Toxicological Studies of Czech Beers and Their Constituents
Abstract
:1. Introduction
- −
- The fruit fly D. melanogaster shows a consistent similarity to humans as approximately 75% of the genes involved in diseases are similar in the fly [18,19]. This well-known eukaryote with the largest scientific history in genetics has also contributed to the understanding of developmental biology, evolutionary concepts and more recently, toxicology [20,21,22,23,24]. Its short life cycle (10–12 days), cost efficiency and easy maintenance are characteristics that make Drosophila to be considered as an ideal model organism.
- −
- The HL-60 human model cell line originated thirty two years ago from a patient with acute myeloid leukaemia [25]. After culturing peripheral blood leukocytes from this female patient, a growth-factor-independent immortal cell line with distinct myeloid characteristics was obtained [26]. This promyelocytic leukaemia cell line has been used world-wide for many toxicity and cancer purposes.
2. Materials and Methods
2.1. Samples Preparation and Sample Compounds
2.2. In Vivo Animal Model and In Vitro Cellular Culture Used
2.2.1. Drosophila Melanogaster
- −
- mwh/mwh: Carries a recessive mutation mwh (multiple wing hairs) which produces several tricomas instead of one per cell when it is in homozygosis [30].
- −
- flr3/In (3LR) TM3, rippsep bx34eesBdS: Carries a flr3 (flare) marker which is a recessive lethal mutation when it is in homozygosis producing deformed tricomas, though it is viable in homozygous somatic cells if and when the larvae start the development [31].
2.2.2. HL-60 Cell Cultures
2.3. Safety In Vivo Assays of Freeze-Dried Samples
2.3.1. Toxicity
2.3.2. Genotoxicity
2.4. Protection In Vivo Tests
2.4.1. Antitoxicity
2.4.2. Antigenotoxicity
2.4.3. Longevity
2.5. Chemopreventive In Vitro Tests
2.5.1. Cytotoxicity Assay
2.5.2. Tumour Cells DNA Damage Evaluation
DNA Fragmentation
Comet Assay
Methylation Status Evaluation
3. Results and Discussion
3.1. Safety of Freeze-Dried Samples
3.2. Genotoxicity of Freeze-Dried Samples
3.3. Antitoxicity
3.4. Antigenotoxicity
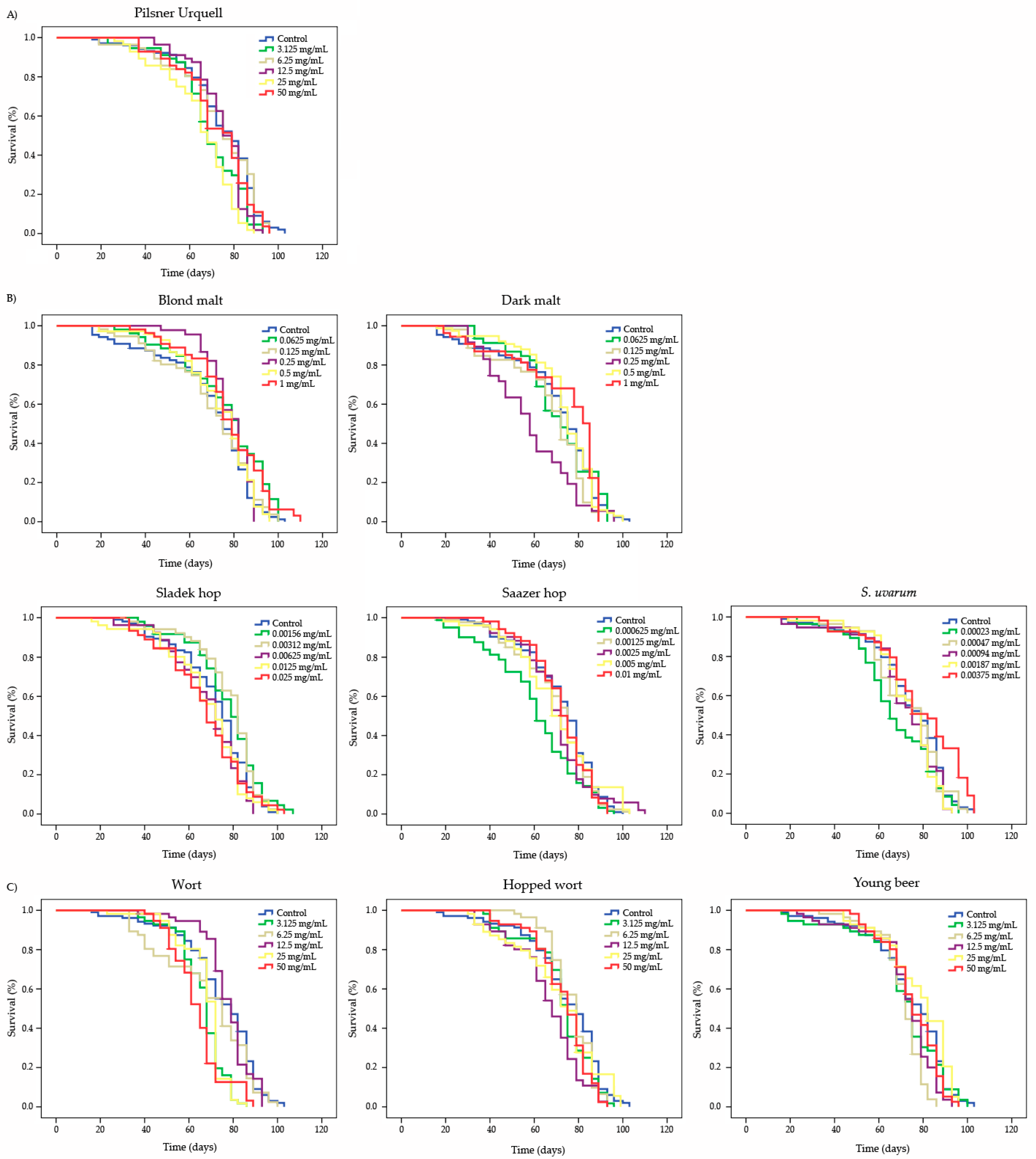
3.5. Longevity
3.6. Cytotoxicity
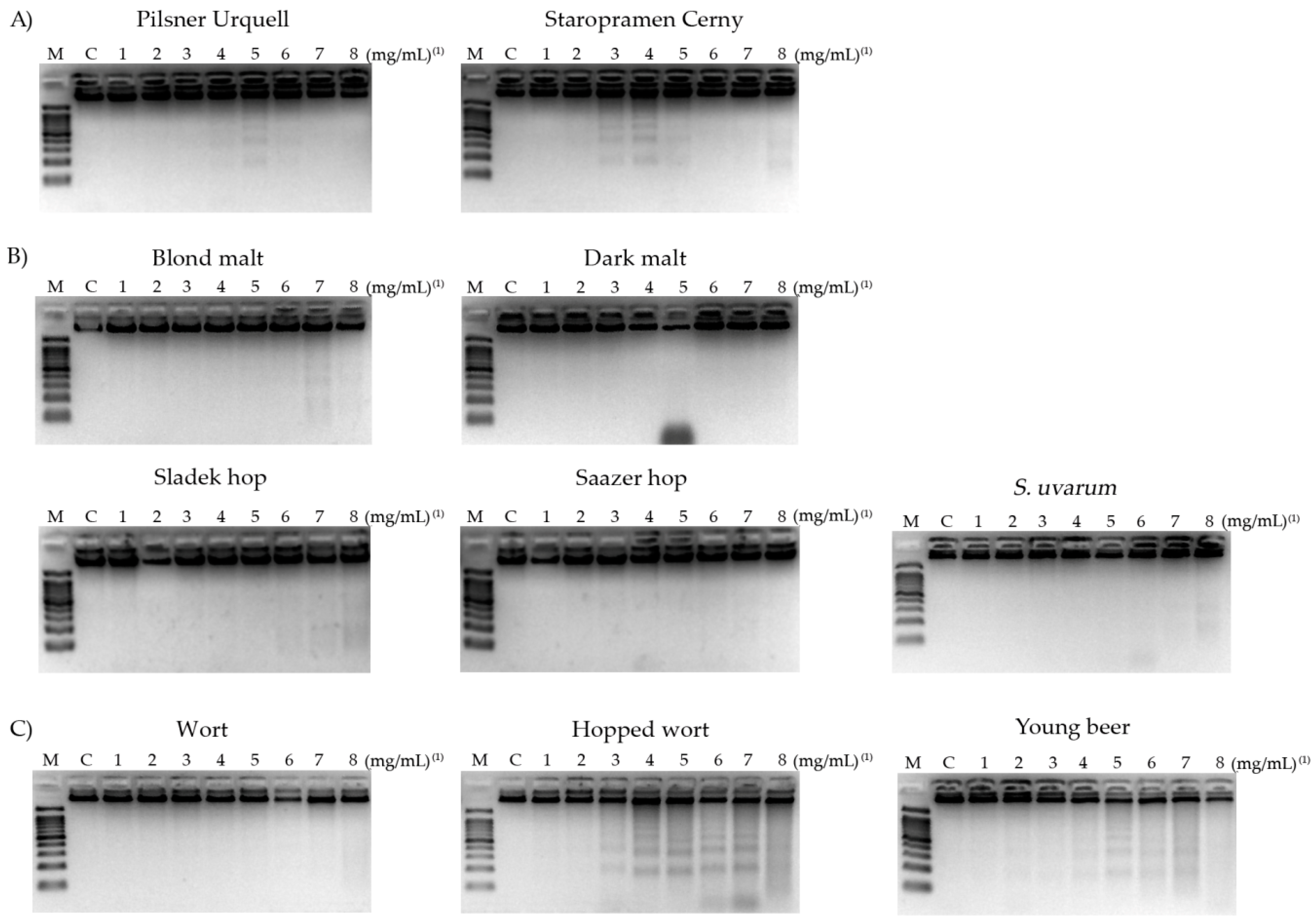
3.7. DNA Fragmentation
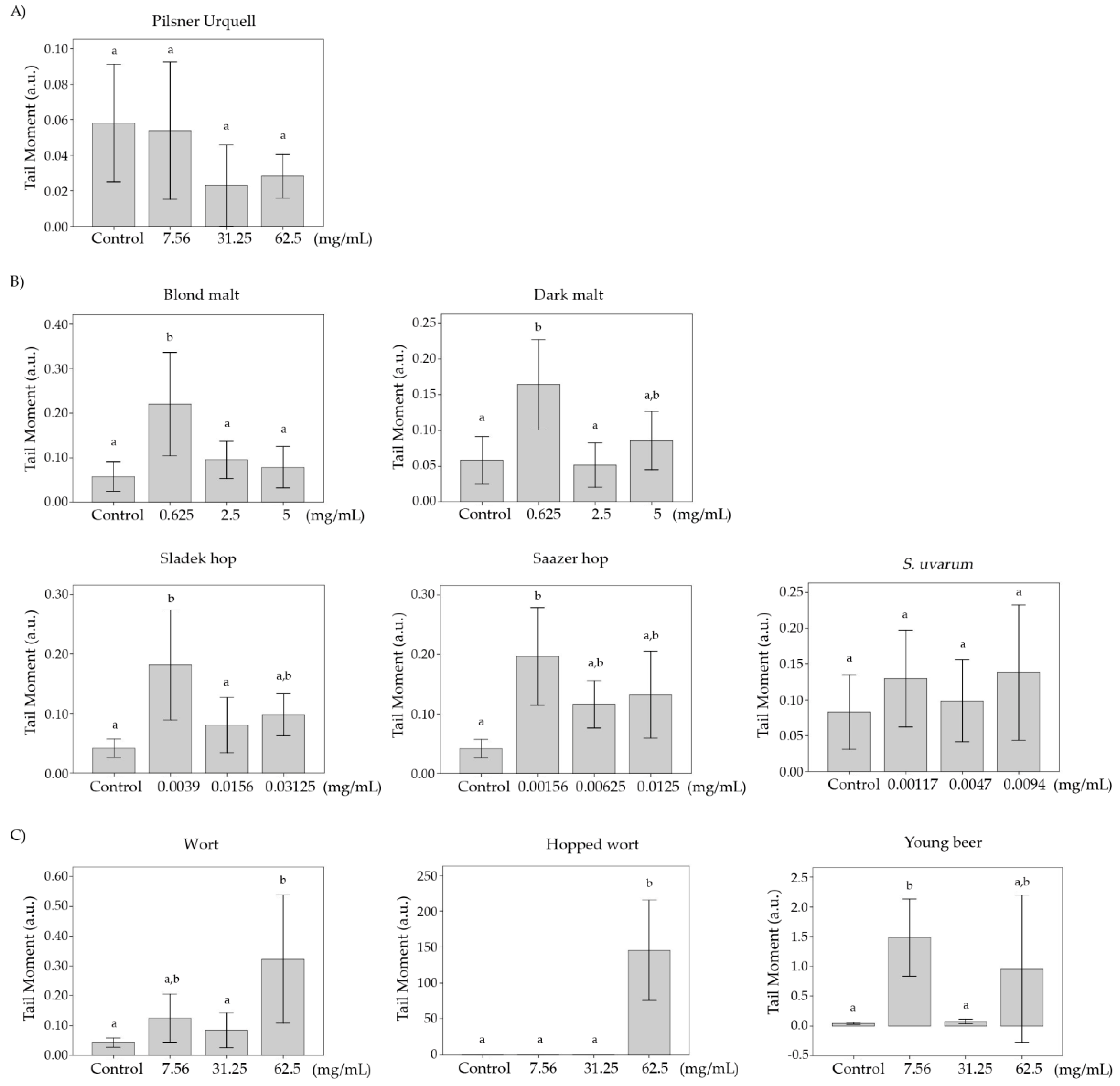
3.8. Comet Assay
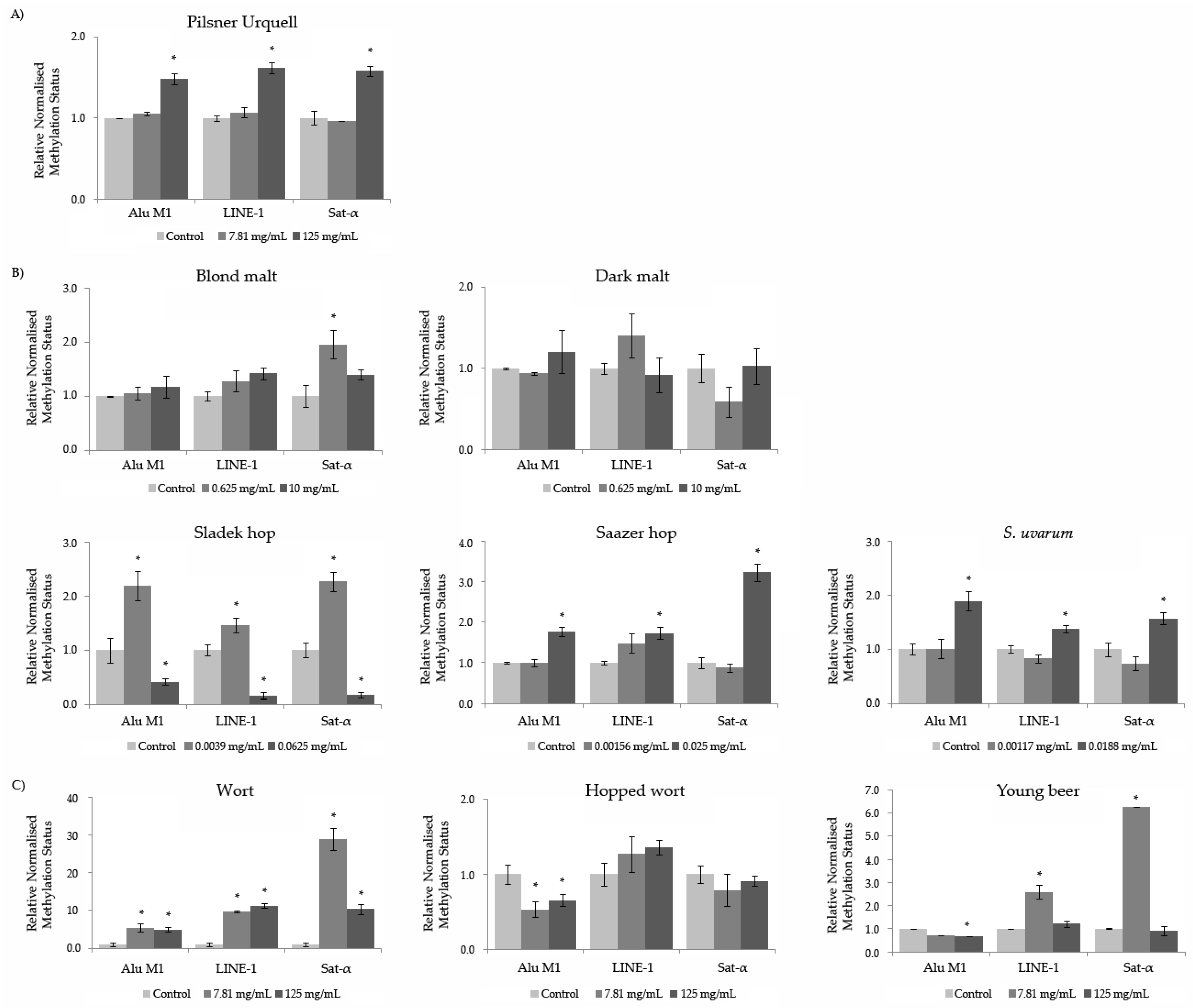
3.9. Methylation Status
