The Development of a Sorghum Bran-Based Biorefining Process to Convert Sorghum Bran into Value Added Products
Abstract
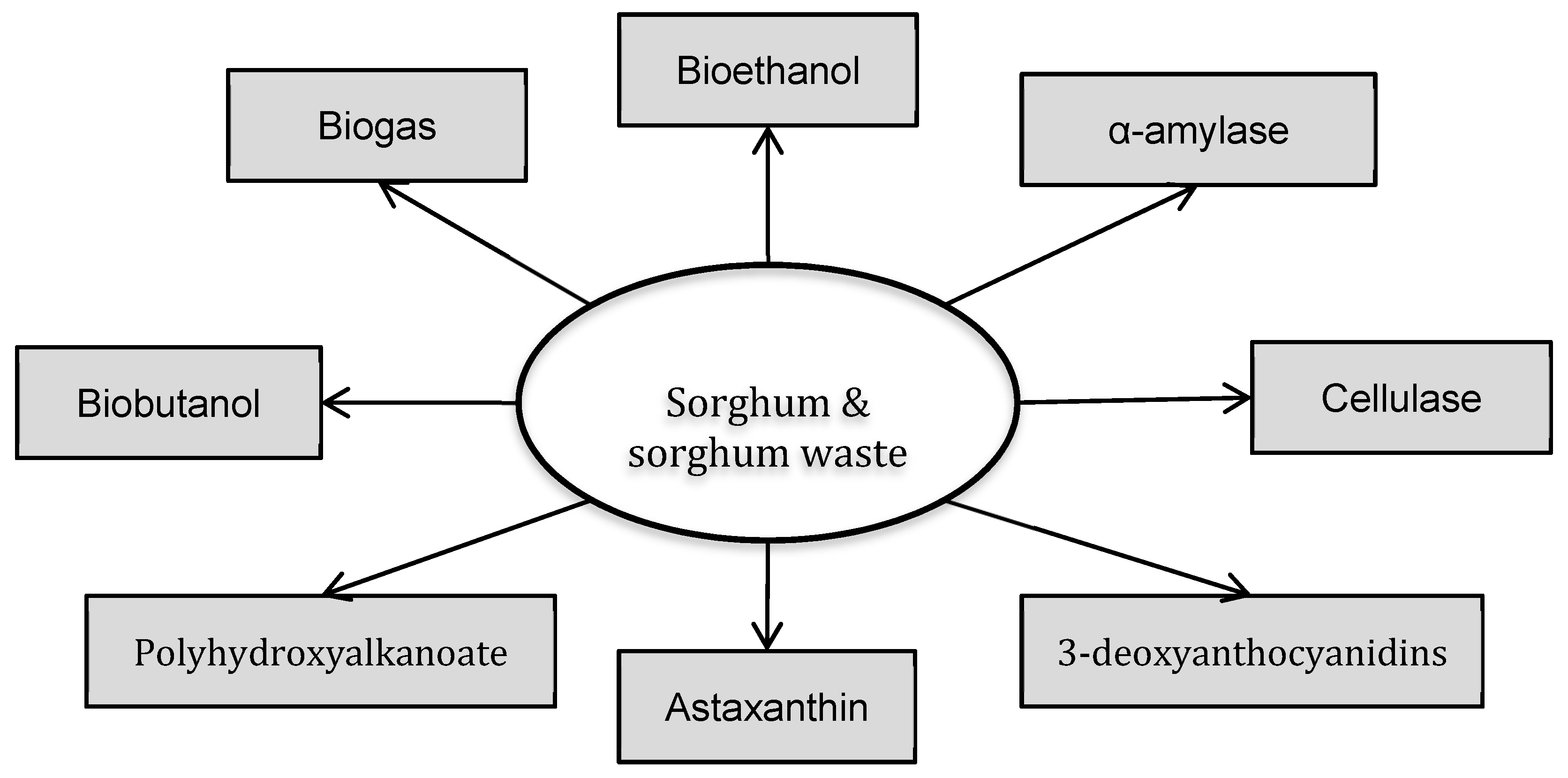
:1. Introduction
2. Materials and Methods
2.1. Sorghum Bran
2.2. Microorganisms
2.3. Sorghum Bran Submerged Fermentation
2.4. Glucoamylase Enzyme
2.5. Enzymatic Hydrolysis of Sorghum Bran
2.6. Sugar Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Starch Content in the Sorghum Brans
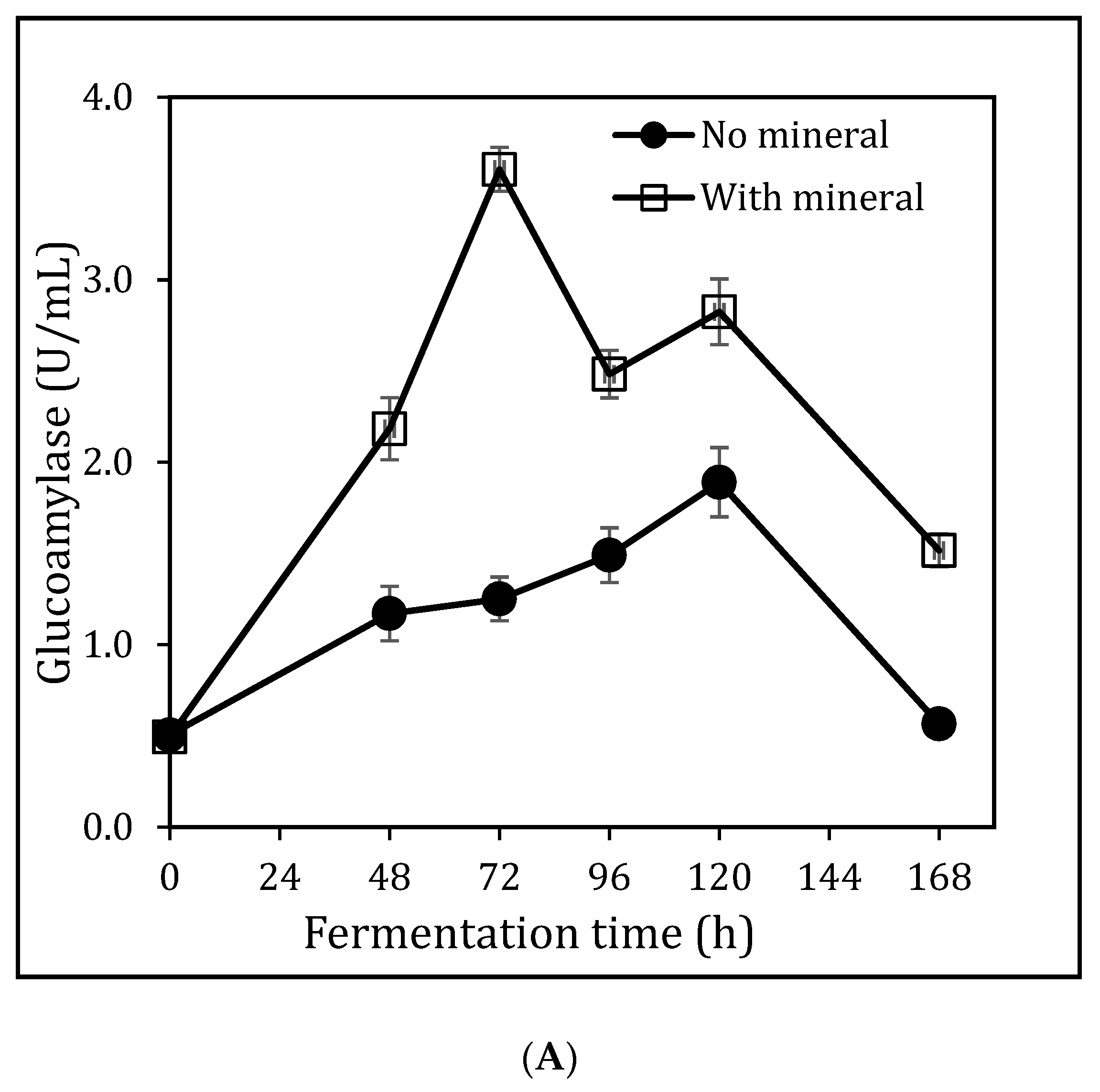
3.2. Glucoamylase Production Using Sorghum Bran Via SmF
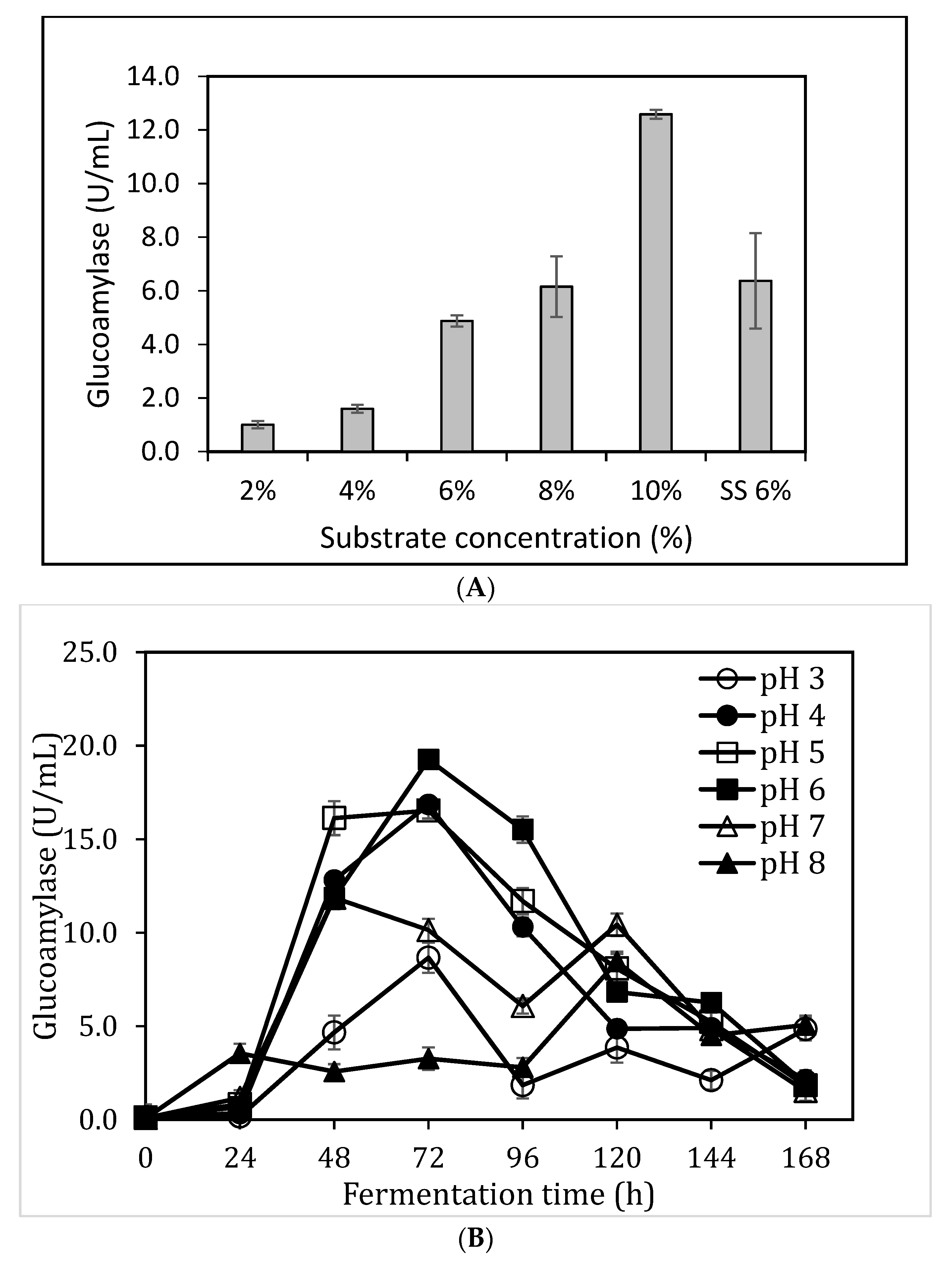
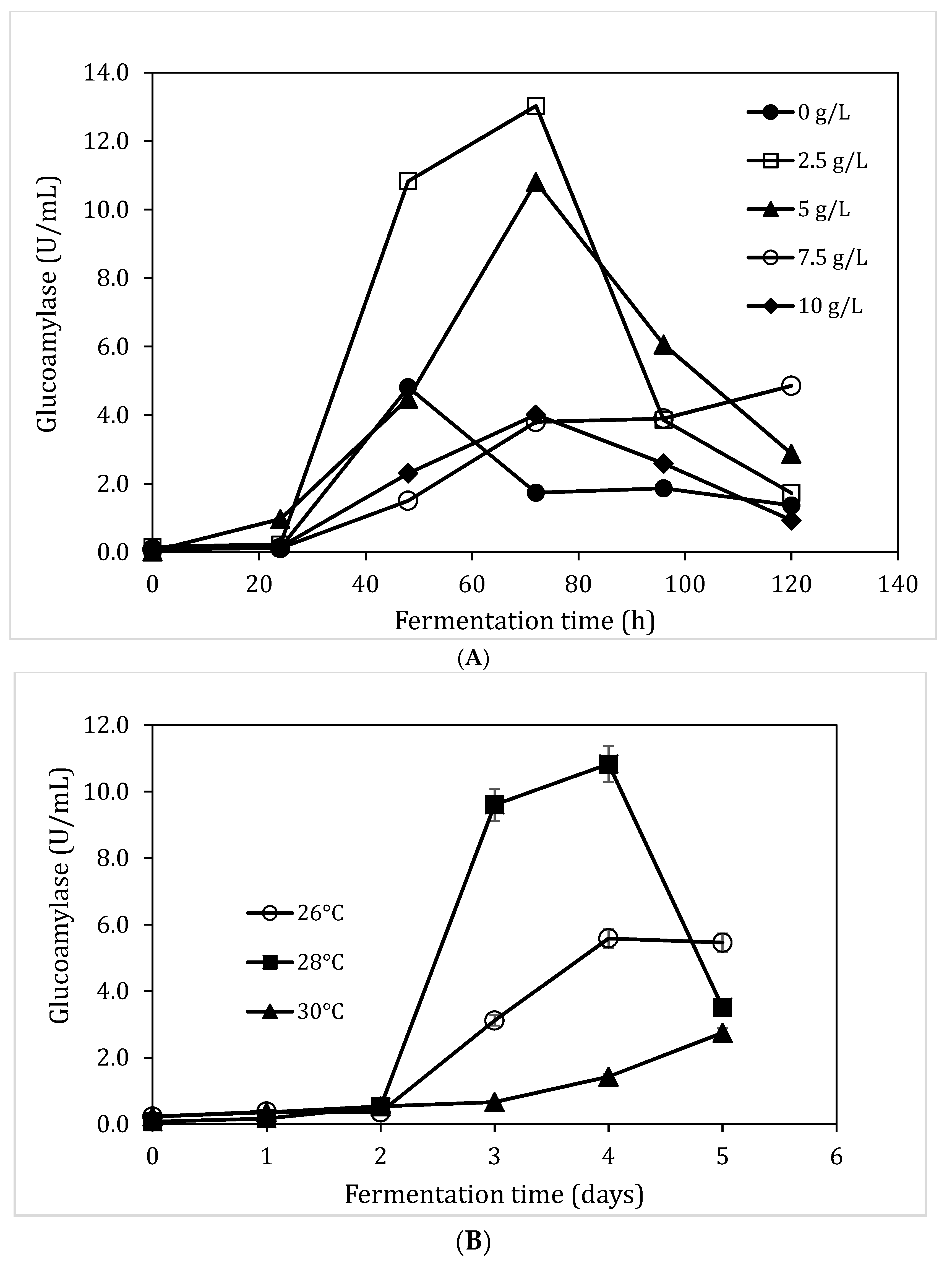
3.3. Impact of Substrate Concentzration and pH on Glucoamylase Production
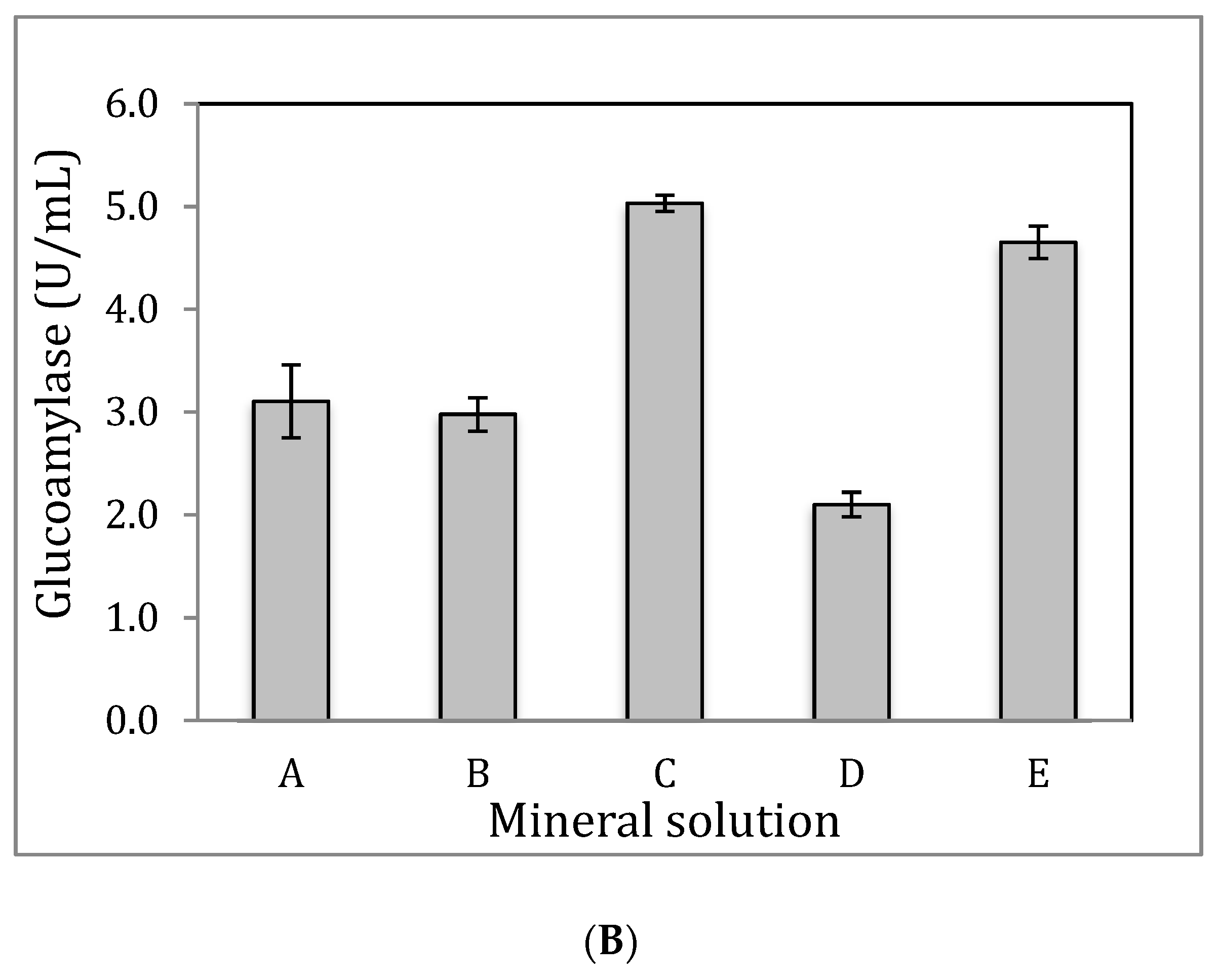
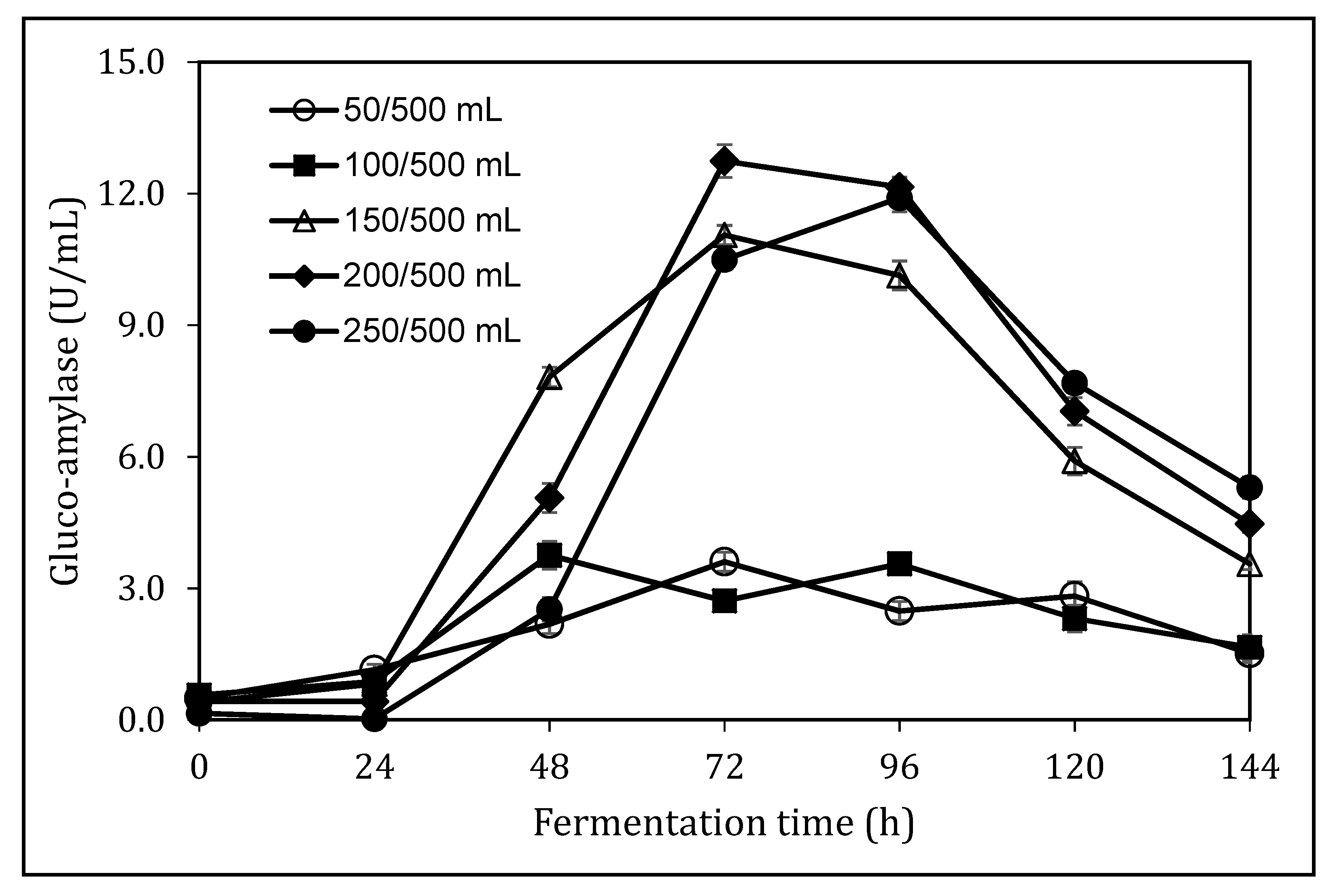
3.4. Impact of Medium Loading Ratio on Glucoamylase Production
3.5. Impact of Yeast Extraction and Temperature on Glucoamylase Production
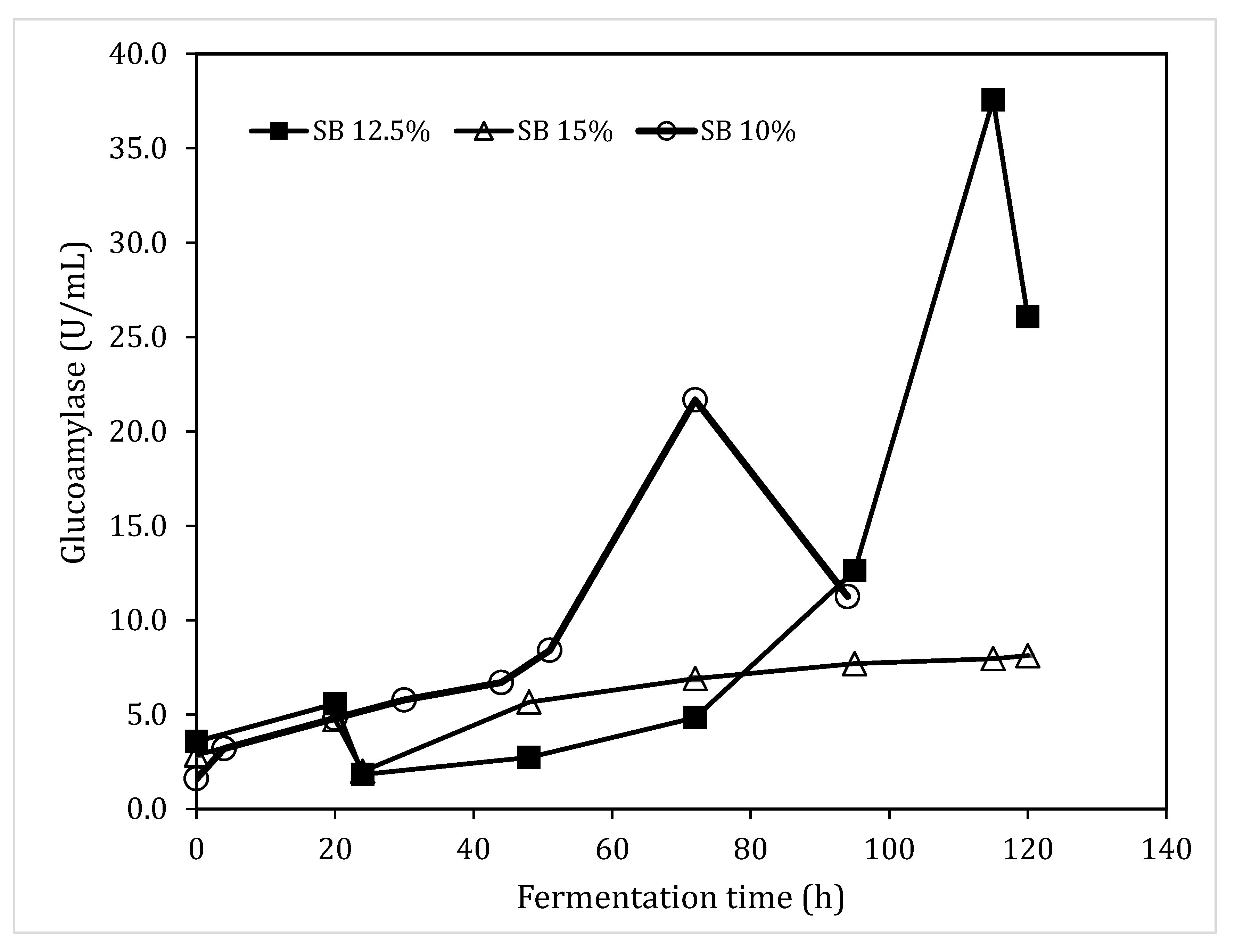
3.6. Glucoamylase Production in Bench Top Fermenters
3.7. Sorghum Bran Hydrolysis Using Crude Glucoamylase Solution
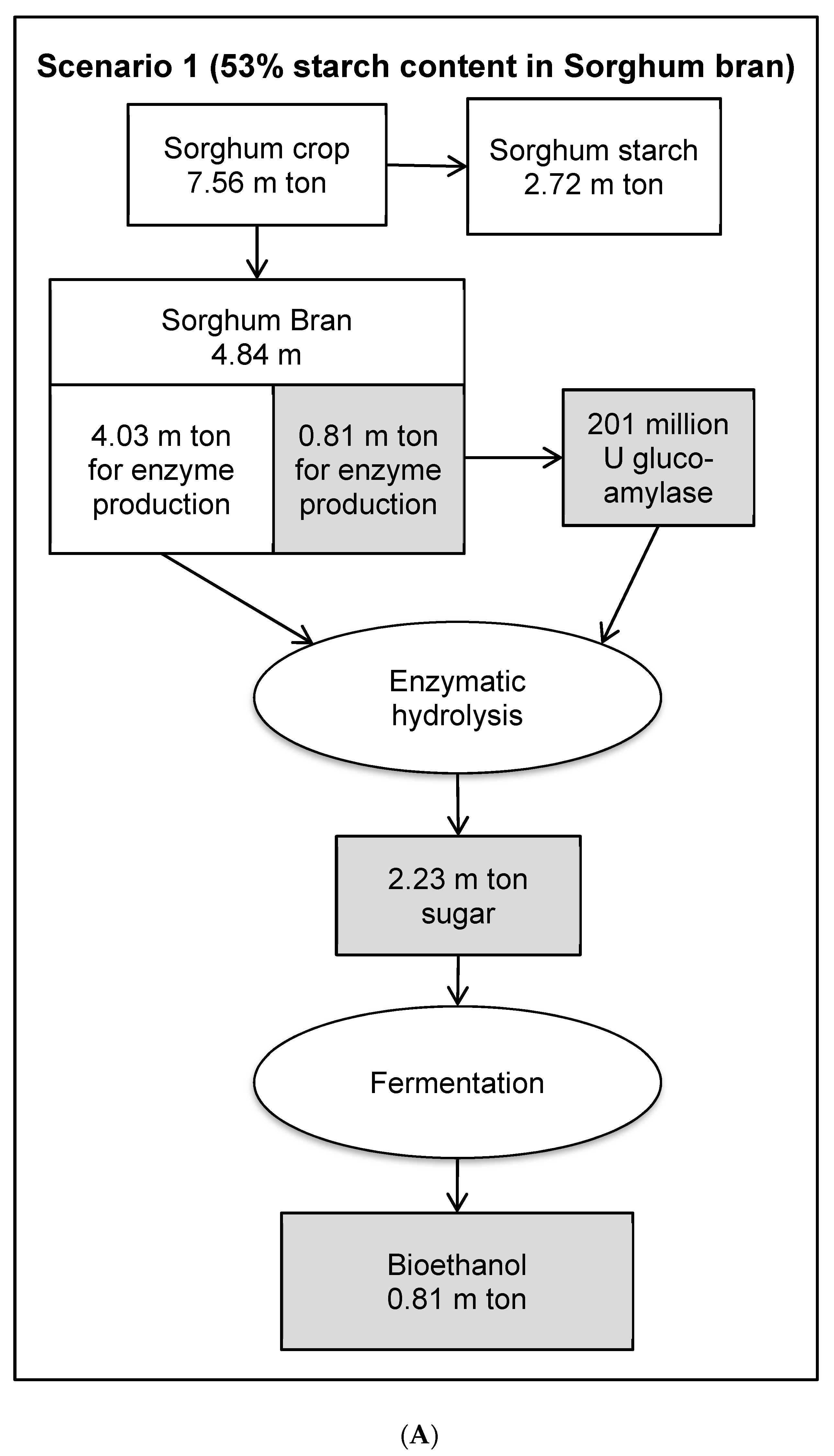
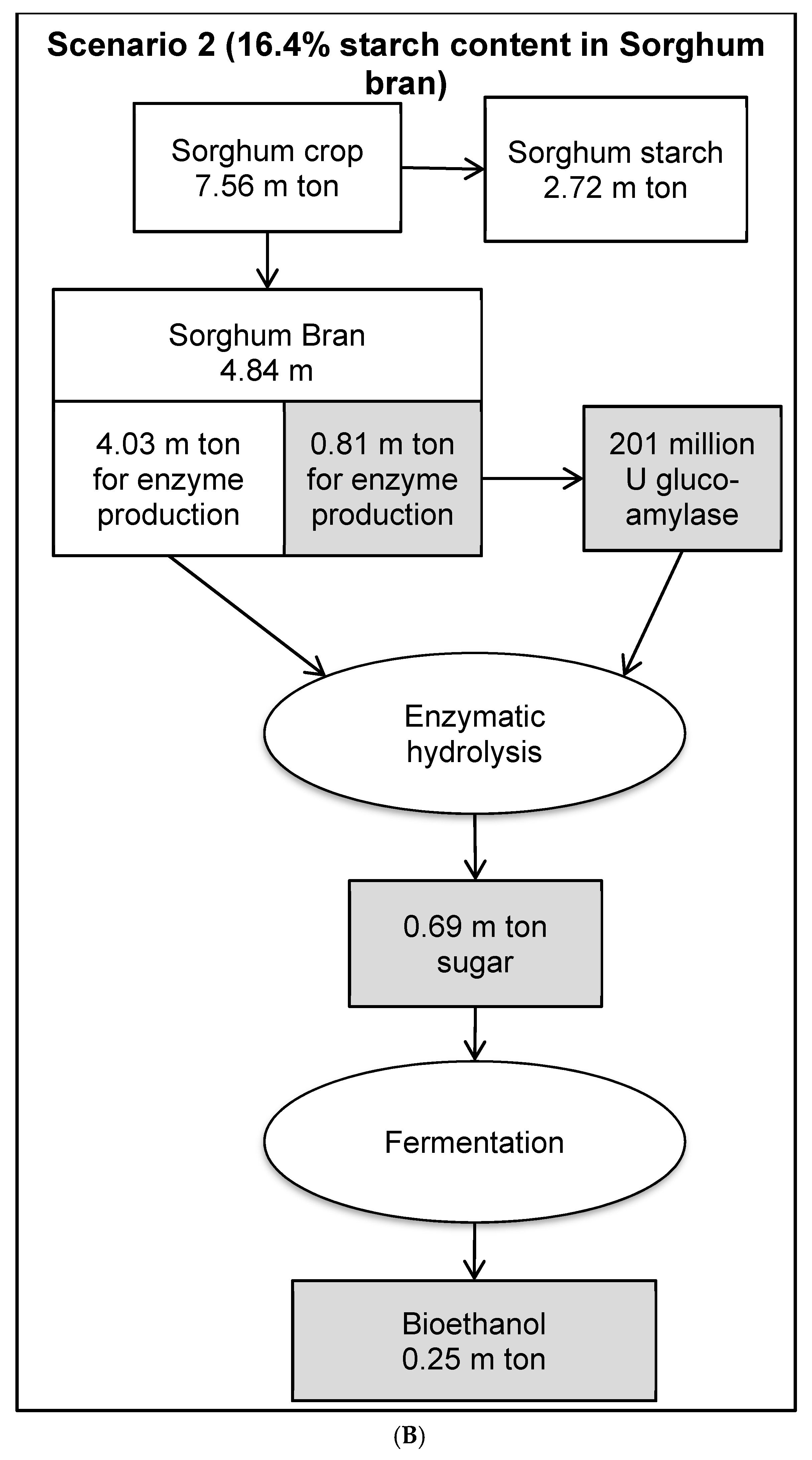
3.8. Economic Evaluation of Glucoamylase Production in a Bioethanol Production Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuller, M.F. Chapter title? In Encyclopedia of Farm Animal Nutrition; CABI: Wallingford, UK, 2014. [Google Scholar]
- Beta, T.; Chisi, M.; Monyo, E.S. Sorghum/harvest, storage and transport. In Encyclopedia of Grain Science; Wingley, C.W., Corke, H., Walker, C.E., Eds.; Elsevier: Oxford, UK, 2004; pp. 119–126. [Google Scholar]
