, 169, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kraujalytė, V.; Pelvan, E.; Alasalvar, C. Volatile compounds and sensory characteristics of various instant teas produced from black tea. Food Chem. 2016, 194, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Hayashi, K.; Yagi, K.; Asai, T.; Mactavish, H.; Picone, J.; Turnbull, C.; Watanabe, N. Biogenesis of 2-phenylethanol in rose flowers: Incorporation of [2H8] L-phenylalanine into 2-phenylethanol and its b-D-glucopyranoside during the flower opening of Rosa ‘Hoh-Jun’ and Rosa damascena Mill. Biosci. Biotechem. Biochem. 2002, 66, 943–947. [Google Scholar] [CrossRef] [PubMed]

| Amino Acid μg/g (Dry Weight) | Plucking | Withering | Rolling | Fermentation | Drying |
|---|---|---|---|---|---|
| P-Ser | 146.89 ± 9.21 a | 136.29 ± 14.76 a | 154.64 ± 2.69 a | 156.27 ± 11.46 a | 96.39 ± 34.04 b |
| PEA | 79.13 ± 16.46 b | 127.90 ± 7.40 a | 94.57 ± 6.85 b | 50.69 ± 5.75 c | 39.06 ± 15.45 c |
| Asp | 13.97 ± 8.92 b | 20.61 ± 4.59 b | 21.85 ± 4.12 ab | 30.57 ± 5.52 b | 40.04 ± 14.91 a |
| Thr | 140.04 ± 25.22 c | 277.19 ± 6.76 a | 264.51 ± 39.46 ab | 229.25 ± 15.56 b | 130.85 ± 3.96 c |
| Ser | 564.34 ± 53.16 c | 880.84 ± 74.88 a | 736.48 ± 56.08 b | 663.63 ± 46.75 b | 383.09 ± 21.37 c |
| Asn | 0.00 ± b | 382.22 ± 662.02 ab | 678.97 ± 145.24 a | 556.45 ± 78.34 ab | 391.01 ± 84.43 ab |
| Glu | 2331.12 ± 310.66 a | 1609.88 ± 101.01 b | 1423.61 ± 22.43 bc | 1224.51 ± 96.73 c | 773.41 ± 12.54 d |
| Thea | 6874.15 ± 1226.16 a | 5284.69 ± 572.10 b | 5100.84 ± 110.42 b | 4618.89 ± 420.34 b | 2559.11 ± 18.16 c |
| α-AAA | 21.32 ± 5.85 d | 126.19 ± 20.76 a | 114.37 ± 11.77 ab | 97.48 ± 10.95 b | 52.69 ± 9.79 c |
| Gly | 67.38 ± 11.69 a | 39.63 ± 2.61 b | 35.06 ± 4.82 bc | 17.42 ± 14.47 cd | 8.21 ± 7.17 d |
| Ala | 248.51 ± 39.53 c | 402.25 ± 12.70 b | 480.03 ± 15.91 a | 470.10 ± 23.93 a | 289.01 ± 29.66 c |
| α-ABA | 15.24 ± 2.67 a | 14.70 ± 2.44 a | 12.89 ± 1.51 ab | 9.07 ± 3.52 b | 7.77 ± 3.27 b |
| Cit | 2.88 ± 0.28 b | 17.34 ± 7.74 a | 13.81 ± 3.77 ab | 16.62 ± 7.14 a | 10.53 ± 3.15 ab |
| Val | 102.20 ± 21.55 d | 581.33 ± 68.70 a | 479.27 ± 33.04 b | 465.69 ± 22.36 b | 282.32 ± 25.48 c |
| Cys | 16.11 ± 10.17 c | 107.07 ± 19.84 a | 64.65 ± 8.54 b | 56.20 ± 16.62 b | 21.31 ± 2.85 c |
| Met | 1.92 ± 2.61 b | 46.54 ± 39.84 a | 10.31 ± 2.71 b | 6.55 ± 0.21 b | 1.82 ± 0.32 b |
| Ile | 14.38 ± 2.06 d | 385.67 ± 41.44 a | 327.93 ± 36.95 b | 307.94 ± 4.83 b | 202.77 ± 14.63 c |