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Damerow, P. Sumerian beer: The origins of brewing technology in ancient Mesopotamia. Cuneif. Digit. Libr. J. 2012, 2, 1–20. [Google Scholar]
- Hornsey, I.S. A History of Beer and Brewing; Royal Society of Chemistry: London, UK, 2003; Volume 34. [Google Scholar]
- Wolf, A.; Bray, G.A.; Popkin, B.M. A short history of beverages and how our body treats them. Obes. Rev. 2008, 9, 151–164. [Google Scholar] [CrossRef]
- Marová, I.; Parilova, K.; Friedl, Z.; Obruca, S.; Duronova, K. Analysis of Phenolic Compounds in Lager Beers of Different Origin: A Contribution to Potential Determination of the Authenticity of Czech Beer. Chromatographia 2011, 73, 83–95. [Google Scholar] [CrossRef]
- Wanninayake, W.; Chovancova, M. Consumer Ethnocentrism and Attitudes towards Foreign Beer Brands: With Evidence from Zlin Region in the Czech Republic. J. Compet. 2012, 4, 3–19. [Google Scholar] [CrossRef]
- Commission, A. Publication of an Application Pursuant to Article 6 (2) of Council Regulation (EC) No 510/2006 on the Protection of Geographical Indications and Designations of Origin for Agricultural Products and Foodstuffs. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do? uri=OJ:C:2008:016:0014:0 022:CS (accessed on 31 March 2017).
- Buiatti, S. Chapter 20-Beer Composition: An Overview; Academic Press: Cambridge, MA, USA, 2009; pp. 212–225. [Google Scholar]
- Chen, W.; Becker, T.; Qian, F.; Ring, J. Beer and beer compounds: Physiological effects on skin health. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 142–150. [Google Scholar] [CrossRef]
- Sanchez, C.L.; Franco, L.; Bravo, R.; Rubio, C.; Rodríguez, A.; Barriga, C.; Cubero, J. Cerveza y salud, beneficios en el sueño. Rev. Española Nutr. Comunitaria 2010, 16, 160–163. [Google Scholar] [CrossRef]
- Rimm, E.; Giovannucci, E.; Willett, W.; Colditz, G.; Ascherio, A.; Rosner, B.; Stampfer, M.; Colditz, G. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991, 338, 464–468. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Spiegelman, D.; Stampfer, M.J. Prospective Study of Beverage Use and the Risk of Kidney Stones. Am. J. Epidemiol. 1996, 143, 240–247. [Google Scholar] [CrossRef]
- Vinson, J.A.; Mandarano, M.; Hirst, M.; Trevithick, J.R.; Bose, P. Phenol Antioxidant Quantity and Quality in Foods: Beers and the Effect of Two Types of Beer on an Animal Model of Atherosclerosis. J. Agric. Food Chem. 2003, 51, 5528–5533. [Google Scholar] [CrossRef]