- Waniska, R.D.; Rooney, L.W.; McDonough, M. Sorghum/utilization. In Encyclopedia of Grain Science; Wingley, C.W., Corke, H., Walker, C.E., Eds.; Elsevier: Oxford, UK, 2004; pp. 126–138. [Google Scholar]
- Adeyemi, I.A. Dry-milling of sorghum for ogi manufacture. J. Cereal Sci. 1983, 1, 221–227. [Google Scholar] [CrossRef]
- Li, S.Z.; Li, G.M.; Zhang, L.; Zhou, Z.; Han, B.; Hou, W.; Wang, J.; Li, T. A demonstration study of ethanol production from sweet sorghum stems with advanced solid state fermentation technology. Appl. Energy 2013, 102, 260–265. [Google Scholar] [CrossRef]
- Jiang, D.; Hao, M.; Fu, J.; Liu, K.; Yan, X. Potential bioethanol production from sweet sorghum on marginal land in China. J. Clean Prod. 2019, 220, 225–234. [Google Scholar] [CrossRef]
- Ahmed El-Imam, A.; Greetham, D.; Du, C.; Dyer, P. The development of a biorefining strategy for the production of biofuel from sorghum milling waste. Biochem. Eng. J. 2019, 150, 107288. [Google Scholar] [CrossRef]
- Cai, D.; Dong, Z.; Han, J.; Yu, H.; Wang, Y.; Qin, P.; Wang, Z.; Tan, T. Co-generation of bio-butanol and bio-lipids under a hybrid process. Green Chem. 2016, 18, 1377–1386. [Google Scholar] [CrossRef]
- Nozari, B.; Mirmohamadsadeghi, S.; Karimi, K. Bioenergy production from sweet sorghum stalks via a biorefinery perspective. Appl. Microbiol. Biotechnol. 2018, 102, 3425–3438. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Guo, C.; Liu, C.-Z. Enhanced hydrogen and volatile fatty acid production from sweet sorghum stalks by two-steps dark fermentation with dilute acid treatment in between. Int. J. Hydrogen Energy 2018, 43, 659–666. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Johnston, D.B.; Nghiem, N.P. Utilization of Sweet Sorghum Juice for the Production of Astaxanthin as a Biorefinery Co-Product by Phaffia rhodozyma. ACS Sustain. Chem. Eng. 2018, 6, 3124–3134. [Google Scholar] [CrossRef]