| Leu | 11.58 ± 7.69 d | 391.21 ± 24.24 a | 338.60 ± 27.36 b | 307.84 ± 13.90 b | 198.18 ± 15.12 c |
| Tyr | 0.00 ± c | 555.68 ± 95.14 a | 542.77 ± 21.18 a | 498.81 ± 35.46 a | 334.49 ± 22.82 b |
| Phe | 45.78 ± 6.33 e | 1806.72 ± 162.61 a | 1396.06 ± 63.67 b | 1220.48 ± 60.33 c | 734.05 ± 60.09 d |
| β-Ala | 6.61 ± 0.94 b | 39.11 ± 27.67 ab | 76.65 ± 38.10 a | 56.08 ± 10.26 a | 31.48 ± 3.90 ab |
| β-ABA | 6.48 ± 0.84 b | 4.37 ± 3.80 b | 5.86 ± 5.27 b | 23.65 ± 12.82 a | 8.90 ± 4.76 b |
| GABA | 2.01 ± 0.53 d | 59.97 ± 23.77 bc | 91.26 ± 6.45 a | 78.41 ± 12.86 ab | 42.44 ± 4.54 c |
| His | 9.40 ± 1.27 c | 80.51 ± 8.41 a | 34.53 ± 4.80 b | 15.17 ± 1.19 c | 19.49 ± 15.88 bc |
| 3Mehis | - | - | - | 10.48 ± 11.16 a | 1.43 ± 1.37 ab |
| 1Mehis | 2.63 ± 0.29 a | 1.97 ± 0.51 a | 0.90 ± 0.08 a | 3.83 ± 3.88 a | 1.17 ± 1.35 a |
| Trp | 37.24 ± 20.68 c | 296.47 ± 44.63 a | 287.80 ± 102.25 a | 174.23 ± 65.19 b | 72.30 ± 9.56 c |
| ORN | 91.31 ± 99.49 c | 360.88 ± 66.09 a | 318.92 ± 42.89 ab | 173.48 ± 151.58 bc | 13.30 ± 6.04 c |
| Lys | 38.72 ± 17.26 d | 368.64 ± 59.13 a | 267.90 ± 23.81 b | 154.20 ± 53.25 c | 56.45 ± 7.42 d |
| Arg | 17.76 ± 5.03 a | 42.87 ± 23.45 a | 26.61 ± 26.08 a | 18.45 ± 8.78 a | 15.89 ± 8014 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zeng, L.; Liao, Y.; Li, J.; Zhou, B.; Yang, Z.; Tang, J. Enzymatic Reaction-Related Protein Degradation and Proteinaceous Amino Acid Metabolism during the Black Tea (Camellia sinensis) Manufacturing Process. Foods 2020, 9, 66. https://doi.org/10.3390/foods9010066
Chen Y, Zeng L, Liao Y, Li J, Zhou B, Yang Z, Tang J. Enzymatic Reaction-Related Protein Degradation and Proteinaceous Amino Acid Metabolism during the Black Tea (Camellia sinensis) Manufacturing Process. Foods. 2020; 9(1):66. https://doi.org/10.3390/foods9010066
Chicago/Turabian StyleChen, Yiyong, Lanting Zeng, Yinyin Liao, Jianlong Li, Bo Zhou, Ziyin Yang, and Jinchi Tang. 2020. "Enzymatic Reaction-Related Protein Degradation and Proteinaceous Amino Acid Metabolism during the Black Tea (Camellia sinensis) Manufacturing Process" Foods 9, no. 1: 66. https://doi.org/10.3390/foods9010066
APA StyleChen, Y., Zeng, L., Liao, Y., Li, J., Zhou, B., Yang, Z., & Tang, J. (2020). Enzymatic Reaction-Related Protein Degradation and Proteinaceous Amino Acid Metabolism during the Black Tea (Camellia sinensis) Manufacturing Process. Foods, 9(1), 66. https://doi.org/10.3390/foods9010066