- World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 1997. [Google Scholar]
- Preedy, V.R. Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Granato, D.; Branco, G.F.; Faria, J.D.A.F.; Cruz, A.G. Characterization of Brazilian lager and brown ale beers based on color, phenolic compounds, and antioxidant activity using chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Pérez-Jiménez, J. Chapter 42-What contribution is beer to the intake of antioxidants in the diet? In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef]
- Lloyd, T.E.; Taylor, J.P. Flightless Flies: Drosophila models of neuromuscular disease. Ann. N. Y. Acad. Sci. 2010, 1184, 1–20. [Google Scholar] [CrossRef]
- Bhargav, D.; Singh, M.P.; Murthy, R.C.; Mathur, N.; Misra, D.; Saxena, D.K.; Chowdhuri, D.K. Toxic potential of municipal solid waste leachates in transgenic Drosophila melanogaster (hsp70-lacZ): hsp70 as a marker of cellular damage. Ecotoxicol. Environ. Saf. 2008, 69, 233–245. [Google Scholar] [CrossRef]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef]
- Dean, B.J. Recent findings on the genetic toxicology of benzene, toluene, xylenes and phenols. Mutat. Res. Genet. Toxicol. 1985, 154, 153–181. [Google Scholar] [CrossRef]
- Hosamani, R. Acute Exposure of Drosophila melanogaster to Paraquat Causes Oxidative Stress and Mitochondrial Dysfunction. Arch. Insect Biochem. Physiol. 2013, 83, 25–40. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Fatima, A.; Jyoti, S.; Naz, F.; Khan, W.; Singh, B.R.; Naqvi, A.H. Evaluation of the toxic potential of graphene copper nanocomposite (GCNC) in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg 9. PLoS ONE 2013, 8, e80944. [Google Scholar] [CrossRef]
- Collins, S.J. The HL-60 promyelocytic leukemia cell line: Proliferation, differentiation, and cellular oncogene expression. Blood 1987, 70, 1233–1244. [Google Scholar]
- Gallagher, R.; Collins, S.; Trujillo, J.; McCredie, K.; Ahearn, M.; Tsai, S.; Metzgar, R.; Aulakh, G.; Ting, R.; Ruscetti, F.; et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 1979, 54, 713–733. [Google Scholar]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; De La Rosa, N.N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef]
- Kirin. Globar Beer Consumption by Country in 2011. Available online: http://www.kirinholdings.co.jp/english/news/2012/1226_01.html (accessed on 31 March 2017).