- Vanamala, J.K.P.; Massey, A.R.; Pinnamaneni, S.R.; Reddivari, L.; Reardon, K.F. Grain and sweet sorghum (Sorghum bicolor L. Moench) serves as a novel source of bioactive compounds for human health. Crit. Rev. Food Sci. Nutr. 2018, 58, 2867–2881. [Google Scholar] [CrossRef]
- Lolasi, F.; Amiri, H.; Asadollahi, M.A.; Karimi, K. Using sweet sorghum bagasse for production of amylases required for its grain hydrolysis via a biorefinery platform. Ind. Crop Prod. 2018, 125, 473–481. [Google Scholar] [CrossRef]
- Kumar, P.; Satyanarayana, T. Microbial glucoamylases: Characteristics and applications. Crit. Rev. Biotechnol. 2009, 29, 225–255. [Google Scholar] [CrossRef] [PubMed]
- Norouzian, D.; Akbarzadeh, A.; Scharer, J.M.; Moo Young, M. Fungal glucoamylases. Biotechnol. Adv. 2006, 24, 80–85. [Google Scholar] [CrossRef] [PubMed]
- López, J.A.; Lázaro, C.D.C.; Castilho, L.D.R.; Freire, D.M.G.; Castro, A.M.D. Characterization of multienzyme solutions produced by solid-state fermentation of babassu cake, for use in cold hydrolysis of raw biomass. Biochem. Eng. J. 2013, 77, 231–239. [Google Scholar] [CrossRef]
- Castro, A.M.D.; Carvalho, D.F.; Freire, D.M.G.; Castilho, L.D.R. Economic analysis of the production of amylases and other hydrolases by Aspergillus awamori in solid-state fermentation of babassu cake. Enzym. Res. 2010, 2010, 576872. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Wang, Q.H.; Liu, Y.Y.; Ma, H.Z. On-site production of crude glucoamylase for kitchen waste hydrolysis. Waste Manag. Res. 2009, 28, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.C.; Pleissner, D.; Lin, C.S.K. Production of fungal glucoamylase for glucose production from food waste. Biomolecules 2013, 3, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Abdalwahab, S.A.; Ibrahim, S.A.; Dawood, E.S. Culture condition forthe production of glucoamylase enzyme by different isolates of Aspergillus spp. Int. Food Res. J. 2012, 19, 1261–1266. [Google Scholar]
- Abu, E.A.; Ado, S.A.; James, D.B. Raw starch degrading amylase production by mixed culture of Aspergillus niger and Saccharomyces cerevisae grown on sorghum pomace. Afr. J. Biotechnol. 2005, 4, 785–790. [Google Scholar]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Stepwise optimisation of enzyme production in solid state fermentation of waste bread pieces. Food Bioprod. Process. 2013, 91, 638–646. [Google Scholar] [CrossRef]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Solid state fermentation of waste bread pieces by Aspergillus awamori Analysing the effects of airflow rate on enzyme production in packed bed bioreactors. Food Bioprod. Process. 2015, 95, 63–75. [Google Scholar] [CrossRef]
- Izmirliogiu, G.; Demirci, A. Strain selection and media optimization for glucoamylase production from industrial potato waste by Aspergillus niger. J. Sci. Food Agric. 2016, 96, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Banerjee, R. Optimization of extraction and purification of gluco-amylase produced by A. awamori in SSF. Biotechnol. Bioprocess. Eng. 2009, 14, 60–66. [Google Scholar] [CrossRef]