- Goldammer, T. Hop varieties. In The Brewers’ Handbook—The Complete Book to Brewing Beer; KVP Publishers: Huntsville, AL, USA, 2000; Volume 53. [Google Scholar]
- Yan, J.; Huen, D.; Morely, T.; Johnson, G.; Gubb, D.; Roote, J.; Adler, P.N. The multiple-wing-hairs Gene Encodes a Novel GBD–FH3 Domain-Containing Protein That Functions Both Prior to and After Wing Hair Initiation. Genetics 2008, 180, 219–228. [Google Scholar] [CrossRef]
- Ren, N.; Charlton, J.; Adler, P.N. The flare Gene, Which Encodes the AIP1 Protein of Drosophila, Functions to Regulate F-Actin Disassembly in Pupal Epidermal Cells. Genetics 2007, 176, 2223–2234. [Google Scholar] [CrossRef] [Green Version]
- Merinas-Amo, T.; Tasset-Cuevas, I.; Díaz-Carretero, A.M.; Alonso-Moraga, Á.; Calahorro, F. Role of Choline in the Modulation of Degenerative Processes: In Vivo and In Vitro Studies. J. Med. Food 2017, 20, 223–234. [Google Scholar] [CrossRef]
- Kozák, V. Analysis of Reasons for Beer Consuption Drop in the Czech Republic; Technical University of Liberec: Liberec, Czechia, 2013. [Google Scholar]
- Graf, U.; Würgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Frei, H.; Würgler, F. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. Mutagen. Relat. Subj. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Frei, H.; Würgler, F.E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat. Res. Mutagen. Relat. Subj. 1995, 334, 247–258. [Google Scholar] [CrossRef]
- Anter, J.; Romero-Jiménez, M.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Analla, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A. Antigenotoxicity, Cytotoxicity, and Apoptosis Induction by Apigenin, Bisabolol, and Protocatechuic Acid. J. Med. Food 2011, 14, 276–283. [Google Scholar] [CrossRef]
- Graf, U.; Abraham, S.K.; Guzmán-Rincón, J.; Würgler, F.E. Antigenotoxicity studies in Drosophila melanogaster. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 203–209. [Google Scholar] [CrossRef]
- Abraham, S.K. Antigenotoxicity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis 1994, 9, 383–386. [Google Scholar] [CrossRef]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; De Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective Effect of Borage Seed Oil and Gamma Linolenic Acid on DNA: In Vivo and In Vitro Studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.W.; Hotic, S.; Arking, R. Dietary restriction in Drosophila is dependent on mitochondrial efficiency and constrained by pre-existing extended longevity. Mech. Ageing Dev. 2007, 128, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, T.; Tasset-Cuevas, I.; Díaz-Carretero, A.M.; Alonso-Moraga, Á.; Calahorro, F. In vivo and in vitro studies of the role of lyophilised blond Lager beer and some bioactive components in the modulation of degenerative processes. J. Funct. Foods 2016, 27, 274–294. [Google Scholar] [CrossRef]
- Mateo-Fernández, M.; Merinas-Amo, T.; Moreno-Millán, M.; Alonso-Moraga, Á.; Demyda-Peyrás, S. In vivo and in vitro genotoxic and epigenetic effects of two types of cola beverages and caffeine: A multi-assay approach. BioMed Res. Int. 2016, 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, T.; Merinas-Amo, R.; Alonso-Moraga, A. A clinical pilot assay of beer consumption: Modulation in the methylation status patterns of repetitive sequences. Sylwan 2017, 161, 134–156. [Google Scholar]
- Deininger, P.L.; Moran, J.V.; A Batzer, M.; Kazazian, H.H. Mobile elements and mammalian genome evolution. Curr. Opin. Genet. Dev. 2003, 13, 651–658. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA Hypomethylation, Cancer, the Immunodeficiency, Centromeric Region Instability, Facial Anomalies Syndrome and Chromosomal Rearrangements. J. Nutr. 2002, 132, 2424–2429. [Google Scholar] [CrossRef]
- Lee, C.; Wevrick, R.; Fisher, R.; Ferguson-Smith, M.; Lin, C. Human centromeric DNAs. Hum. Genet. 1997, 100, 291–304. [Google Scholar] [CrossRef]
- Weiner, A.M. SINEs and LINEs: The art of biting the hand that feeds you. Curr. Opin. Cell Boil. 2002, 14, 343–350. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Campan, M.; Long, T.I.; Kim, M.; Woods, C.; Fiala, E.; Ehrlich, M.; Laird, P.W. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005, 33, 6823–6836. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, G.; Raji, O.Y.; Markopoulou, S.; Gosney, J.R.; Bryan, J.; Warburton, C.; Walshaw, M.; Sheard, J.; Field, J.K.; Liloglou, T. DNA methylation biomarkers offer improved diagnostic efficiency in lung cancer. Cancer Res. 2012, 72, 5692–5701. [Google Scholar] [CrossRef] [PubMed]
- Liloglou, T.; Bediaga, N.G.; Brown, B.R.; Field, J.K.; Davies, M.P.; Davies, M.P.A. Epigenetic biomarkers in lung cancer. Cancer Lett. 2014, 342, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Arimoto-Kobayashi, S.; Sugiyama, C.; Harada, N.; Takeuchi, M.; Takemura, M.; Hayatsu, H. Inhibitory Effects of Beer and Other Alcoholic Beverages on Mutagenesis and DNA Adduct Formation Induced by Several Carcinogens. J. Agric. Food Chem. 1999, 47, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Anter, J.; Campos-Sánchez, J.; El Hamss, R.; Rojas-Molina, M.; Muñoz-Serrano, A.; Analla, M.; Alonso-Moraga, Á. Modulation of genotoxicity by extra-virgin olive oil and some of its distinctive components assessed by use of the Drosophila wing-spot test. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 703, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Yang, X.; Tian, Y. In vitro and in vivo antioxidant and antimutagenic activities of polyphenols extracted from hops (Humulus lupulus L.). J. Sci. Food Agric. 2014, 94, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Arimoto-Kobayashi, S.; Ishida, R.; Nakai, Y.; Idei, C.; Takata, J.; Takahashi, E.; Okamoto, K.; Negishi, T.; Konuma, T. Inhibitory Effects of Beer on Mutation in the Ames Test and DNA Adduct Formation in Mouse Organs Induced by 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Biol. Pharm. Bull. 2006, 29, 67–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press (OUP): Oxford, UK, 2015. [Google Scholar]
- Callemien, D.; Collin, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer a review. Food Rev. Int. 2010, 26, 1–84. [Google Scholar] [CrossRef]
- Callemien, D.; Jerkovic, V.; Rozenberg, R.; Collin, S. Hop as an Interesting Source of Resveratrol for Brewers: Optimization of the Extraction and Quantitative Study by Liquid Chromatography/Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 424–429. [Google Scholar] [CrossRef]
- Goupy, P.; Hugues, M.; Boivin, P.; Amiot, M.J. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agric. 1999, 79, 1625–1634. [Google Scholar] [CrossRef]
- Vinson, J.A.; Jang, J.; Yang, J.; Dabbagh, Y.; Liang, X.; Serry, M.; Proch, J.; Cai, S. Vitamins and Especially Flavonoids in Common Beverages Are Powerful in Vitro Antioxidants Which Enrich Lower Density Lipoproteins and Increase Their Oxidative Resistance after ex Vivo Spiking in Human Plasma. J. Agric. Food Chem. 1999, 47, 2502–2504. [Google Scholar] [CrossRef]
- Woffenden, H.M.; Ames, J.M.; Chandra, S. Relationships between Antioxidant Activity, Color, and Flavor Compounds of Crystal Malt Extracts. J. Agric. Food Chem. 2001, 49, 5524–5530. [Google Scholar] [CrossRef]
- Friedman, M. Food Browning and Its Prevention: An Overview†. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Henle, T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids 2005, 29, 313–322. [Google Scholar] [CrossRef]
- Silván, J.M.; Van De Lagemaat, J.; Olano, A.; Del Castillo, M.D. Analysis and biological properties of amino acid derivates formed by Maillard reaction in foods. J. Pharm. Biomed. Anal. 2006, 41, 1543–1551. [Google Scholar] [CrossRef]
- Yen, G.C.; Chau, C.F.; Lii, J.D. Isolation and characterization of the most antimutagenic Maillard reaction products derived from xylose and lysine. J. Agric. Food Chem. 1993, 41, 771–776. [Google Scholar] [CrossRef]
- Yen, G.-C.; Tsai, L.-C. Antimutagenicity of a partially fractionated Maillard reaction product. Food Chem. 1993, 47, 11–15. [Google Scholar] [CrossRef]
- Vagnarelli, P.; De Sario, A.; Cuzzoni, M.T.; Mazza, P.; De Carli, L. Cytotoxicity and clastogenic activity of ribose-lysine browning model system. J. Agric. Food Chem. 1991, 39, 2237–2239. [Google Scholar] [CrossRef]
- Rivero, D.; Perez-Magariño, S.; Gonzalez-SanJose, M.L.; Valls-Belles, V.; Codoñer, P.; Muñiz, P. Inhibition of Induced DNA Oxidative Damage by Beers: Correlation with the Content of Polyphenols and Melanoidins. J. Agric. Food Chem. 2005, 53, 3637–3642. [Google Scholar] [CrossRef]
- Morales, F.J.; Babbel, M.-B. Antiradical Efficiency of Maillard Reaction Mixtures in a Hydrophilic Media. J. Agric. Food Chem. 2002, 50, 2788–2792. [Google Scholar] [CrossRef]
- Merinas-Amo, T.; Martinez-Jurado, M.; Merinas-Amo, R.; Mateo-Fenandez, M.; Alonso-Moraga, Á. In vivo and in vitro toxicological and biological activities of alpha-acid humulone, isoxanthohumol and stout beer. Toxicol. Lett. 2014, 229, S179–S180. [Google Scholar] [CrossRef]
- Merinas-Amo, T.; Martínez-Jurado, M.; Merinas-Amo, R.; García-Zorrilla, V.; Fernández-Bedmar, Z.; Alonso-Moraga, A.; Mateo-Fernández, M. Comparison of in vivo and in vitro activities of alcohol and non-alcohol beer. Toxicol. Lett. 2015, 238, 65–66. [Google Scholar] [CrossRef]
- Krofta, K. Comparison of quality parameters of Czech and foreign hop varieties. Plant Soil Environ. 2003, 49, 261–268. [Google Scholar] [CrossRef]
- Krofta, K.; Mikyška, A.; Hašková, D. Antioxidant characteristics of hops and hop products. J. Inst. Brew. 2008, 114, 160–166. [Google Scholar] [CrossRef]