- Imran, M.; Asad, M.J.; Gulfraz, M.; Qureshi, R.; Gul, H.; Manzoor, N.; Choudhary, A.N. Glucoamylase production from Aspergillus niger by using solid state fermentation process. Pak. J. Bot. 2010, 44, 2103–2110. [Google Scholar]
- Du, C.; Lin, S.K.C.; Koutinas, A.; Wang, R.; Dorado, P.; Webb, C. A wheat biorefining strategy based on solid-state fermentation for fermentative production of succinic acid. Bioresour. Technol. 2018, 99, 8310–8315. [Google Scholar] [CrossRef] [PubMed]
- Pavezzi, F.C.; Gomes, E.; Silva, R.D. Production and characterization of glucoamylase from fungus Aspergillus awamori expressed in yeast Saccharomyces cerevisiae using different carbon sources. Braz. J. Microbiol. 2008, 39, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Satyanarayana, T. Optimization of culture variables for improving glucoamylase production by alginate-entrapped Thermomucor indicae-seudaticae using statistical methods. Bioresour. Technol. 2007, 98, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Zambare, V. Solid state fermentation of Aspergillus oryzae for gluco-amylase production on agro residues. Int. J. Life Sci. 2010, 4, 16–25. [Google Scholar] [CrossRef]
- Koutinas, A.; Belafi-Bako, K.; Kabiri-Badr, A.; Toth, A.; Gubicza, L.; Webb, C. Enzymatic hydrolysis of polysaccharides, Hydrolysis of starch by an enzyme complex from fermentation by Aspergillus awamori. Food Bioprod. Process. 2001, 79, 41–45. [Google Scholar] [CrossRef]