- Romero-Jiménez, M.; Campos-Sánchez, J.; Analla, M.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Genotoxicity and anti-genotoxicity of some traditional medicinal herbs. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 585, 147–155. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Tapsell, L.C. Food synergy: The key to a healthy diet. Proc. Nutr. Soc. 2013, 72, 200–206. [Google Scholar] [CrossRef]
- Larsson, S.; Kaluza, J.; Wolk, A. Combined impact of healthy lifestyle factors on lifespan: Two prospective cohorts. J. Intern. Med. 2017, 282, 209–219. [Google Scholar] [CrossRef]
- Fleming, J.; Reveillaud, I.; Niedzwiecki, A. Role of oxidative stress in Drosophila aging. Mutat. Res. 1992, 275, 267–279. [Google Scholar] [CrossRef]
- Schriner, S.E.; Coskun, V.; Hogan, S.P.; Nguyen, C.T.; Lopez, T.E.; Jafari, M. Extension of Drosophila Lifespan by Rhodiola rosea Depends on Dietary Carbohydrate and Caloric Content in a Simplified Diet. J. Med. Food 2016, 19, 318–323. [Google Scholar] [CrossRef]
- Hevia, D.; Mayo, J.C.; Quiros, I.; Sainz, R. Beer constituents inhibit prostate cancer cells proliferation. Eur. J. Cancer Suppl. 2008, 6, 142. [Google Scholar] [CrossRef]
- Stevens, J.F.; E Page, J. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Nozawa, H.; Yoshida, A.; Tajima, O.; Katayama, M.; Sonobe, H.; Wakabayashi, K.; Kondo, K. Intake of beer inhibits azoxymethane-induced colonic carcinogenesis in male Fischer 344 rats. Int. J. Cancer 2004, 108, 404–411. [Google Scholar] [CrossRef]
- Maietti, A.; Brighenti, V.; Bonetti, G.; Tedeschi, P.; Prencipe, F.P.; Benvenuti, S.; Brandolini, V.; Pellati, F. Metabolite profiling of flavonols and in vitro antioxidant activity of young shoots of wild Humulus lupulus L. (hop). J. Pharm. Biomed. Anal. 2017, 142, 28–34. [Google Scholar] [CrossRef]
- Luzak, B.; Kassassir, H.; Rój, E.; Stanczyk, L.; Watala, C.; Golanski, J. Xanthohumol from hop cones (Humulus lupulus L.) prevents ADP-induced platelet reactivity. Arch. Physiol. Biochem. 2017, 123, 54–60. [Google Scholar] [CrossRef]
- Wyllie, A.; Kerr, J.; Currie, A. Cell Death: The Significance of Apoptosis. Adv. Clin. Chem. 1980, 68, 251–306. [Google Scholar]
- Merinas-Amo, T.; Merinas-Amo, R.; Valenzuela-Gómez, F.; Bailón, A.D.H.; Lozano-Baena, M.; Mateo-Fernández, M.; Fernández-Bedmar, Z.; Alonso-Moraga, Á. Toxicological in vivo and in vitro evaluation of three diet abundant phenols: Tyrosol, ferulic and vanillic acids. Toxicol. Lett. 2016, 258, 177–178. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Mahli, A.; Weiskirchen, S.; Hellerbrand, C. The hop constituent xanthohumol exhibits hepatoprotective effects and inhibits the activation of hepatic stellate cells at different levels. Front. Physiol. 2015, 6, 140. [Google Scholar] [CrossRef]
- Pichler, C.; Ferk, F.; Al-Serori, H.; Huber, W.; Jäger, W.; Waldherr, M.; Mišík, M.; Kundi, M.; Nersesyan, A.; Herbacek, I. Xanthohumol prevents DNA damage by dietary carcinogens: Results of a human intervention trial. Cancer Prev. Res. 2017, 10, 153–160. [Google Scholar] [CrossRef]
- Poe, B.S.; O’Neill, K.L. Caffeine modulates heat shock induced apoptosis in the human promyelocytic leukemia cell line HL-60. Cancer Lett. 1997, 121, 1–6. [Google Scholar] [CrossRef]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Morozzi, G. Genotoxicity of alkene epoxides in human peripheral blood mononuclear cells and HL60 leukaemia cells evaluated with the comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 747, 1–6. [Google Scholar] [CrossRef]
- Fairbairn, D.W.; Olive, P.L.; O’Neill, K.L. The comet assay: A comprehensive review. Mutat. Res. Genet. Toxicol. 1995, 339, 37–59. [Google Scholar] [CrossRef]
- Herman, J.G.; Baylin, S.B. Gene Silencing in Cancer in Association with Promoter Hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef]
- Esteller, M. DNA methylation and cancer therapy: New developments and expectations. Curr. Opin. Oncol. 2005, 17, 55–60. [Google Scholar] [CrossRef]
- Román-Gómez, J.; Jiménez-Velasco, A.; Agirre, X.; Castillejo, J.A.; Navarro, G.; José-Enériz, E.S.; Garate, L.; Cordeu, L.; Cervantes, F.; Prosper, F.; et al. Repetitive DNA hypomethylation in the advanced phase of chronic myeloid leukemia. Leuk. Res. 2008, 32, 487–490. [Google Scholar] [CrossRef]
- Yang, C.S.; Fang, M.; Chen, D. Dietary Polyphenols May Affect DNA Methylation. J. Nutr. 2007, 137, 223S–228S. [Google Scholar] [Green Version]
- Lindsay, R.C.; Withycombe, D.A.; Micketts, R.J. Comparison of Gas Chromatographic Methods for Analysis of Beer Flavors. Proc. Annu. Meet. Am. Soc. Brew. Chem. 1972, 30, 4–7. [Google Scholar] [CrossRef]
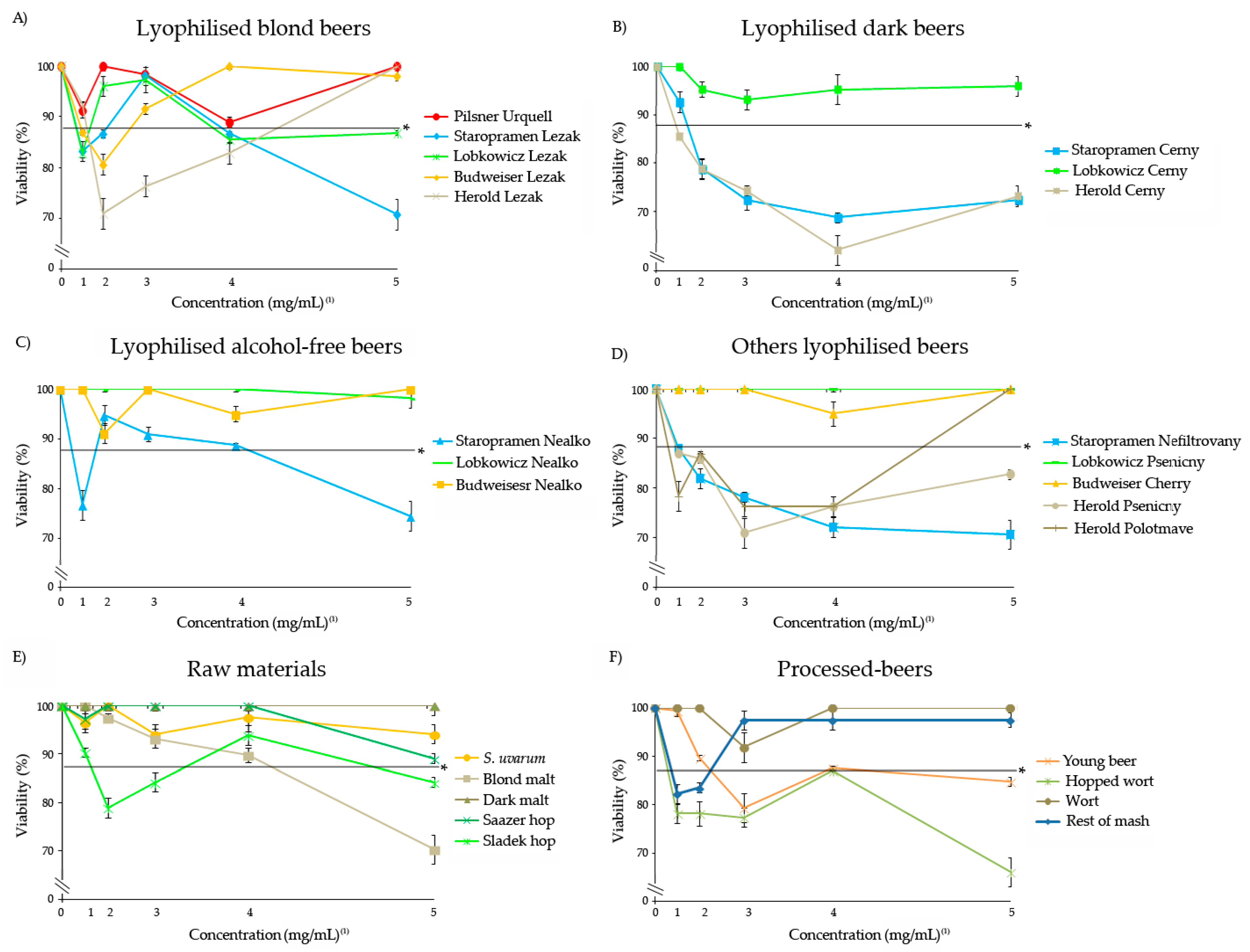
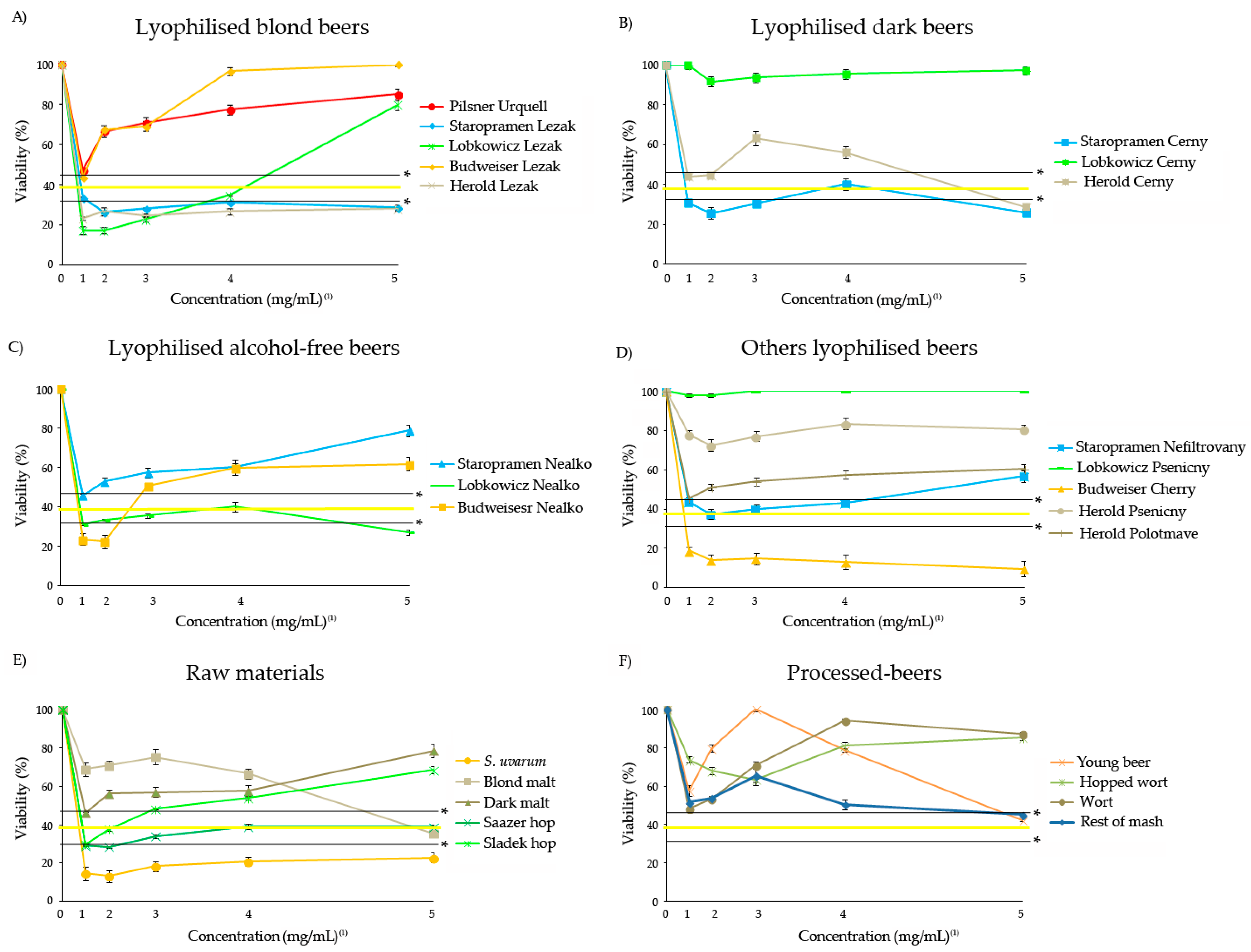
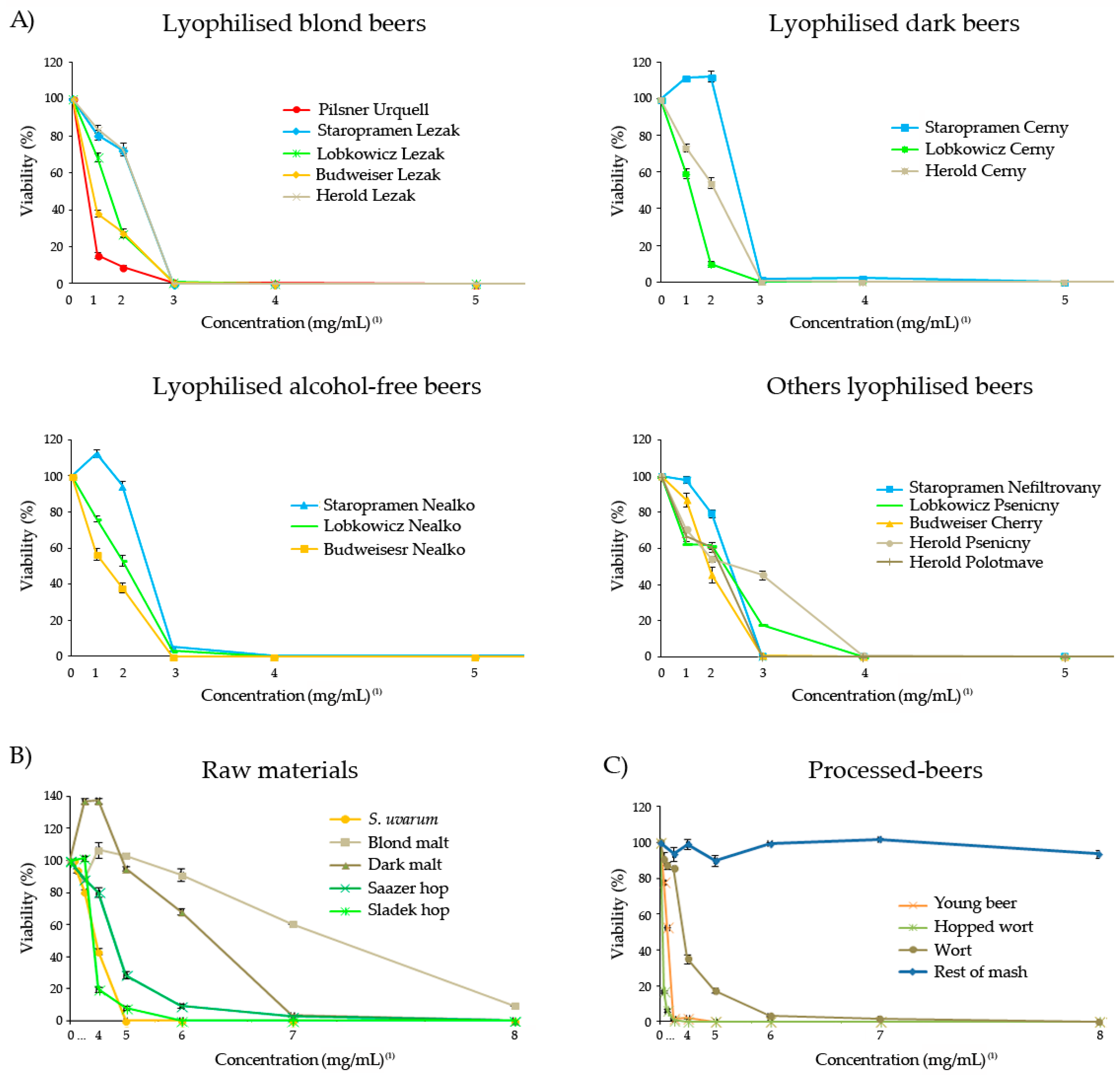
| Beer Trade Mark | Named in the Manuscript | Beer Type/Ingredient Origin |