- Pensupa, N.; Jin, M.; Kokolski, M.; Archer, D.B.; Du, C. A solid state fungal fermentation-based strategy for the hydrolysis of wheat straw. Bioresour. Technol. 2013, 149, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Bancerz, R.; Osińska-Jaroszuk, M.; Jaszek, M.; Janusz, G.; Stefaniuk, D.; Sulej, J.; Jarosz-Wilkołazka, A.; Rogalski, J. New alkaline lipase from Rhizomucor variabilis: Biochemical properties and stability in the presence of microbial EPS. Biotechnol. Appl. Biochem. 2016, 63, 67–76. [Google Scholar] [CrossRef]
- Yang, S.Q.; Xiong, H.; Yang, H.Y.; Yan, Q.J.; Jiang, Z.Q. High-level production of beta-1, 3–1, 4-glucanase by Rhizomucor miehei under solid-state fermentation and its potential application in the brewing industry. J. Appl. Microbiol. 2015, 118, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bernfeld, P. Amylases alpha and beta. Meth. Enzymol. 1955, 1, 149–158. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Corredor, D.Y.; Bean, S.; Wang, D. Pretreatment and enzymatic hydrolysis of sorghum bran. Cereal Chem. 2007, 84, 61–66. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Kalaiyarasi, M.; Vincent, S.G.P. Cow dung is an ideal fermentation medium for amylase production in solid state fermentation. J. Gene. Eng. Biotechnol. 2015, 13, 111–117. [Google Scholar] [CrossRef]
- Khan, J.A.; Yadav, S.K. Production of alpha amylase by Aspergillus niger using cheaper substrates employing solis state fermentation. Int. J. Plant Anim. Environ. Sci. 2011, 13, 100–108. [Google Scholar]
- Saleem, A.; Ebrahim, M.K. Production of amylase by fungi isolated from legume seeds collected in Almadinah Almunawwarah, Saudi Arabia. J. Taibah. Univ. Sci. 2014, 8, 90–97. [Google Scholar] [CrossRef]
- Simair, A.A.; Qureshi, A.S.; Khushk, I.; Ali, C.H.; Lashari, S.; Bhutto, M.A.; Mangrio, G.S.; Lu, C. Production and partial characterization of alpha amylase enzyme from Bacillus sp bcc 01–50 and potential application. Biomed. Res. Int. 2017, 2017, 9173040. [Google Scholar] [CrossRef]
- Haqh, I.R.; Albdullah, A.; Shah, A.H. Isolation and screening of fungi for the biosynthesis of alpha amylase. Biotechnology 2002, 12, 61–66. [Google Scholar]
| Substrate | Strain | Fermentation Type | Gluco-Amylase Production | Ref. |
|---|---|---|---|---|
| Babassu cake (kernel residue) | A. awamori | SSF/4 days | 22.8 U/mL | [16] |
| Babassu cake, castor seed, sunflower & canola cakes | A. awamori, A. wenti, P. verrucosum | SSF/4 days | 29.8 U/g | [17] |
| Kitchen waste/Wheat bran | A. niger | SSF/5 days | 1838 U/g | [18] |
| Pastry waste & mixed food waste | A. awamori | SSF/10 days | 76.1 ± 6.1 U/mL | [19] |
| Rice bran | A. awamori, A. niger, A. terreus, A. tamarii | SmF | 264.5 U/g | [20] |
| Sorghum pomace | A. niger and Saccharomyces cerevisae | SmF/3 days | 3.3 U/mg protein | [21] |
| Waste bread | A. awamori | SSF/4–5 days | 114–130.8 U/g | [22,23] |
| Waste potato mash | A. niger | SSF | 274.4 U/mL | [24] |
| Wheat bran | A. awamori | SSF/4 days | 9157 U/g | [25] |
| Wheat bran | A. niger | SSF/4 days | 1.345 ± 0.009 IU/mL/min | [26] |
| Wheat milling by-product | A. awamori | SSF/4 days | 48 U/g 4.4 U/mL | [27] |
| Mineral Solution | Composition | REF |
|---|---|---|
| A | (NH4)2SO4 1 g/L, KH2PO4 0.5 g/L, K2HPO4 0.5 g/L, MgSO4 0.2 g/L | [32] |
| B | KH2PO4 6 g/L, MgSO4·7H2O 1 g/L, FeCl3·4H2O 10 mg/L | [33] |
| C | KH2PO4 1 g/L, MgSO4·7H2O 0.3 g/L, CaCl2 0.3 g/L | [34] |