|---|---|---|
| Pilsner Urquell | Pilsner Urquell | Blond |
| Staropramen Lezak | Staropramen Lezak | Blond |
| Staropramen Cerny Lezak | Staropramen Cerny | Stout |
| Staropramen Nealko | Staropramen Nealko | Alcohol-free |
| Staropramen Nefiltrovany | Staropramen Nefiltrovany | Blond |
| Lobkowicz Lezak Premium | Lobkowicz Lezak | Blond |
| Lobkowicz Cerny Premium | Lobkowicz Cerny | Stout |
| Lobkowicz Nealkoholicky Premium | Lobkowicz Nealko | Alcohol-free |
| Lobkowicz Psenicny Premium | Lobkowicz Psenicny | Wheat |
| Budweiser Budvar B:Original, Svetly lezak | Budweiser Lezak | Blond |
| Budweiser Budvar B:Free, Nealkoholicke pivo | Budweiser Nealko | Alcohol-free |
| Budweiser Budvar B:Cherry, Tmavy lezak s visni | Budweiser Cherry | Cherry flavoured |
| Herold Svétly Breznicky Lezak | Herold Lezak | Blond |
| Herold Tmave Speciálni Pivo | Herold Cerny | Stout |
| Herold Psenicny Kvasnicovy Lezak | Herold Psenicny | Wheat |
| Herold Polotmave Specialni Pivo | Herold Polotmave | Pomegranate flavoured |
| Rest of mash (1) | Rest of mash | CULS (8) Prague brewery |
| Blond malt | Blond malt | CULS Prague brewery |
| Dark malt | Dark malt | CULS Prague brewery |
| Sladek hop (2) | Sladek hop | CULS Prague brewery |
| Saazer hop (3) | Saazer hop | CULS Prague brewery |
| Saccharomyces uvarum(4) | S. uvarum | CULS Prague brewery |
| Wort (5) | Wort | CULS Prague brewery |
| Hopped wort (6) | Hopped wort | CULS Prague brewery |
| Young beer (7) | Young beer | CULS Prague brewery |
| Reaction ID | GenBank Number | Amplicon Start | Amplicon End | Forward Primer Sequence 5′ to 3′ (N) | Reverse Primer Sequence 5′ to 3′ (N) | GC-Content (%) | |
|---|---|---|---|---|---|---|---|
| Forward Reverse | |||||||
| Alu C4 | Consensus Sequence | 1 | 98 | GGTTAGGTATAGTGGTTTATATTTGTAATTTTAGTA (36) | ATTAACTAAACTAATCTTAAACTCCTAACCTCA (33) | 25 | 27.3 |
| Alu M1 | Y07755 | 5059 | 5164 | ATTATGTTAGTTAGGATGGTTTCGATTTT (29) | CAATCGACCGAACGCGA (17) | 27.6 | 58.8 |
| LINE-1 | X52235 | 251 | 331 | GGACGTATTTGGAAAATCGGG (21) | AATCTCGCGATACGCCGTT (19) | 47.6 | 52.6 |
| Sat-α | M38468 | 139 | 260 | TGATGGAGTATTTTTAAAATATACGTTTTGTAGT (34) | AATTCTAAAAATATTCCTCTTCAATTACGTAAA (33) | 23.5 | 21.2 |
| Compound | Clones per Wing (nº Spots) (1) | |||||
|---|---|---|---|---|---|---|
| N° of Wings | Small Single Clones (1–2 Cells) m = 2 | Large Simple Clones (>2 Cells) m = 5 | Twin Clones m = 5 | Total Clones m = 2 | U-Test (2) | |
| H2O | 38 | 0.105 (4) | 0.053 (2) | 0 | 0.158 (6) | |
| H2O2 | 40 | 0.2 (8) | 0.2 (8) | 0 | 0.400 (16) + | |
| Pilsner Urquell (mg/mL) | ||||||
| 3.125 | 40 | 0.175 (7) | 0.100 (4) | 0 | 0.275 (11) i | ∆ |
| 50 | 40 | 0.225 (9) | 0.075 (3) | 0 | 0.300 (12) i | ∆ |
| Blond malt (mg/mL) | ||||||
| 0.0625 | 40 | 0.225 (9) | 0.025 (1) | 0 | 0.250 (10) i | ∆ |
| 1 | 40 | 0.250 (10) | 0.075 (3) | 0 | 0.325 (13) i | ∆ |
| Dark malt (mg/mL) | ||||||
| 0.0625 | 40 | 0.200 (8) | 0.025 (1) | 0 | 0.225 (9) i | ∆ |
| 1 | 40 | 0.100 (4) | 0.025 (1) | 0 | 0.125 (5) i | ∆ |
| Sladek hop (mg/mL) | ||||||
| 0.00156 | 40 | 0.125 (5) | 0.05 (2) | 0 | 0.175 (7) i | ∆ |
| 0.025 | 38 | 0.316 (12) | 0.052 (2) | 0 | 0.368 (14) i | ∆ |
| Saazer hop (mg/mL) | ||||||
| 0.000625 | 38 | 0.211 (8) | 0.052 (2) | 0 | 0.263 (10) i | ∆ |
| 0.01 | 38 | 0.131 (5) | 0.079 (3) | 0 | 0.210 (8) i | ∆ |
| S. uvarum (mg/mL) | ||||||
| 0.00023 | 40 | 0.200 (8) | 0.075 (3) | 0 | 0.275 (11) i | ∆ |
| 0.00375 | 34 | 0.294 (10) | 0.059 (2) | 0 | 0.353 (12) i | ∆ |
| Wort (mg/mL) | ||||||
| 3.125 | 40 | 0.300 (12) | 0 | 0.025 (1) | 0.325 (13) i | ∆ |
| 50 | 38 | 0.132 (5) | 0.026 (1) | 0 | 0.158 (6) i | ∆ |
| Hopped wort (mg/mL) | ||||||
| 3.125 | 40 | 0.200 (8) | 0.025 (1) | 0 | 0.225 (9) i | ∆ |
| 50 | 38 | 0.131 (5) | 0.158 (6) | 0 | 0.289 (11) i | ∆ |
| Young beer (mg/mL) | ||||||
| 3.125 | 40 | 0.255 (9) | 0 | 0 | 0.255 (9) i | ∆ |
| 50 | 34 | 0.353 (12) | 0 | 0 | 0.353 (12) i | ∆ |
| Clones per Wing (nº Spots) (1) | Mann Whitney U-Test (2) | Inhibition Percentage (%) (3) | |||||
|---|---|---|---|---|---|---|---|
| Compound | N° of Wings | Small Single Clones (1–2 Cells) m = 2 | Large Simple Clones (More Than 2 Cells) m = 5 | Twin Clones m = 5 | Total Clones m = 2 | ||
| H2O | 40 | 0.175 (7) | 0 | 0 | 0.175 (7) | ||
| H2O2 | 40 | 0.2 (8) | 0.2 (8) | 0 | 0.400 (16) + | ||
| Pilsner Urquell (mg/mL) | |||||||
| 3.125 | 38 | 0.263 (10) | 0.026 (1) | 0 | 0.289 (11) β | 27.75 | |
| 50 | 40 | 0.200 (8) | 0.025 (1) | 0 | 0.225 (9) β | 43.75 | |
| Blond malt (mg/mL) | |||||||
| 0.0625 | 40 | 0.225 (9) | 0.050 (2) | 0 | 0.275 (11) β | 31.25 | |
| 1 | 38 | 0.315 (12) | 0.106 (4) | 0 | 0.421 (16) λ | ∆ | −5.25 |
| Dark malt (mg/mL) | |||||||
| 0.0625 | 40 | 0.175 (7) | 0.05 (2) | 0 | 0.225 (9) β | 43.75 | |
| 1 | 38 | 0.131 (5) | 0.052 (2) | 0 | 0.183 (7) β | 54.25 | |
| Sladek hop (mg/mL) | |||||||
| 0.00156 | 38 | 0.131 (5) | 0.079 (3) | 0 | 0.210 (8) β | 47.50 | |
| 0.025 | 40 | 0.325 (13) | 0.075 (3) | 0 | 0.400 (16) λ | ∆ | 0.00 |
| Saazer hop (mg/mL) | |||||||
| 0.000625 | 38 | 0.079 (3) | 0.052 (2) | 0 | 0.131 (5) β | 67.25 | |
| 0.01 | 40 | 0.050 (2) | 0.050 (2) | 0 | 0.100 (4) β | 75.00 | |
| S. uvarum (mg/mL) | |||||||
| 0.00023 | 18 | 0.278 (5) | 0.222 (4) | 0 | 0.500 (9) λ | ∆ | −25.00 |
| 0.00375 | 27 | 0.222 (6) | 0.125 (3) | 0 | 0.333 (9) β | 16.75 | |
| Wort (mg/mL) | |||||||
| 3.125 | 40 | 0.125 (5) | 0.025 (1) | 0 | 0.150 (6) β | 62.50 | |
| 50 | 40 | 0.250 (10) | 0.025 (1) | 0 | 0.275 (11) β | 31.25 | |
| Hopped wort (mg/mL) | |||||||
| 3.125 | 40 | 0.325 (13) | 0.075 (3) | 0 | 0.400 (16) λ | ∆ | 00.00 |
| 50 | 40 | 0.100 (4) | 0.100 (4) | 0 | 0.200 (8) β | 50.00 | |
| Young beer (mg/mL) | |||||||
| 3.125 | 40 | 0.425 (17) | 0.075 (3) | 0 | 0.500 (20) λ | ∆ | −25.00 |
| 50 | 26 | 0.500 (13) | 0.192 (5) | 0 | 0.692 (18) λ | ∆ | −73.00 |
| Compound | Concentration | Mean Lifespan (1) (Days) | Mean Healthspan (1) (Days) | ||
|---|---|---|---|---|---|
| Pilsner Urquell | Control | 74.319 | 47.048 | ||
| 3.125 mg/mL | 69.096 | * | 48.615 | ns | |
| 6.25 mg/mL | 73.179 | ns | 45.385 | ns | |
| 12.5 mg/mL | 74.571 | ns | 57.923 | * | |
| 25 mg/mL | 64.857 | * | 44.765 | ns | |
| 50 mg/mL | 72.413 | ns | 49.462 | ns | |
| Blond malt | Control | 69.888 | 35.959 | ||
| 0.0625 mg/mL | 76.349 | * | 47.769 | * | |
| 0.125 mg/mL | 70.412 | ns | 39.714 | ns | |
| 0.25 mg/mL | 78.448 | ns | 65.617 | * | |
| 0.5 mg/mL | 73.514 | ns | 48.067 | * | |
| 1 mg/mL | 77.946 | * | 53.583 | * | |
| Dark malt | Control | 69.888 | 35.959 | ||
| 0.0625 mg/mL | 70.557 | ns | 50.129 | * | |
| 0.125 mg/mL | 66.314 | * | 34.135 | ns | |
| 0.25 mg/mL | 58.281 | * | 35.692 | ns | |
| 0.5 mg/mL | 73.077 | ns | 44.001 | ns | |
| 1 mg/mL | 72.433 | ns | 38.176 | ns | |
| Sladek hop | Control | 72.107 | 44.211 | ||
| 0.00156 mg/mL | 77.439 | * | 50.792 | ns | |
| 0.00312 mg/mL | 78.083 | * | 54.987 | * | |
| 0.00625 mg/mL | 68.774 | ns | 45.750 | ns | |
| 0.0125 mg/mL | 68.280 | ns | 42.154 | ns | |
| 0.025 mg/mL | 67.556 | ns | 36.667 | * | |
| Saazer hop | Control | 72.107 | 44.211 | ||
| 0.000625 mg/mL | 60.714 | * | 29.929 | * | |
| 0.00125 mg/mL | 69.993 | ns | 43.947 | ns | |
| 0.0025 mg/mL | 70.314 | ns | 48.909 | ns | |
| 0.005 mg/mL | 70.143 | ns | 44.754 | ns | |
| 0.01 mg/mL | 72.098 | ns | 53.091 | * | |
| S. uvarum | Control | 74.319 | 47.048 | ||
| 0.00023 mg/mL | 67.590 | * | 42.545 | * | |
| 0.00047 mg/mL | 73.738 | ns | 53.167 | ns | |
| 0.00094 mg/mL | 71.876 | ns | 51.533 | * | |
| 0.00187 mg/mL | 73.223 | ns | 55.292 | * | |
| 0.00375 mg/mL | 79.060 | * | 54.393 | * | |
| Wort | Control | 74.319 | 47.048 | ||
| 3.125 mg/mL | 66.179 | * | 51.929 | ns | |
| 6.25 mg/mL | 68.399 | ns | 38.143 | * | |
| 12.5 mg/mL | 77.628 | ns | 64.238 | * | |
| 25 mg/mL | 67.464 | * | 52.571 | ns | |
| 50 mg/mL | 63.885 | * | 48.250 | ns | |
| Hopped wort | Control | 74.319 | 47.048 | ||
| 3.125 mg/mL | 72.232 | ns | 50.077 | ns | |
| 6.25 mg/mL | 76.922 | ns | 63.375 | * | |
| 12.5 mg/mL | 66.256 | * | 41.167 | ns | |
| 25 mg/mL | 71.095 | ns | 40.541 | * | |
| 50 mg/mL | 73.004 | ns | 53.833 | ns | |
| Young beer | Control | 74.319 | 47.048 | ||
| 3.125 mg/mL | 71.696 | ns | 47.357 | ns | |
| 6.25 mg/mL | 70.747 | * | 57.357 | * | |
| 12.5 mg/mL | 72.117 | * | 52.224 | * | |
| 25 mg/mL | 78.426 | ns | 57.786 | * | |
| 50 mg/mL | 75.314 | ns | 58.314 | * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merinas-Amo, T.; Merinas-Amo, R.; García-Zorrilla, V.; Velasco-Ruiz, A.; Chladek, L.; Plachy, V.; del Río-Celestino, M.; Font, R.; Kokoska, L.; Alonso-Moraga, Á. Toxicological Studies of Czech Beers and Their Constituents. Foods 2019, 8, 328. https://doi.org/10.3390/foods8080328
Merinas-Amo T, Merinas-Amo R, García-Zorrilla V, Velasco-Ruiz A, Chladek L, Plachy V, del Río-Celestino M, Font R, Kokoska L, Alonso-Moraga Á. Toxicological Studies of Czech Beers and Their Constituents. Foods. 2019; 8(8):328. https://doi.org/10.3390/foods8080328
Chicago/Turabian StyleMerinas-Amo, Tania, Rocío Merinas-Amo, Victoria García-Zorrilla, Alejandro Velasco-Ruiz, Ladislav Chladek, Vladimir Plachy, Mercedes del Río-Celestino, Rafael Font, Ladislav Kokoska, and Ángeles Alonso-Moraga. 2019. "Toxicological Studies of Czech Beers and Their Constituents" Foods 8, no. 8: 328. https://doi.org/10.3390/foods8080328
APA StyleMerinas-Amo, T., Merinas-Amo, R., García-Zorrilla, V., Velasco-Ruiz, A., Chladek, L., Plachy, V., del Río-Celestino, M., Font, R., Kokoska, L., & Alonso-Moraga, Á. (2019). Toxicological Studies of Czech Beers and Their Constituents. Foods, 8(8), 328. https://doi.org/10.3390/foods8080328