| D | KH2PO4 1 g/L, MgSO4·7H2O 0.5 g/L | Designed in this study |
| E | NH4NO3 1.5 g/L, K2HPO4 2.5 g/L, KH2PO4 1.5 g/L, MgSO4 0.12 g/L, NaCl 0.25 g/L | Designed in this study |
| Sorghum species | Milling Processing | Starch Content (w/w) | REF |
|---|---|---|---|
| Red sorghum | Peanut butter maker | 16.4 ± 1.3% | This study |
| Red sorghum | Blender | 13.0 ± 0.8% | This study |
| Red sorghum | Knife mill | 81.9 ± 3.2% | This study |
| Red sorghum | A tangential abrasive dehulling device | 30% | [37] |
| Red sorghum | Buhler mill/hammer mill | 24% | [4] |
| Red sorghum | Wet milling | 52.96 ± 1.43% | [7] |
| White sorghum | Wet milling | 49.7 ± 0.86% | [7] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makanjuola, O.; Greetham, D.; Zou, X.; Du, C. The Development of a Sorghum Bran-Based Biorefining Process to Convert Sorghum Bran into Value Added Products. Foods 2019, 8, 279. https://doi.org/10.3390/foods8080279
Makanjuola O, Greetham D, Zou X, Du C. The Development of a Sorghum Bran-Based Biorefining Process to Convert Sorghum Bran into Value Added Products. Foods. 2019; 8(8):279. https://doi.org/10.3390/foods8080279
Chicago/Turabian StyleMakanjuola, Oyenike, Darren Greetham, Xiaoyan Zou, and Chenyu Du. 2019. "The Development of a Sorghum Bran-Based Biorefining Process to Convert Sorghum Bran into Value Added Products" Foods 8, no. 8: 279. https://doi.org/10.3390/foods8080279
APA StyleMakanjuola, O., Greetham, D., Zou, X., & Du, C. (2019). The Development of a Sorghum Bran-Based Biorefining Process to Convert Sorghum Bran into Value Added Products. Foods, 8(8), 279. https://doi.org/10.3390/foods8080279






